New Concepts in the Development and Malformation of the Arterial Valves
Abstract
:1. Anatomy, Histology and Nomenclature of the Mature Arterial (Semilunar) Valves
2. Positioning of the Arterial Valves
3. The Transition Zone and the Arterial-Myocardial Boundary
4. EndMT and Cushion Formation
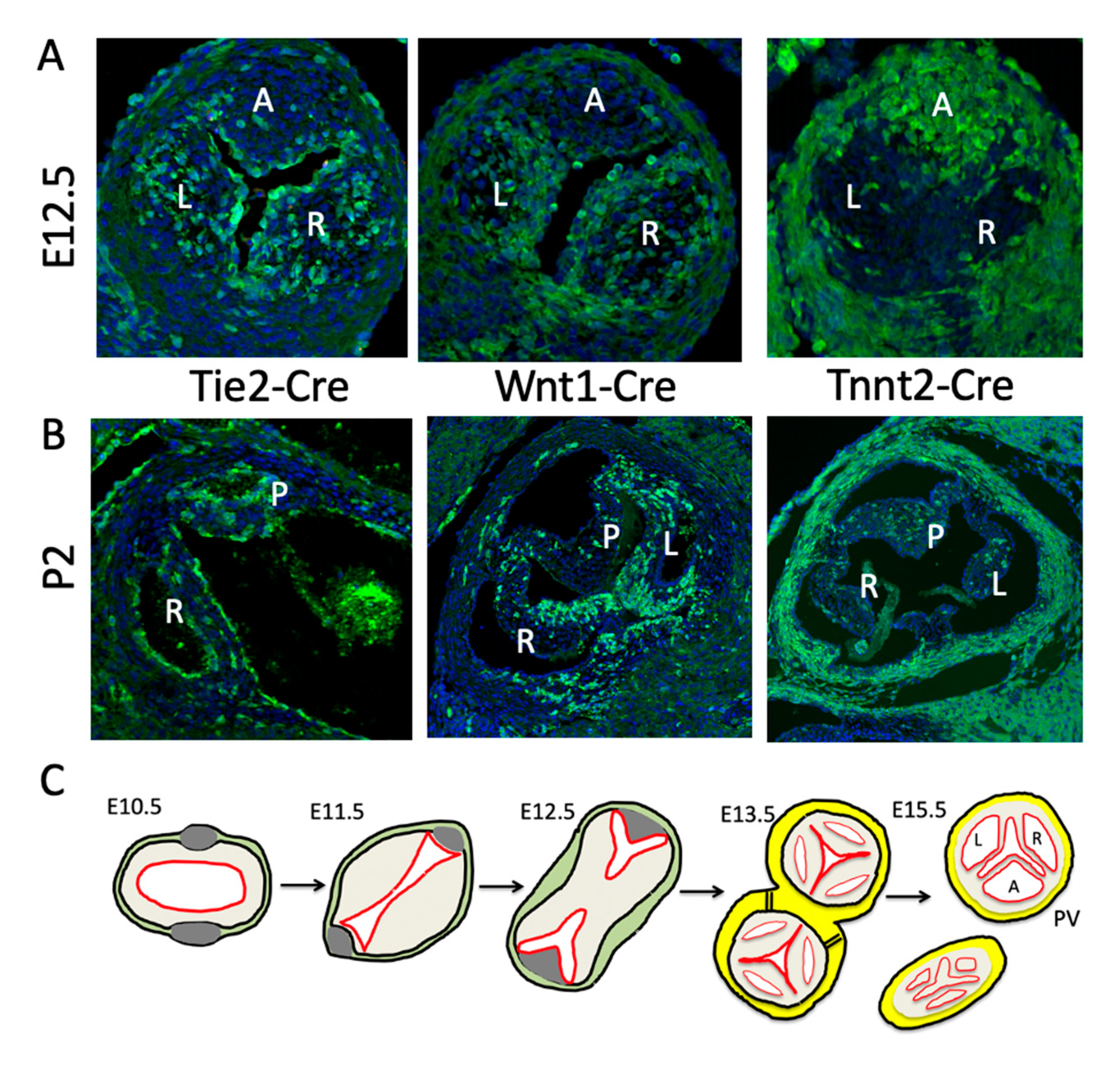
5. Neural Crest Cells, Outflow Tract Septation and Valve Development
6. Direct Differentiation of SHF Progenitors into Valve Mesenchyme
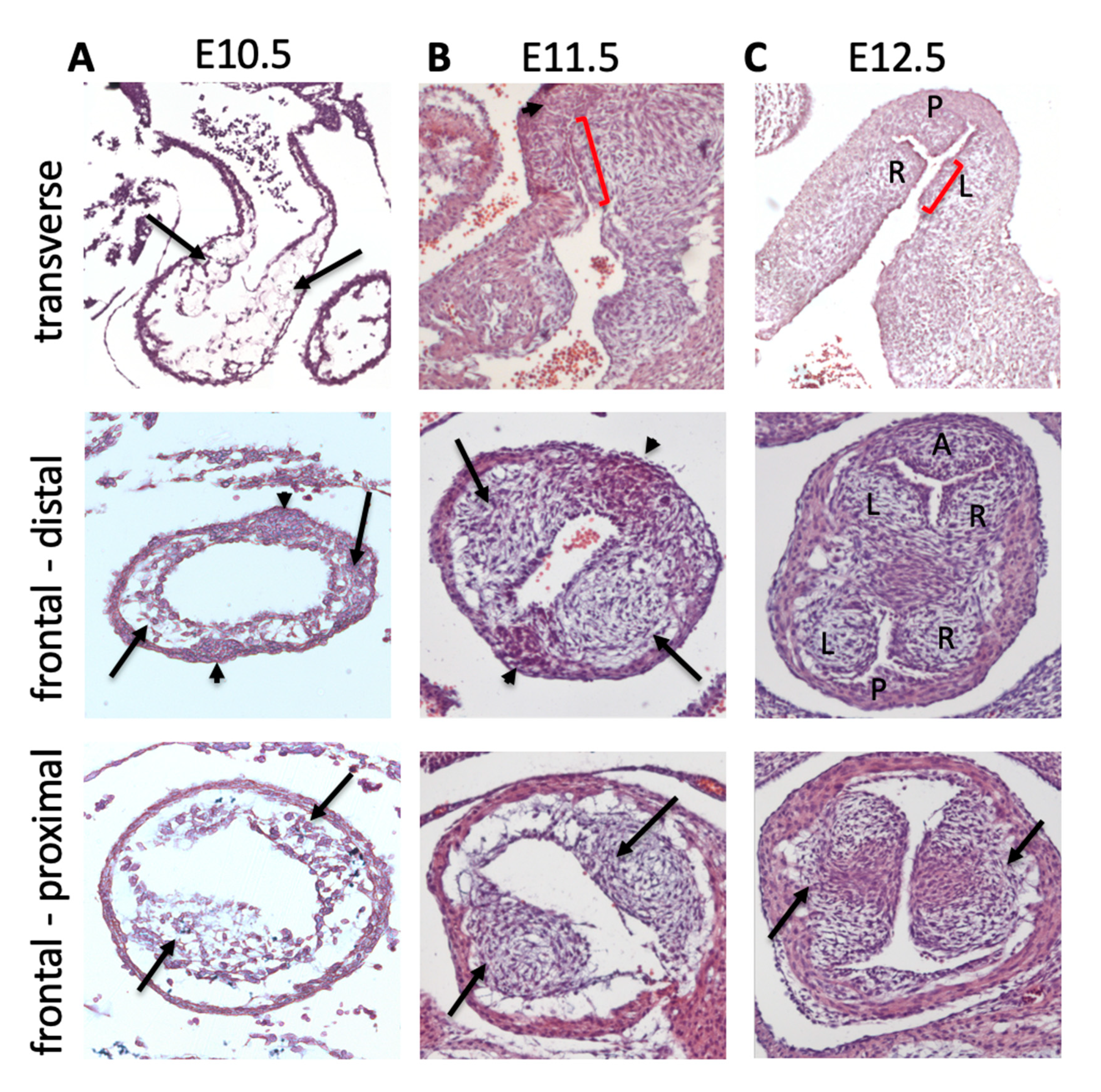
7. Cushion Expansion to Form Arterial Valve Primordia
8. Valve Sculpting
9. Maturation of the Arterial Valve Leaflets
10. Mechanisms Underpinning BAV
11. Hyperplastic Cushions/Leaflets and Excessive Fusion
12. Displaced Cushions
13. Bicuspid Valve without Raphe and the Absent Leaflet
14. Abnormal Leaflet Numbers in the Setting of Defects in Outflow Tract Septation
15. Bicuspid Valves and Congenital Heart Malformations
16. The Genomics of BAV
17. Final Conclusions
Funding
Conflicts of Interest
References
- Webb, S.; Qayyum, S.R.; Anderson, R.H.; Lamers, W.H.; Richardson, M.K. Septation and separation within the outflow tract of the developing heart. J. Anat. 2003, 202, 327–342. [Google Scholar] [CrossRef]
- Anderson, R.H.; Mori, S.; Spicer, D.E.; Brown, N.A.; Mohun, T.J. Development and Morphology of the Ventricular Outflow Tracts. World J. Pediatr. Congenit. Hear. Surg. 2016, 7, 561–577. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.; Eley, L.; Donald-Wilson, C.; Davis, J.; Curley, N.; Alqahtani, A.; Murphy, L.; Anderson, R.H.; Henderson, D.J.; Chaudhry, B. Development and maturation of the fibrous components of the arterial roots in the mouse heart. J. Anat. 2017, 232, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Sievers, H.H.; Hemmer, W.; Beyersdorf, F.; Moritz, A.; Moosdorf, R.; Lichtenberg, A.; Misfeld, M.; Charitos, E.I.; Working Group for Aortic Valve Surgery of German Society of Thoracic and Cardiovascular Surgery. The everyday used nomenclature of the aortic root components: The tower of Babel? Eur. J. Cardiothorac. Surg. 2012, 41, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.H.; Devine, W.A.; Ho, S.Y.; Smith, A.; McKay, R. The myth of the aortic annulus: The anatomy of the subaortic outflow tract. Ann. Thorac. Surg. 1991, 52, 640–646. [Google Scholar] [CrossRef]
- Crucean, A.; Alqahtani, A.; Barron, D.J.; Brawn, W.J.; Richardson, R.V.; O’Sullivan, J.; Anderson, R.H.; Henderson, D.J.; Chaudhry, B. Re-evaluation of hypoplastic left heart syndrome from a developmental and morphological perspective. Orphanet J. Rare Dis. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Choudhary, B.; Zhou, J.; Li, P.; Thomas, S.; Kaartinen, V.; Sucov, H.M. Absence of TGFβ signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis 2009, 47, 115–121. [Google Scholar] [CrossRef]
- Harmon, A.; Nakano, A. Nkx2-5 lineage tracing visualizes the distribution of second heart field-derived aortic smooth muscle. Genesis 2013, 51, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Von Gise, A.; Pu, W.T. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ. Res. 2012, 110, 1628–1645. [Google Scholar] [CrossRef]
- MacGrogan, D.; Luxán, G.; Driessen-Mol, A.; Bouten, C.V.; Baaijens, F.; De La Pompa, J.L. How to Make a Heart Valve: From Embryonic Development to Bioengineering of Living Valve Substitutes. Cold Spring Harb. Perspect. Med. 2014, 4, a013912. [Google Scholar] [CrossRef]
- O’Donnell, A.; Yutzey, K.E. Mechanisms of heart valve development and disease. Development 2020, 147, dev183020. [Google Scholar] [CrossRef]
- Phillips, H.M.; Mahendran, P.; Singh, E.; Anderson, R.H.; Chaudhry, B.; Henderson, D.J. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaflets. Cardiovasc. Res. 2013, 99, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Courchaine, K.; Gray, M.J.; Beel, K.; Thornburg, K.L.; Rugonyi, S. 4-D Computational Modeling of Cardiac Outflow Tract Hemodynamics over Looping Developmental Stages in Chicken Embryos. J. Cardiovasc. Dev. Dis. 2019, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Costell, M.; Carmona, R.; Gustafsson, E.; González-Iriarte, M.; Faessler, R.; Muñoz-Chápuli, R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ. Res. 2002, 91, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.H.; Chaudhry, B.; Mohun, T.J.; Bamforth, S.D.; Hoyland, D.; Phillips, H.M.; Webb, S.; Moorman, A.F.; Brown, N.A.; Henderson, D.J. Normal and abnormal development of the intrapericardial arterial trunks in humans and mice. Cardiovasc. Res. 2012, 95, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Waldo, K.L.; Hutson, M.R.; Ward, C.C.; Zdanowicz, M.; Stadt, H.A.; Kumiski, D.; Abu-Issa, R.; Kirby, M.L. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev. Biol. 2005, 281, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Ramsbottom, S.A.; Sharma, V.; Rhee, H.J.; Eley, L.; Phillips, H.M.; Rigby, H.F.; Dean, C.; Chaudhry, B.; Henderson, D.J. Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells is Required for Outflow Tract Lengthening during Cardiac Development. PLoS Genet. 2014, 10, e1004871. [Google Scholar] [CrossRef] [Green Version]
- Sizarov, A.; Lamers, W.H.; Mohun, T.J.; Brown, N.A.; Anderson, R.H.; Moorman, A.F. Three-dimensional and molecular analysis of the arterial pole of the developing human heart. J. Anat. 2012, 220, 336–349. [Google Scholar] [CrossRef]
