The Anatomy, Development, and Evolution of the Atrioventricular Conduction Axis
Abstract
:1. Introduction
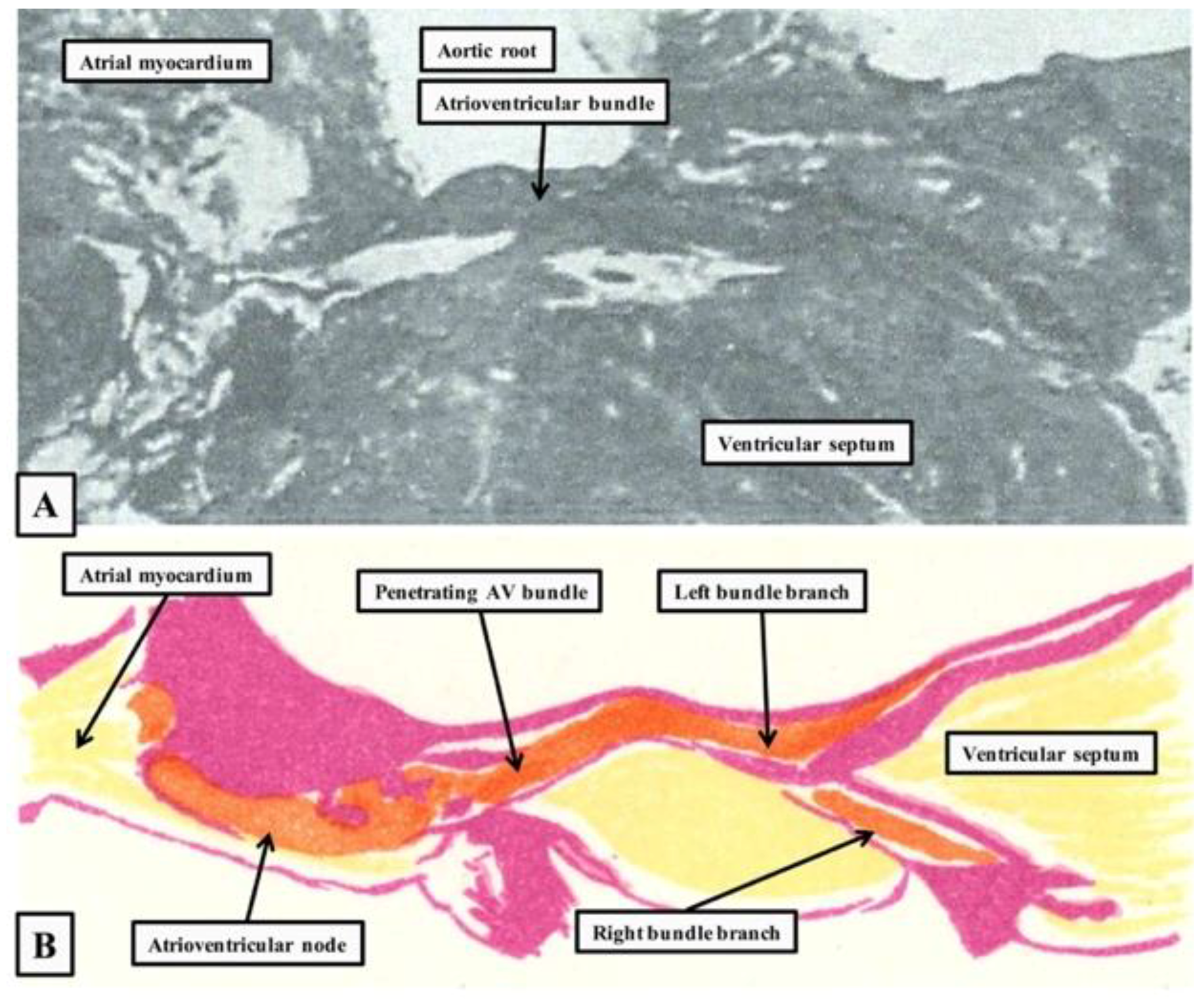
