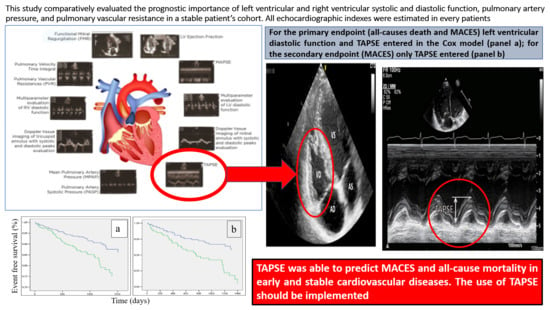
The Prognostic Importance of TAPSE in Early and in Stable Cardiovascular Diseases
Abstract
:1. Introduction
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Limits
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Back Matter
References
- Ghio, S.; Temporelli, P.L.; Klersy, C.; Simioniuc, A.; Girardi, B.; Scelsi, L.; Rossi, A.; Cicoira, M.; Genta, F.T.; Dini, F.L. Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur. J. Heart Fail. 2013, 15, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishram, J.K. Prognostic interactions between cardiovascular risk factors. Dan. Med. J. 2014, 61, B4982. [Google Scholar]
- Kuznetsova, T.; Thijs, L.; Knez, J.; Herbots, L.; Zhang, Z.; Staessen, J.A. Prognostic value of left ventricular diastolic dysfunction in a general population. J. Am. Heart Assoc. 2014, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Moller, J.E.; Egstrup, K.; Kober, L.; Poulsen, S.H.; Nyvad, O.; Torp-Pedersen, C. Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am. Heart J. 2003, 145, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, F.; Detaint, D.; Avierinos, J.F.; Scott, C.; Tajik, J.; Enriquez-Sarano, M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Bleasdale, R.A.; Frenneaux, M.P. Prognostic importance of right ventricular dysfunction. Heart 2002, 88, 323–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biering-Sørensen, T.; Biering- Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.K. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbility and mortality in a low risk general population: The copenhagen city heart study. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. American society of echocardiography’s nomenclature and standards committee; task force on chamber quantification; American college of cardiology echocardiography committee; american heart association; european association of echocardiography, european society of cardiology. recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar]
- Quiñones, M.A.; Otto, C.M.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A. Recommendations for quantification of doppler echocardiography: A report from the doppler quantification task force of the nomenclature and standards committee of the American society of echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European Association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Abbas, A.E.; Fortuin, F.D.; Schiller, N.B.; Appleton, C.P.; Moreno, C.A.; Lester, S.J. A simple method for noninvasive estimation of pulmonary vascular resistance. J. Am. Coll. Cardiol. 2003, 41, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Liu, L.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015, 132, 402–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Liu, D.; Herrmann, S.; Niemann, M.; Gaudron, P.D.; Voelker, W.; Ertl, G.; Bijnens, B.; Weidemann, F. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Kaul, S.; Tei, C.; Hopkins, J.M.; Shah, P.M. Assessment of right ventricular function using two-dimensional echocardiography. Am. Heart J. 1984, 107, 526–531. [Google Scholar] [CrossRef]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Baron-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012): The joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar]
- Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S. Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar]
- Chemla, D.; Castelain, V.; Humbert, M.; Hébert, J.L.; Simonneau, G.; Lecarpentier, Y.; Hervé, P. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest 2004, 126, 1313–1317. [Google Scholar] [CrossRef]
- Chalal, N.S.; Lim, T.K.; Chambers, J.C. Normative reference values for tissue doppler imaging parameters of left ventricular function: A population based study. Eur. J. Echocardiogr. 2010, 11, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Saxena, N.; Rajagopalan, N.; Edelman, K.; Lòpez-Candales, A. Tricuspid annular systolic velocity: A useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography 2006, 23, 750–755. [Google Scholar] [CrossRef]