- Kramer, T.C. The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. Am. J. Anat. 1942, 71, 343–370. [Google Scholar] [CrossRef] [Green Version]
- Eley, L.; Alqahtani, A.; MacGrogan, D.; Richardson, R.V.; Murphy, L.; Salguero-Jiménez, A.; Pedro, M.S.R.S.; Tiurma, S.; McCutcheon, L.; Gilmore, A.; et al. A novel source of arterial valve cells linked to bicuspid aortic valve without raphe in mice. eLife 2018, 7, e34110. [Google Scholar] [CrossRef]
- Combs, M.D.; Yutzey, K.E. Heart Valve Development. Circ. Res. 2009, 105, 408–421. [Google Scholar] [CrossRef] [Green Version]
- De Vlaming, A.; Sauls, K.; Hajdu, Z.; Visconti, R.P.; Mehesz, A.N.; Levine, R.A.; Slaugenhaupt, S.A.; Hagege, A.; Chester, A.H.; Markwald, R.R.; et al. Atrioventricular valve development: New perspectives on an old theme. Differentiation 2012, 84, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Puceat, M. Embryological origin of the endocardium and derived valve progenitor cells: From developmental biology to stem cell-based valve repair. Biochim. Biophys. Acta 2013, 1833, 917–922. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.-L.; Liang, X.; Shi, Y.; Chu, P.-H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 Identifies a Cardiac Progenitor Population that Proliferates Prior to Differentiation and Contributes a Majority of Cells to the Heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef] [Green Version]
- Verzi, M.P.; McCulley, D.J.; De Val, S.; Dodou, E.; Black, B.L. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 2005, 287, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Feliciano, J.; Lee, K.-H.; Kong, S.W.; Rajagopal, S.; Ma, Q.; Springer, Z.; Izumo, S.; Tabin, C.J.; Pu, W.T. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development 2006, 133, 3607–3618. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Wang, Y.; Lui, W.; Langworthy, M.; Tompkins, K.L.; Hatzopoulos, A.K.; Baldwin, H.S.; Zhou, B. Nfatc1 Coordinates Valve Endocardial Cell Lineage Development Required for Heart Valve Formation. Circ. Res. 2011, 109, 183–192. [Google Scholar] [CrossRef] [Green Version]
- De La Pompa, J.L.; Timmerman, L.A.; Takimoto, H.; Yoshida, H.; Elia, A.J.; Samper, E.; Potter, J.; Wakeham, A.; Marengere, L.; Langille, B.L.; et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 1998, 392, 182–186. [Google Scholar] [CrossRef]
- Ranger, A.M.; Grusby, M.J.; Hodge, M.R.; Gravallese, E.M.; De La Brousse, F.C.; Hoey, T.; Mickanin, C.; Baldwin, H.S.; Glimcher, L.H. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 1998, 392, 186–190. [Google Scholar] [CrossRef]
- Chang, C.-P.; Neilson, J.R.; Bayle, J.; Gestwicki, J.E.; Kuo, A.; Stankunas, K.; Graef, I.A.; Crabtree, G.R. A Field of Myocardial-Endocardial NFAT Signaling Underlies Heart Valve Morphogenesis. Cell 2004, 118, 649–663. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, L.A.; Grego-Bessa, J.; Raya, A.; Bertran, E.; Pérez-Pomares, J.; Díez, J.; Aranda, S.; Palomo-Ponce, S.; McCormick, F.; Izpisúa-Belmonte, J.C.; et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004, 18, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Luna-Zurita, L.; Prados, B.; Grego-Bessa, J.; Luxán, G.; Del Monte, G.; Benguria, A.; Adams, R.H.; Pérez-Pomares, J.; De La Pompa, J.L. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J. Clin. Investig. 2010, 120, 3493–3507. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Lu, M.-F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef] [Green Version]
- Kokudo, T.; Suzuki, Y.; Yoshimatsu, Y.; Yamazaki, T.; Watabe, T.; Miyazono, K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J. Cell Sci. 2008, 121 Pt 20, 3317–3324. [Google Scholar] [CrossRef] [Green Version]
- Lakkis, M.M.; Epstein, J.A. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development 1998, 125, 4359–4367. [Google Scholar]
- Gitler, A.D.; Zhu, Y.; Ismat, F.A.; Lu, M.M.; Yamauchi, Y.; Parada, L.F.; Epstein, J.A. Nf1 has an essential role in endothelial cells. Nat. Genet. 2002, 33, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Araki, T.; Chan, G.; Newbigging, S.; Morikawa, L.; Bronson, R.T.; Neel, B.G. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proc. Natl. Acad. Sci. USA 2009, 106, 4736–4741. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, J.; Alfieri, C.M.; Yutzey, K.E. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev. Dyn. 2004, 230, 239–250. [Google Scholar] [CrossRef]
- Kisanuki, Y.Y.; Ehammerbc, R.; Miyazaki, J.; Williamsba, S.C.; Arichardsonef, J.; Yanagisawabea, M. Tie2-Cre Transgenic Mice: A New Model for Endothelial Cell-Lineage Analysis In Vivo. Dev. Biol. 2001, 230, 230–242. [Google Scholar] [CrossRef] [Green Version]
- MacGrogan, D.; D’Amato, G.; Travisano, S.; Martínez-Poveda, B.; Luxán, G.; Del Monte-Nieto, G.; Papoutsi, T.; Sbroggiò, M.; Bou, V.; Arco, P.G.-D.; et al. Sequential Ligand-Dependent Notch Signaling Activation Regulates Valve Primordium Formation and Morphogenesis. Circ. Res. 2016, 118, 1480–1497. [Google Scholar] [CrossRef]
- Shigeta, A.; Huang, V.; Zuo, J.; Besada, R.; Nakashima, Y.; Lu, Y.; Ding, Y.; Pellegrini, M.; Kulkarni, R.P.; Hsiai, T.; et al. Endocardially Derived Macrophages are Essential for Valvular Remodeling. Dev. Cell 2019, 48, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Kirby, M.L.; Gale, T.F.; Stewart, D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983, 220, 1059–1061. [Google Scholar] [CrossRef]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607–1616. [Google Scholar]
- Simões-Costa, M.; Bronner, M.E. Establishing neural crest identity: A gene regulatory recipe. Development 2015, 142, 242–257. [Google Scholar] [CrossRef] [Green Version]
- Leonard, C.E.; Taneyhill, L.A. The road best traveled: Neural crest migration upon the extracellular matrix. Semin. Cell Dev. Biol. 2020, 100, 177–185. [Google Scholar] [CrossRef]
- Dupin, E.; Calloni, G.W.; Coelho-Aguiar, J.D.M.; Le Douarin, N.M. The issue of the multipotency of the neural crest cells. Dev. Biol. 2018, 444, S47–S59. [Google Scholar] [CrossRef]
- Scholl, A.M.; Kirby, M.L.; Reinhold, A.M. Signals controlling neural crest contributions to the heart. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Keyte, A.L.; Alonzo-Johnsen, M.; Hutson, M.R. Evolutionary and developmental origins of the cardiac neural crest: Building a divided outflow tract. Birth Defects Res. C Embryo Today 2014, 102, 309–323. [Google Scholar] [CrossRef] [Green Version]
- Plein, A.; Fantin, A.; Ruhrberg, C. Neural Crest Cells in Cardiovascular Development. Mech. Regen. 2015, 111, 183–200. [Google Scholar] [CrossRef]
- Bockman, D.E.; Redmond, M.E.; Kirby, M.L. Alteration of early vascular development after ablation of cranial neural crest. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1989, 225, 209–217. [Google Scholar] [CrossRef]
- Bockman, D.E.; Redmond, M.E.; Kirby, M.L. Altered Development of Pharyngeal Arch Vessels after Neural Crest Ablation. Ann. N. Y. Acad. Sci. 1990, 588, 296–304. [Google Scholar] [CrossRef]
- Bradshaw, L.; Chaudhry, B.; Hildreth, V.; Webb, S.; Henderson, D.J. Dual role for neural crest cells during outflow tract septation in the neural crest-deficient mutant Splotch2H. J. Anat. 2009, 214, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.B.; Feiner, L.; Lu, M.M.; Li, J.; Ma, X.; Webber, A.L.; Jia, L.; Raper, J.A.; Epstein, J.A. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 2001, 128, 3071–3080. [Google Scholar] [PubMed]
- Luo, T.; Lee, Y.-H.; Saint-Jeannet, J.-P.; Sargent, T.D. Induction of neural crest in Xenopus by transcription factor AP2. Proc. Natl. Acad. Sci. USA 2003, 100, 532–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poelmann, R.; Groot, A.G.-D. A Subpopulation of Apoptosis-Prone Cardiac Neural Crest Cells Targets to the Venous Pole: Multiple Functions in Heart Development? Dev. Biol. 1999, 207, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Hoff, M.J.V.D.; Moorman, A.F.; Ruijter, J.M.; Lamers, W.H.; Bennington, R.W.; Markwald, R.R.; Wessels, A. Myocardialization of the Cardiac Outflow Tract. Dev. Biol. 1999, 212, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Hoff, M.J.V.D.; Kruithof, B.P.; Moorman, A.F.; Markwald, R.R.; Wessels, A. Formation of Myocardium after the Initial Development of the Linear Heart Tube. Dev. Biol. 2001, 240, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Mifflin, J.J.; Dupuis, L.E.; Alcala, N.E.; Russell, L.G.; Kern, C.B. Intercalated cushion cells within the cardiac outflow tract are derived from the myocardial troponin T type 2 (Tnnt2) Cre lineage. Dev. Dyn. 2018, 247, 1005–1017. [Google Scholar] [CrossRef]
- Peterson, J.C.; Chughtai, M.; Wisse, L.J.; Groot, A.C.G.-D.; Feng, Q.; Goumans, M.-J.; VanMunsteren, J.C.; Jongbloed, M.R.M.; DeRuiter, M.C. Bicuspid aortic valve formation: Nos3 mutation leads to abnormal lineage patterning of neural crest cells and the second heart field. Dis. Model. Mech. 2018, 11, dmm034637. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Chaboissier, M.-C.; Behringer, R.R.; Rowitch, D.H.; Schedl, A.; Epstein, J.A.; De Crombrugghe, B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc. Natl. Acad. Sci. USA 2004, 101, 6502–6507. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.; Kulessa, H.; Tompkins, K.; Zhou, Y.; Batts, L.; Baldwin, H.S.; Hogan, B. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003, 17, 2362–2367. [Google Scholar] [CrossRef] [Green Version]
- Théveniau-Ruissy, M.; Dandonneau, M.; Mesbah, K.; Ghez, O.; Mattei, M.; Miquerol, L.; Kelly, R.G. The del22q11.2 Candidate GeneTbx1Controls Regional Outflow Tract Identity and Coronary Artery Patterning. Circ. Res. 2008, 103, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.S.; Sridurongrit, S.; Ruiz-Lozano, P.; Kaartinen, V. Deficient Signaling via Alk2 (Acvr1) Leads to Bicuspid Aortic Valve Development. PLoS ONE 2012, 7, e35539. [Google Scholar] [CrossRef] [Green Version]
- Mommersteeg, M.T.; Yeh, M.L.; Parnavelas, J.G.; Andrews, W.D. Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves. Cardiovasc. Res. 2015, 106, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Miquerol, L.; Langille, B.L.; Nagy, A. Embryonic development is disrupted by modest increases in vascularendothelial growth factor gene expression. Development 2000, 127, 3941–3946. [Google Scholar]
- Dor, Y.; Camenisch, T.D.; Itin, A.I.; Fishman, G.; McDonald, J.A.; Carmeliet, P.; Keshet, E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development 2001, 128, 1531–1538. [Google Scholar]