- Giovanardi, P.; Tincani, E.; Stefanelli, G.; Turrini, F.; Magnavacchi, P.; Sansoni, S.; Zennaro, M.; Pinelli, G.; Tondi, S. Right ventricular presystolic peak velocity represents right ventricular function in stable patients. Minerva Cardioangiol 2017, 65, 134–139. [Google Scholar] [PubMed]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcari, A.; Magnacca, S.; Bracone, F.; Costanzo, S.; Persichillo, M.; Di Castelnuovo, A.; De Curtis, A.; Zito, F.; Schünemann, H.J.; Donati, M.B.; et al. Relation between pulmonary function and 10-year risk for cardiovascular disease among healthy men and woman in Italy: The Moli-Sani project. Eur. J. Prev. Cardiol. 2013, 20, 862–871. [Google Scholar] [CrossRef]
- Moceri, P.; Baudouy, D.; Chiche, O.; Cerboni, P.; Bouvier, P.; Chaussade, C.; Ferrari, E. Imaging in pulmonary hypertension: Focus on the role of echocardiography. Arch. Cardiovasc. Dis. 2014, 107, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granstam, S.O.; Björuklund, E.; Wikström, G.; Roos, M.W. Use of echocardiographic pulmonary acceleration time and estimated vascular resistance for the evaluation of possible pulmonary hypertension. Cardiovasc. Ultrasound 2013, 11, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedberg, M.K. Imaging right-left ventricular interactions. J. Am. Coll. Cardiol. Imaging 2018, 11, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Giovanardi, P.; Tincani, E.; Rossi, R.; Agnoletto, V.; Bondi, M.; Modena, M.G. Right ventricular function predicts cardiovascular events in outpatients with stable cardiovascular diseases: Preliminary results. Intern. Emerg. Med. 2012, 7, 251–256. [Google Scholar] [CrossRef]
- Lam, C.S.; Borlaug, B.A.; Kane, G.C.; Enders, F.T.; Rodeheffer, R.J.; Redfield, M.M. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009, 119, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Modin, D.; Mogelvang, R.; Andersen, D.M.; Biering-Sorensen, T. Right ventricular function evaluated by tricuspid annular plane systolic excursion predicts cardiovascular death in the general population. J. Am. Heart Assoc. 2019, 8, e012197. [Google Scholar] [CrossRef]

| Clinical Characteristics: | Prevalence of Cardiovascular Risk Factors: | Cardiovascular Diseases: | |||
|---|---|---|---|---|---|
| Patients who completed the follow-up § | 369 | Diabetes § | 76 (20.6%) | Prior stroke § | 34 (9.2%) |
| Caucasian § | 367 (99.4%) | Arterial hypertension § | 284 (77%) | Prior myocardial infarction/unstable angina § | 53 (14.4%) |
| Men § | 198 (53.7%) | Dyslipidemia § | 254 (68.8%) | Prior overt heart failure § | 26 (7%) |
| Mean age at enrollment date (years) * | 68.1 ± 13.7 | Current tobacco use § | 92 (24.9%) | Patients with previous MACES ° | 113 |
| Mean follow-up (days) * | 1178 ± 391 | Medications for Cardiovascular Conditions: | Patients without previous MACES | 256 | |
| Mean systolic blood pressure (mmHg) * | 132 ± 7 | Antiplatelet agents § | 196 (53.1%) | Exclusion Criteria: | |
| Mean diastolic blood pressure (mmHg) * | 83 ± 4.2 | ACE-I ^§ | 234 (63.4%) | Recent heart failure decompensation § | 490 |
| Absence of LV # hypertrophy § | 175 (47.5%) | ARBs ^^§ | 67 (18.2%) | Atrial fibrillation § | 255 |
| Mild LV hypertrophy § | 141 (38.2%) | Beta blockers § | 133 (36%) | Recent acute myocardial infarction § | 328 |
| Moderate LV hypertrophy § | 47 (12.7%) | Calcium channel blockers § | 199 (53.9%) | COPD \, severe pulmonary hypertension and severe valvular diseases § | 140 |
| Severe LV hypertrophy § | 6 (1.6%) | Lipid lowering drugs § | 303 (82.1%) | Congenital heart diseases § | 33 |
| Body mass index (BMI) * | 23.2 ± 4.2 | Hypoglycemic agents § | 70 (19%) | Bad acoustic window § | 52 |
| LV+ Echocardiographic Parameters: | RV # Echocardiographic Parameters: | ||||||