- Lee, Y.M.; Cope, J.J.; Ackermann, G.E.; Goishi, K.; Armstrong, E.J.; Paw, B.H.; Bischoff, J. Vascular endothelial growth factor receptor signaling is required for cardiac valve formation in zebrafish. Dev. Dyn. 2006, 235, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Sugi, Y.; Ito, N.; Szebenyi, G.; Myers, K.; Fallon, J.F.; Mikawa, T.; Markwald, R.R. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev. Biol. 2003, 258, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Bronson, R.T.; Klaman, L.D.; Hampton, T.G.; Wang, J.-F.; Green, P.J.; Magnuson, T.; Douglas, P.S.; Morgan, J.P.; Neel, B.G. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat. Genet. 2000, 24, 296–299. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Ishii, M.; Sun, J.; Sucov, H.M.; Maxson, R.E.; Maxson, R.E. Msx1 and Msx2 regulate survival of secondary heart field precursors and post-migratory proliferation of cardiac neural crest in the outflow tract. Dev. Biol. 2007, 308, 421–437. [Google Scholar] [CrossRef]
- Srinivasan, D.K.; Dheen, S.T.; Tay, S.S.-W. Maternal diabetes induces congenital heart defects in mice by altering the expression of genes involved in cardiovascular development. Cardiovasc. Diabetol. 2007, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Von Gise, A.; Liu, Q.; Hu, T.; Tian, X.; He, L.; Pu, W.; Huang, X.; He, L.; Cai, C.-L.; et al. Yap1 is Required for Endothelial to Mesenchymal Transition of the Atrioventricular Cushion. J. Biol. Chem. 2014, 289, 18681–18692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mjaatvedt, C.; Yamamura, H.; Capehart, A.; Turner, D.; Markwald, R. The Cspg2 Gene, Disrupted in thehdfMutant, is Required for Right Cardiac Chamber and Endocardial Cushion Formation. Dev. Biol. 1998, 202, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camenisch, T.D.; Spicer, A.P.; Brehm-Gibson, T.; Biesterfeldt, J.; Augustine, M.L.; Calabro, A.; Kubalak, S.; Klewer, S.E.; McDonald, J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Investig. 2000, 106, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, L.E.; McCulloch, D.R.; McGarity, J.D.; Bahan, A.; Wessels, A.; Weber, D.; Diminich, A.M.; Nelson, C.M.; Apte, S.S.; Kern, C.B. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev. Biol. 2011, 357, 152–164. [Google Scholar] [CrossRef] [Green Version]
- Durst, R.; Sauls, K.; Peal, D.S.; DeVlaming, A.; Toomer, K.; Leyne, M.; Salani, M.; Talkowski, M.E.; Brand, H.; Perrocheau, M.; et al. Mutations in DCHS1 cause mitral valve prolapse. Nature 2015, 525, 109–113. [Google Scholar] [CrossRef]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef]
- Toomer, K.A.; Fulmer, D.; Guo, L.; Drohan, A.; Peterson, N.; Swanson, P.; Brooks, B.; Mukherjee, R.; Body, S.; Lipschutz, J.H.; et al. A role for primary cilia in aortic valve development and disease. Dev. Dyn. 2017, 246, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Irigoín, F.; Badano, J.L. Keeping the Balance Between Proliferation and Differentiation: The Primary Cilium. Curr. Genom. 2011, 12, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Hinton, R.B.; Lincoln, J.; Deutsch, G.H.; Osinska, H.; Manning, P.B.; Benson, D.W.; Yutzey, K.E. Extracellular Matrix Remodeling and Organization in Developing and Diseased Aortic Valves. Circ. Res. 2006, 98, 1431–1438. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-J.; Chen, C.-H.; Zhou, B.; Chang, C.-P. Partitioning the heart: Mechanisms of cardiac septation and valve development. Development 2012, 139, 3277–3299. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Baldwin, H.S.; Zhou, B. Nfatc1 directs the endocardial progenitor cells to make heart valve primordium. Trends Cardiovasc. Med. 2013, 23, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Nomura-Kitabayashi, A.; Phoon, C.K.; Kishigami, S.; Rosenthal, J.; Yamauchi, Y.; Abe, K.; Yamamura, K.-I.; Samtani, R.; Lo, C.W.; Mishina, Y. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am. J. Physiol. Circ. Physiol. 2009, 297, H1617–H1628. [Google Scholar] [CrossRef] [Green Version]
- Keyes, W.M.; Sanders, E.J. Regulation of apoptosis in the endocardial cushions of the developing chick heart. Am. J. Physiol. Physiol. 2002, 282, C1348–C1360. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Wessels, A.; Gourdie, R.G.; Thompson, R.P. Spatiotemporal and tissue specific distribution of apoptosis in the developing chick heart. Dev. Dyn. 2002, 223, 119–133. [Google Scholar] [CrossRef]
- Abdelwahid, E.; Rice, D.P.; Pelliniemi, L.J.; Jokinen, E. Overlapping and differential localization of Bmp-2, Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001, 305, 67–78. [Google Scholar] [CrossRef]
- Zhao, Z.; Rivkees, S.A. Programmed cell death in the developing heart: Regulation by BMP4 and FGF2. Dev. Dyn. 2000, 217, 388–400. [Google Scholar] [CrossRef]
- Sharma, P.R.; Anderson, R.H.; Copp, A.J.; Henderson, D.J. Spatiotemporal analysis of programmed cell death during mouse cardiac septation. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2004, 277, 355–369. [Google Scholar] [CrossRef]
- Hurle, J.; Blanco, A.M. Development of mouse semilunar valves. Brain Struct. Funct. 1980, 160, 83–91. [Google Scholar] [CrossRef]
- Hurle, J. Scanning and light microscope studies of the development of the chick embryo semilunar heart valves. Brain Struct. Funct. 1979, 157, 69–80. [Google Scholar] [CrossRef]
- Alfieri, C.M.; Cheek, J.; Chakraborty, S.; Yutzey, K.E. Wnt signaling in heart valve development and osteogenic gene induction. Dev. Biol. 2010, 338, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol 1995, 172, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Biechler, S.V.; Junor, L.; Evans, A.N.; Eberth, J.F.; Price, R.L.; Potts, J.D.; Yost, M.J.; Goodwin, R.L. The impact of flow-induced forces on the morphogenesis of the outflow tract. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- Goddard, L.M.; Duchemin, A.-L.; Ramalingan, H.; Wu, B.; Chen, M.; Bamezai, S.; Yang, J.; Li, L.; Morley, M.P.; Wang, T.; et al. Hemodynamic Forces Sculpt Developing Heart Valves through a KLF2-WNT9B Paracrine Signaling Axis. Dev. Cell 2017, 43, 274–289. [Google Scholar] [CrossRef]
- Hsu, J.J.; Vedula, V.; Baek, K.I.; Chen, C.; Chen, J.; Chou, M.I.; Lam, J.; Subhedar, S.; Wang, J.; Ding, Y.; et al. Contractile and hemodynamic forces coordinate Notch1b-mediated outflow tract valve formation. JCI Insight 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.; Eberth, J.F.; Goodwin, R.L.; Potts, J.D. Altered Hemodynamics in the Embryonic Heart Affects Outflow Valve Development. J. Cardiovasc. Dev. Dis. 2015, 2, 108–124. [Google Scholar] [CrossRef]
- Menon, V.; Eberth, J.F.; Junor, L.; Potts, A.J.; Belhaj, M.; DiPette, N.J.; Jenkins, M.W.; Potts, J.D. Removing vessel constriction on the embryonic heart results in changes in valve gene expression, morphology, and hemodynamics. Dev. Dyn. 2017, 247, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Steed, E.; Boselli, F.; Vermot, J. Hemodynamics driven cardiac valve morphogenesis. Biochim. Biophys. Acta 2016, 1863, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Poelmann, R.E.; Groot, A.C.G.-D. Hemodynamics in Cardiac Development. J. Cardiovasc. Dev. Dis. 2018, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckel, E.; Boselli, F.; Roth, S.; Krudewig, A.; Belting, H.-G.; Charvin, G.; Vermot, J. Oscillatory Flow Modulates Mechanosensitive klf2a Expression through trpv4 and trpp2 during Heart Valve Development. Curr. Biol. 2015, 25, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Faucherre, A.; Maati, H.M.O.; Nasr, N.; Pinard, A.; Theron, A.; Odelin, G.; Desvignes, J.-P.; Salgado, D.; Collod-Béroud, G.; Avierinos, J.-F.; et al. Piezo1 is required for outflow tract and aortic valve development. J. Mol. Cell. Cardiol. 2020, 143, 51–62. [Google Scholar] [CrossRef]
- Henderson, D.J.; Copp, A.J. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ. Res. 1998, 83, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Fenderson, B.A.; Stamenkovic, I.; Aruffo, A. Localization of hyaluronan in mouse embryos during implantation, gastrulation and organogenesis. Differentiation 1993, 54, 85–98. [Google Scholar] [CrossRef]
- Hurle, J.; Kitten, G.; Sakai, L.Y.; Volpin, D.; Solursh, M. Elastic extracellular matrix of the embryonic chick heart: An immunohistological study using laser confocal microscopy. Dev. Dyn. 1994, 200, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Votteler, M.; Berrio, D.A.C.; Horke, A.; Sabatier, L.; Reinhardt, D.P.; Nsair, A.; Aikawa, E.; Schenke-Layland, K. Elastogenesis at the onset of human cardiac valve development. Development 2013, 140, 2345–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulin, A.; Hortells, L.; Gomez-Stallons, M.V.; O’Donnell, A.; Chetal, K.; Adam, M.; Lancellotti, P.; Oury, C.; Potter, S.S.; Salomonis, N.; et al. Maturation of heart valve cell populations during postnatal remodeling. Development 2019, 146, dev173047. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Wang, Y.; Xiao, F.; Butcher, J.T.; Yutzey, K.E.; Zhou, B. Developmental Mechanisms of Aortic Valve Malformation and Disease. Annu. Rev. Physiol. 2017, 79, 21–41. [Google Scholar] [CrossRef]
- Cujec, B.; Pollick, C. Isolated Thickening of One Aortic Cusp: Preferential Thickening of the Noncoronary Cusp. J. Am. Soc. Echocardiogr. 1988, 1, 430–432. [Google Scholar] [CrossRef]
- Nakamura, T.; Colbert, M.C.; Robbins, J. Neural Crest Cells Retain Multipotential Characteristics in the Developing Valves and Label the Cardiac Conduction System. Circ. Res. 2006, 98, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- Anstine, L.J.; Horne, T.E.; Horwitz, E.M.; Lincoln, J. Contribution of Extra-Cardiac Cells in Murine Heart Valves is Age-Dependent. J. Am. Hear. Assoc. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Hulin, A.; Anstine, L.J.; Kim, A.J.; Potter, S.J.; DeFalco, T.J.; Lincoln, J.; Yutzey, K.E. Macrophage Transitions in Heart Valve Development and Myxomatous Valve Disease. Arter. Thromb. Vasc. Biol. 2018, 38, 636–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, A.; Wang, S.H.; Skelding, K.; Miller, D.; Simper, D.; Caplice, N. Bone marrow-derived myofibroblasts are present in adult human heart valves. J. Hear. Valve Dis. 2005, 14, 674–678. [Google Scholar]
- Tao, G.; Kotick, J.D.; Lincoln, J. Heart Valve Development, Maintenance, and Disease. Mech. Regen. 2012, 100, 203–232. [Google Scholar] [CrossRef]
- Krepp, J.M.; Roman, M.J.; Devereux, R.B.; Bruce, A.; Prakash, S.K.; Morris, S.A.; Milewicz, D.M.; Holmes, K.W.; Ravekes, W.; Shohet, R.V.; et al. Bicuspid and unicuspid aortic valves: Different phenotypes of the same disease? Insight from the GenTAC Registry. Congenit. Hear. Dis. 2017, 12, 740–745. [Google Scholar] [CrossRef]