|---|---|---|---|---|---|---|---|
| Entire Cohort | Previous MACES ” | No Previous MACES | Entire Cohort | Previous MACES | No Previous MACES | ||
| LV ejection fraction (%) * | 52.7 ± 5.5 | 49.9 ± 7.5 | 54 ± 3.8 | TAPSE ^ (cm) * | 2.5 ± 0.4 | 2.4 ± 0.5 | 2.5 ± 0.4 |
| MAPSE ## (cm) * | 1.5 ± 0.8 | 1.4 ± 0.3 | 1.5 ± 1 | DTI ^^ RV Presystolic Peak (cm s−1) * | 18.4 ± 8 | 17.6 ± 8.2 | 18.8 ± 7.9 |
| DTI LV Systolic Peak (cm s−1) * | 11 ± 4.4 | 10 ± 4.2 | 11.4 ± 4.5 | DTI RV Systolic Peak (cm s−1) * | 16.4 ± 5 | 15.7 ± 5 | 16.8 ± 5.1 |
| Functional Mitral Regurgitation: | PVTI ° (cm) * | 21.4 ± 4.4 | 20.7 ± 4.4 | 21.7 ± 4.4 | |||
| absent § | 97 (26.3%) | 16 (4.3%) | 81 (22%) | TRV °° (m s−1) * | 2.4 ± 0.5 | 2.4 ± 0.5 | 2.4 ± 0.5 |
| mild § | 222 (60.2%) | 72 (19.5%) | 150 (40.7%) | MPAP | (mmHg) * | 21.2 ± 5.6 | 21.9 ± 6.1 | 20.9 ± 5.3 |
| moderate § | 50 (13.5%) | 25 (6.8%) | 25 (6.8%) | PVR -- (Woods Unit) * | 1.32 ± 0.3 | 1.4 ± 0.4 | 1.3 ± 0.3 |
| LV diastolic function: | RV diastolic function: | ||||||
| normal § | 51 (13.8%) | 6 (1.6%) | 45 (12.2%) | normal § | 71 (19.3%) | 18 (4.9%) | 53 (14.4%) |
| impaired relaxation § | 280 (75.9%) | 85 (23.1%) | 195 (52.8%) | impaired relaxation § | 295 (79.9%) | 94 (25.5%) | 201 (54.5%) |
| pseudonormal § | 34 (9.2%) | 18 (4.9%) | 16 (4.3%) | restrictive § | 3 (0.8%) | 1 (0.2%) | 2 (0.5%) |
| restrictive § | 4 (1.1%) | 4 (1.1%) | -- | ||||
| MACES# or Death at Follow-Up | No MACES or Death at Follow-Up | p Value | |
|---|---|---|---|
| LV+ ejection fraction (%) * | 49.9 ± 7.3 | 53.5 ± 4.8 | <0.001 |
| MAPSE ” (cm) * | 1.37 ± 0.3 | 1.52 ± 0.9 | 0.195 |
| DTI ^^ LV systolic peak (cm s−1) * | 10.2 ± 4.4 | 11.2 ± 4.6 | 0.064 |
| Functional mitral regurgitation: | <0.001 | ||
| absent § | 10 (2.7%) | 87 (23.6%) | |
| mild § | 43 (11.8%) | 179 (48.6%) | |
| moderate § | 22 (6%) | 28 (7.3%) | |
| LV diastolic function: | <0.001 | ||
| normal § | 1 (0.3%) | 50 (13.6%) | |
| impaired relaxation § | 56 (15.2%) | 224 (60.7%) | |
| pseudonormal § | 16 (4.3%) | 18 (4.9%) | |
| restrictive § | 2 (0.5%) | 2 (0.5%) | |
| TAPSE ^ (cm) * | 2.3 ± 0.5 | 2.5 ± 0.4 | 0.001 |
| DTI RV ## presystolic peak (cm s−1) * | 17.5 ± 7 | 18.7 ± 8.2 | 0.24 |
| DTI RV systolic peak (cm s−1) * | 15.5 ± 4.3 | 16.7 ± 5.3 | 0.064 |
| PVTI ° (cm) * | 21.1 ± 4.4 | 21.5 ± 4.4 | 0.498 |
| TRV °° (m s−1) * | 2.5 ± 0.5 | 2.3 ± 0.5 | 0.001 |
| MPAP | (mmHg) * | 23 ± 6 | 20.7 ± 5.4 | 0.001 |
| PVR -- (woods unit) * | 1.4 ± 0.4 | 1.3 ± 0.3 | 0.001 |
| RV diastolic function: | 0.258 | ||
| normal § | 13 (3.5%) | 60 (16.3%) | |
| impaired relaxation § | 60 (16.2%) | 233 (63.1%) | |
| restrictive § | 2 (0.6%) | 1 (0.3%) |
| Cox Regression Analysis for Primary Composite Endpoint (All-Causes Death and MACES #) | |||||
| Hazard Ratio | 95% Confidence Interval | p Value | |||
| Lower | Upper | ||||
| LV + diastolic function | 17.07 | 2.179–133.985 | 0.007 | ||
| TAPSE ^ (cm) | 0.555 | 0.325–0.95 | 0.032 | ||
| Cox Regression Analysis for Secondary Endpoint (MACES) | |||||
| Hazard Ratio | 95% Confidence Interval | p Value | |||
| Lower | Upper | ||||
| TAPSE (cm) | 0.493 | 0.261–0.931 | 0.029 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanardi, P.; Tincani, E.; Maioli, M.; Tondi, S. The Prognostic Importance of TAPSE in Early and in Stable Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2020, 7, 4. https://doi.org/10.3390/jcdd7010004
Giovanardi P, Tincani E, Maioli M, Tondi S. The Prognostic Importance of TAPSE in Early and in Stable Cardiovascular Diseases. Journal of Cardiovascular Development and Disease. 2020; 7(1):4. https://doi.org/10.3390/jcdd7010004
Chicago/Turabian StyleGiovanardi, Paolo, Enrico Tincani, Marco Maioli, and Stefano Tondi. 2020. "The Prognostic Importance of TAPSE in Early and in Stable Cardiovascular Diseases" Journal of Cardiovascular Development and Disease 7, no. 1: 4. https://doi.org/10.3390/jcdd7010004