- Lopez, A.; Fernández, M.C.; Durán, A.C.; Sans-Coma, V.; Fernández, B. Quadricuspid aortic valves in Syrian hamsters and their formation according to current knowledge on valvulogenesis. Jpn. J. Veter. Res. 2015, 63, 37–43. [Google Scholar]
- Sievers, H.-H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef] [Green Version]
- Urena, M.; Doyle, D.; Dumont, É.; Ribeiro, H.B.; Bilodeau, S.; Rodés-Cabau, J. Transcatheter Aortic Valve Replacement with a Balloon-expandable Valve for the Treatment of Noncalcified Bicuspid Aortic Valve Disease. Rev. Española Cardiol. 2014, 67, 327–329. [Google Scholar] [CrossRef]
- Kinoshita, T.; Naito, S.; Tomoaki, S.; Asai, T. Valve Phenotype and Risk Factors of Aortic Dilatation After Aortic Valve Replacement in Japanese Patients With Bicuspid Aortic Valve. Circ. J. 2016, 80, 1356–1361. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.J.; Jin, X.; Song, J.-K.; Lee, S.; Lee, J.H.; Park, J.-B.; Lee, S.-P.; Kim, D.-H.; Park, S.-J.; Kim, Y.-J.; et al. Clinical Characteristics of Korean Patients with Bicuspid Aortic Valve Who Underwent Aortic Valve Surgery. Korean Circ. J. 2018, 48, 48. [Google Scholar] [CrossRef] [Green Version]
- Niaz, T.; Poterucha, J.T.; Olson, T.M.; Johnson, J.N.; Craviari, C.; Nienaber, T.; Palfreeman, J.; Cetta, F.; Hagler, D.J. Characteristic Morphologies of the Bicuspid Aortic Valve in Patients with Genetic Syndromes. J. Am. Soc. Echocardiogr. 2017, 31, 194–200. [Google Scholar] [CrossRef]
- Koenraadt, W.M.C.; Grewal, N.; Gaidoukevitch, O.Y.; DeRuiter, M.C.; Groot, A.C.G.-D.; Bartelings, M.M.; Holman, E.R.; Klautz, R.J.M.; Schalij, M.J.; Jongbloed, M.R. The extent of the raphe in bicuspid aortic valves is associated with aortic regurgitation and aortic root dilatation. Neth. Hear. J. 2016, 24, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, W.M. Ueber Abweichungen in der Zahl der Semilunar-klappen. Beitr. Pathol. Anat. 1918, 64, 39–54. [Google Scholar]
- Simonds, J.P. Congenital Malformations of the Aortic and Pulmonary Valves. Am. J. Med Sci. 1923, 166, 584–595. [Google Scholar] [CrossRef]
- Koletsky, S. Congenital bicuspid pulmonary valves. Arch. Pathol. 1941, 31, 338–353. [Google Scholar]
- Shaner, R.F. Abnormal pulmonary and aortic semilunar valves in embryos. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1963, 147, 5–13. [Google Scholar] [CrossRef]
- Sans-Coma, V.; Fernández, B.; Durán, A.C.; Thiene, G.; Arqué, J.M.; Muñoz-Chápuli, R.; Cardo, M. Fusion of valve cushions as a key factor in the formation of congenital bicuspid aortic valves in Syrian hamsters. Anat. Rec. 1996, 244, 490–498. [Google Scholar] [CrossRef]
- Soto-Navarrete, M.T.; López-Unzu, M.Á.; Durán, A.C.; Fernandez, B. Embryonic development of bicuspid aortic valves. Prog. Cardiovasc. Dis. 2020, 25. [Google Scholar] [CrossRef]
- Luxán, G.; D’Amato, G.; MacGrogan, D.; De La Pompa, J.L. Endocardial Notch Signaling in Cardiac Development and Disease. Circ. Res. 2016, 118. [Google Scholar] [CrossRef]
- Sans-Coma, V.; Cardo, M.; Thiene, G.; Fernández, B.; Arqué, J.M.; Durán, A.C. Bicuspid aortic and pulmonary valves in the Syrian hamster. Int. J. Cardiol. 1992, 34, 249–254. [Google Scholar] [CrossRef]
- Fernández, B.; Fernandez, M.C.; Durán, A.C.; Lopez, D.; Martire, A.; Sans-Coma, V. Anatomy and formation of congenital bicuspid and quadricuspid pulmonary valves in Syrian hamsters. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1998, 250, 70–79. [Google Scholar] [CrossRef]
- Fernández, B.; Durán, A.C.; Thiene, G.; Cardo, M.; Arqué, J.M.; Sans-Coma, V. Embryological evidence for the formation of a quadricuspid aortic valve in the Syrian hamster. Cardiovasc. Pathol. 1994, 3, 287–291. [Google Scholar] [CrossRef]
- Sans-Coma, V.; Fernandez, M.C.; Fernández, B.; Durán, A.C.; Anderson, R.H.; Arqué, J.M. Genetically alike Syrian hamsters display both bifoliate and trifoliate aortic valves. J. Anat. 2011, 220, 92–101. [Google Scholar] [CrossRef]
- Fernandez, B.; Durán, A.; Martire, A.; López, D.; Sans-Coma, V. New Embryological Evidence for the Formation of Quadricuspid Aortic Valves in the Syrian Hamster (Mesocricetus auratus). J. Comp. Pathol. 1999, 121, 89–94. [Google Scholar] [CrossRef]
- Odelin, G.; Faure, E.; Kober, F.; Maurel-Zaffran, C.; Theron, A.; Coulpier, F.; Guillet, B.; Bernard, M.; Avierinos, J.-F.; Charnay, P.; et al. Loss of Krox20 results in aortic valve regurgitation and impaired transcriptional activation of fibrillar collagen genes. Cardiovasc. Res. 2014, 104, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Odelin, G.; Faure, E.; Coulpier, F.; Di Bonito, M.; Bajolle, F.; Studer, M.; Avierinos, J.-F.; Charnay, P.; Topilko, P.; Zaffran, S. Krox20 defines a subpopulation of cardiac neural crest cells contributing to arterial valves and bicuspid aortic valve. Development 2017, 145, dev151944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milos, N.C.; Nordstrom, D.B.; Ongaro, I.; Chow, A.K. Variations in structure of the outflow tract of the human embryonic heart: A new hypothesis for generating bicuspid aortic semilunar valves. Ann. Anat. Anat. Anz. 2017, 211, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, B.; Durán, A.C.; Fernández-Gallego, T.; Fernandez, M.C.; Such, M.; Arqué, J.M.; Sans-Coma, V. Bicuspid Aortic Valves With Different Spatial Orientations of the Leaflets Are Distinct Etiological Entities. J. Am. Coll. Cardiol. 2009, 54, 2312–2318. [Google Scholar] [CrossRef] [Green Version]
- Odelin, G.; Faure, E.; Maurel-Zaffran, C.; Zaffran, S. Krox20 Regulates Endothelial Nitric Oxide Signaling in Aortic Valve Development and Disease. J. Cardiovasc. Dev. Dis. 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Koenraadt, W.M.C.; Bartelings, M.M.; Groot, A.C.G.-D.; Bökenkamp, R.; DeRuiter, M.C.; Schalij, M.J.; Jongbloed, M.R. Pulmonary Valve Morphology in Patients with Bicuspid Aortic Valves. Pediatr. Cardiol. 2018, 39, 690–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laforest, B.; Andelfinger, G.; Nemer, M. Loss of Gata5 in mice leads to bicuspid aortic valve. J. Clin. Investig. 2011, 121, 2876–2887. [Google Scholar] [CrossRef] [Green Version]
- Franz, T. Persistent truncus arteriosus in the Splotch mutant mouse. Brain Struct. Funct. 1989, 180, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Sundeen, J.T.; Bloom, S. Sinus of valsalva aneurysm associated with multiple conotruncal congenital malformations. Hum. Pathol. 1987, 18, 96–99. [Google Scholar] [CrossRef]
- Kappetein, A.; Groot, A.G.-D.; Zwinderman, A.; Rohmer, J.; Poelmann, R.; Huysmans, H. The neural crest as a possible pathogenetic factor in coarctation of the aorta and bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 1991, 102, 830–836. [Google Scholar] [CrossRef]
- Jain, R.; Engleka, K.A.; Rentschler, S.L.; Manderfield, L.J.; Li, L.; Yuan, L.; Epstein, J.A. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J. Clin. Investig. 2011, 121, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, M. Cardio-cephalic neural crest syndrome: A novel hypothesis of vascular neurocristopathy. Interv. Neuroradiol. 2017, 23, 572–576. [Google Scholar] [CrossRef]
- Quintessenza, J.A. Tetralogy of Fallot: Management of the Pulmonary Valve. In Surgery of Conotruncal Anomalies; Lacour-Gayet, F., Bove, E.L., Hraska, V., Morell, V.O., Spray, T.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 139–147. [Google Scholar]
- Lammer, E.J.; Opitz, J.M.; Reynolds, J.F. The DiGeorge anomaly as a developmental field defect. Am. J. Med. Genet. 1986, 25, 113–127. [Google Scholar] [CrossRef]
- Van Mierop, L.H.; Kutsche, L.M. Cardiovascular anomalies in digeorge syndrome and importance of neural crest as a possible pathogenetic factor. Am. J. Cardiol. 1986, 58, 133–137. [Google Scholar] [CrossRef]
- Keyte, A.; Hutson, M.R. The neural crest in cardiac congenital anomalies. Differentiation 2012, 84, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Parisot, P.; Mesbah, K.; Théveniau-Ruissy, M.; Kelly, R.G. Tbx1, subpulmonary myocardium and conotruncal congenital heart defects. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 477–484. [Google Scholar] [CrossRef]
- Hasten, E.; McDonald-McGinn, D.M.; Crowley, T.B.; Zackai, E.; Emanuel, B.S.; Morrow, B.E.; Racedo, S.E. Dysregulation of TBX1 dosage in the anterior heart field results in congenital heart disease resembling the 22q11.2 duplication syndrome. Hum. Mol. Genet. 2018, 27, 1847–1857. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Guo, C.; Chang, W.; Zhuang, J.; Zhu, P.; Li, X. Temporally Distinct Six2-Positive Second Heart Field Progenitors Regulate Mammalian Heart Development and Disease. Cell Rep. 2017, 18, 1019–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, S.; Choi, M.; Wakimoto, H.; Ma, L.; Jiang, J.; Overton, J.D.; Romano-Adesman, A.; Bjornson, R.D.; Breitbart, R.E.; Brown, K.K.; et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature 2013, 498, 220–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homsy, J.; Zaidi, S.; Shen, Y.; Ware, J.S.; Samocha, K.E.; Karczewski, J.; DePalma, S.R.; Mckean, D.; Wakimoto, H.; Gorham, J.; et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015, 350, 1262–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glessner, J.T.; Bick, A.G.; Ito, K.; Homsy, J.G.; Rodriguez-Murillo, L.; Fromer, M.; Mazaika, E.; Vardarajan, B.; Italia, M.; Leipzig, J.; et al. Increased Frequency of De Novo Copy Number Variants in Congenital Heart Disease by Integrative Analysis of Single Nucleotide Polymorphism Array and Exome Sequence Data. Circ. Res. 2014, 115, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Sifrim, A.; Hitz, M.-P.; Wilsdon, A.; Breckpot, J.; Al Turki, S.H.; Thienpont, B.; McRae, J.; Fitzgerald, T.W.; Singh, T.; Swaminathan, G.J.; et al. Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing. Nat. Genet. 2016, 48, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Wooten, E.C.; Iyer, L.K.; Montefusco, M.C.; Hedgepeth, A.K.; Payne, D.D.; Kapur, N.K.; Housman, D.E.; Mendelsohn, M.E.; Huggins, G.S. Application of gene network analysis techniques identifies AXIN1/PDIA2 and endoglin haplotypes associated with bicuspid aortic valve. PLoS ONE 2010, 5, e8830. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Pilipenko, V.; Kaufman, K.M.; Cripe, L.H.; Kottyan, L.; Keddache, M.; Dexheimer, P.; Weirauch, M.T.; Benson, D.W. Whole Exome Sequencing for Familial Bicuspid Aortic Valve Identifies Putative Variants. Circ. Cardiovasc. Genet. 2014, 7, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonachea, E.M.; Zender, G.; White, P.; Corsmeier, D.J.; Newsom, D.; Fitzgerald-Butt, S.; Garg, V.; McBride, K.L. Use of a targeted, combinatorial next-generation sequencing approach for the study of bicuspid aortic valve. BMC Med. Genom. 2014, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Dargis, N.; Lamontagne, M.; Gaudreault, N.; Sbarra, L.; Henry, C.; Pibarot, P.; Mathieu, P.; Bossé, Y. Identification of Gender-Specific Genetic Variants in Patients With Bicuspid Aortic Valve. Am. J. Cardiol. 2016, 117, 420–426. [Google Scholar] [CrossRef]
- Gharibeh, L.; Komati, H.; Bossé, Y.; Boodhwani, M.; Heydarpour, M.; Fortier, M.; Hassanzadeh, R.; Ngu, J.; Mathieu, P.; Body, S.; et al. GATA6 Regulates Aortic Valve Remodeling, and Its Haploinsufficiency Leads to Right-Left Type Bicuspid Aortic Valve. Circulation 2018, 138, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Giusti, B.; Sticchi, E.; De Cario, R.; Magi, A.; Nistri, S.; Pepe, G. Genetic Bases of Bicuspid Aortic Valve: The Contribution of Traditional and High-Throughput Sequencing Approaches on Research and Diagnosis. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Zhou, W.; Jiao, J.; Nielsen, J.B.; Mathis, M.R.; Heydarpour, M.; Lettre, G.; Folkersen, L.; Prakash, S.; Schurmann, C.; et al. Protein-altering and regulatory genetic variants near GATA4 implicated in bicuspid aortic valve. Nat. Commun. 2017, 8, 15481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKellar, S.H.; Tester, D.J.; Yagubyan, M.; Majumdar, R.; Ackerman, M.J.; Sundt, T.M. Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2007, 134, 290–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, S.A.; Aherrahrou, Z.; Liptau, H.; Erasmi, A.W.; Hagemann, C.; Wrobel, S.; Borzym, K.; Schunkert, H.; Sievers, H.-H.; Erdmann, J. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem. Biophys. Res. Commun. 2006, 345, 1460–1465. [Google Scholar] [CrossRef]
- Gould, R.A.; Genomics, B.-H.C.F.M.; Aziz, H.; Woods, C.E.; Seman-Senderos, M.A.; Sparks, E.; Preuss, C.; Wünnemann, F.; Bedja, D.; Moats, C.R.; et al. ROBO4 variants predispose individuals to bicuspid aortic valve and thoracic aortic aneurysm. Nat. Genet. 2018, 51, 42–50. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Chamberlain, A.A.; Lui, W.; Koirala, P.; Susztak, K.; Klein, D.; Taylor, V.; Zhou, B. Endocardial to Myocardial Notch-Wnt-Bmp Axis Regulates Early Heart Valve Development. PLoS ONE 2013, 8, e60244. [Google Scholar] [CrossRef] [Green Version]
- Koenig, S.N.; Bosse, K.; Majumdar, U.; Bonachea, E.M.; Radtke, F.; Garg, V. Endothelial Notch1 is Required for Proper Development of the Semilunar Valves and Cardiac Outflow Tract. J. Am. Hear. Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Li, R.-G.; Xu, Y.; Wang, J.; Liu, X.-Y.; Yuan, F.; Huang, R.-T.; Xue, S.; Li, L.; Liu, H.; Li, Y.-J.; et al. GATA4 Loss-of-Function Mutation and the Congenitally Bicuspid Aortic Valve. Am. J. Cardiol. 2018, 121, 469–474. [Google Scholar] [CrossRef]
- Musfee, F.I.; Guo, D.; Pinard, A.C.; Hostetler, E.M.; Blue, E.E.; Nickerson, D.A.; Bamshad, M.J.; Milewicz, D.M.; Prakash, S.K. Rare deleterious variants of NOTCH1, GATA4, SMAD6, and ROBO4 are enriched in BAV with early onset complications but not in BAV with heritable thoracic aortic disease. Mol. Genet. Genom. Med. 2020, e1406. [Google Scholar] [CrossRef]
- Moskowitz, I.P.; Wang, J.; Peterson, M.A.; Pu, W.T.; MacKinnon, A.C.; Oxburgh, L.; Chu, G.C.; Sarkar, M.; Berul, C.; Smoot, L.; et al. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. Proc. Natl. Acad. Sci. USA 2011, 108, 4006–4011. [Google Scholar] [CrossRef] [Green Version]
- Misra, C.; Chang, S.-W.; Basu, M.; Huang, N.; Garg, V. Disruption of myocardial Gata4 and Tbx5 results in defects in cardiomyocyte proliferation and atrioventricular septation. Hum. Mol. Genet. 2014, 23, 5025–5035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, J.; Xiang, M.; Olson, P.; Guzzetta, A.; Zhang, K.; Moskowitz, I.P.; Xie, L. Gata4 potentiates second heart field proliferation and Hedgehog signaling for cardiac septation. Proc. Natl. Acad. Sci. USA 2017, 114, E1422–E1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Di, R.-M.; Qiao, Q.; Li, X.-M.; Huang, R.-T.; Xue, S.; Liu, X.-Y.; Wang, J.; Yang, Y.-Q. GATA6 loss-of-function mutation contributes to congenital bicuspid aortic valve. Gene 2018, 663, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.A.; Martín, M.; Arias, L.M.; Coto, E.; Naves-Díaz, M.; Moris, C.; Andía, J.B.C.; Rodríguez, I. Variants in cardiac GATA genes associated with bicuspid aortic valve. Eur. J. Clin. Investig. 2018, 48, e13027. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, D.J.; Eley, L.; Chaudhry, B. New Concepts in the Development and Malformation of the Arterial Valves. J. Cardiovasc. Dev. Dis. 2020, 7, 38. https://doi.org/10.3390/jcdd7040038
Henderson DJ, Eley L, Chaudhry B. New Concepts in the Development and Malformation of the Arterial Valves. Journal of Cardiovascular Development and Disease. 2020; 7(4):38. https://doi.org/10.3390/jcdd7040038
Chicago/Turabian StyleHenderson, Deborah J., Lorraine Eley, and Bill Chaudhry. 2020. "New Concepts in the Development and Malformation of the Arterial Valves" Journal of Cardiovascular Development and Disease 7, no. 4: 38. https://doi.org/10.3390/jcdd7040038