2. The Location and Architecture of the Normal Atrioventricular Conduction Axis
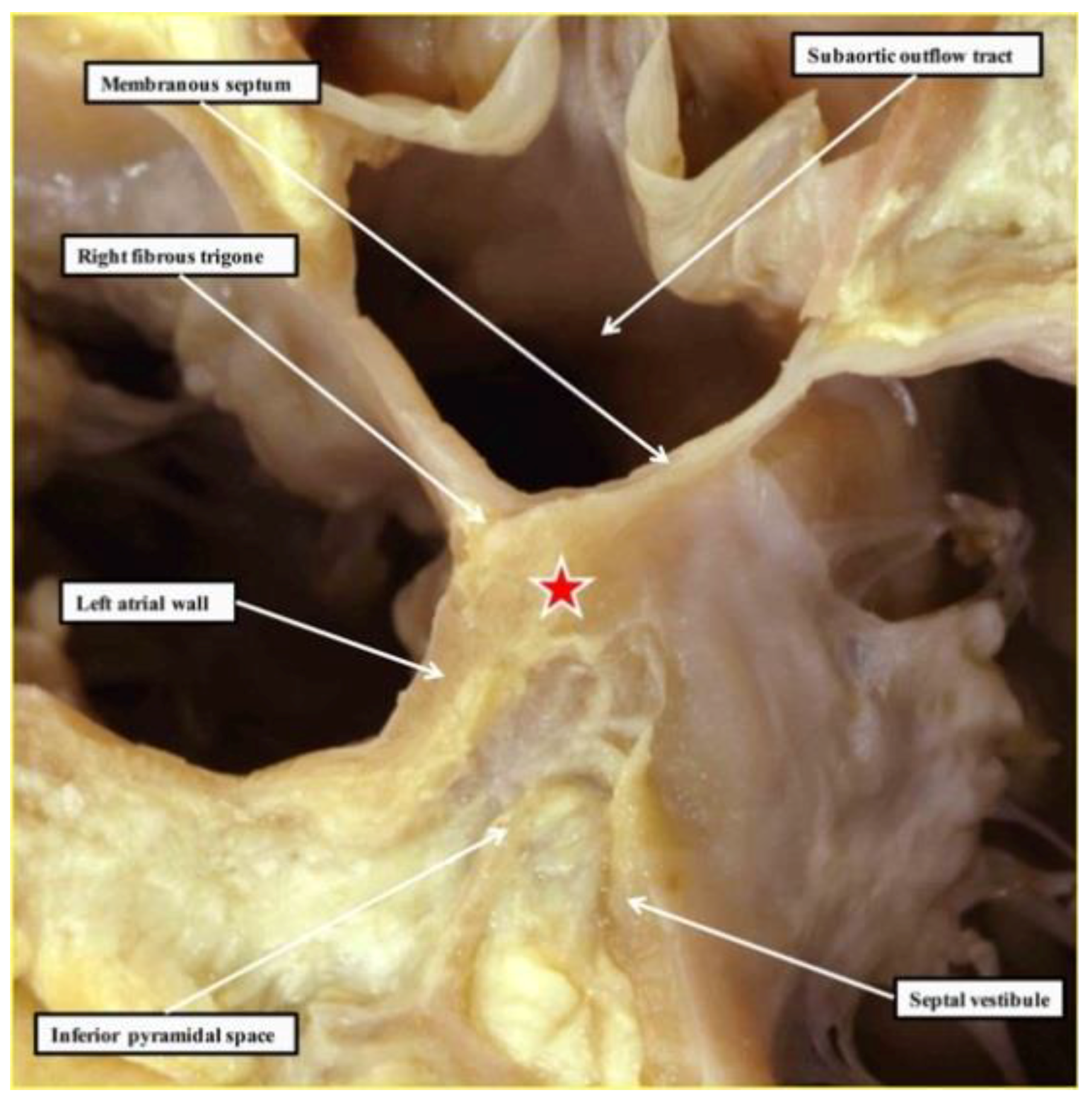
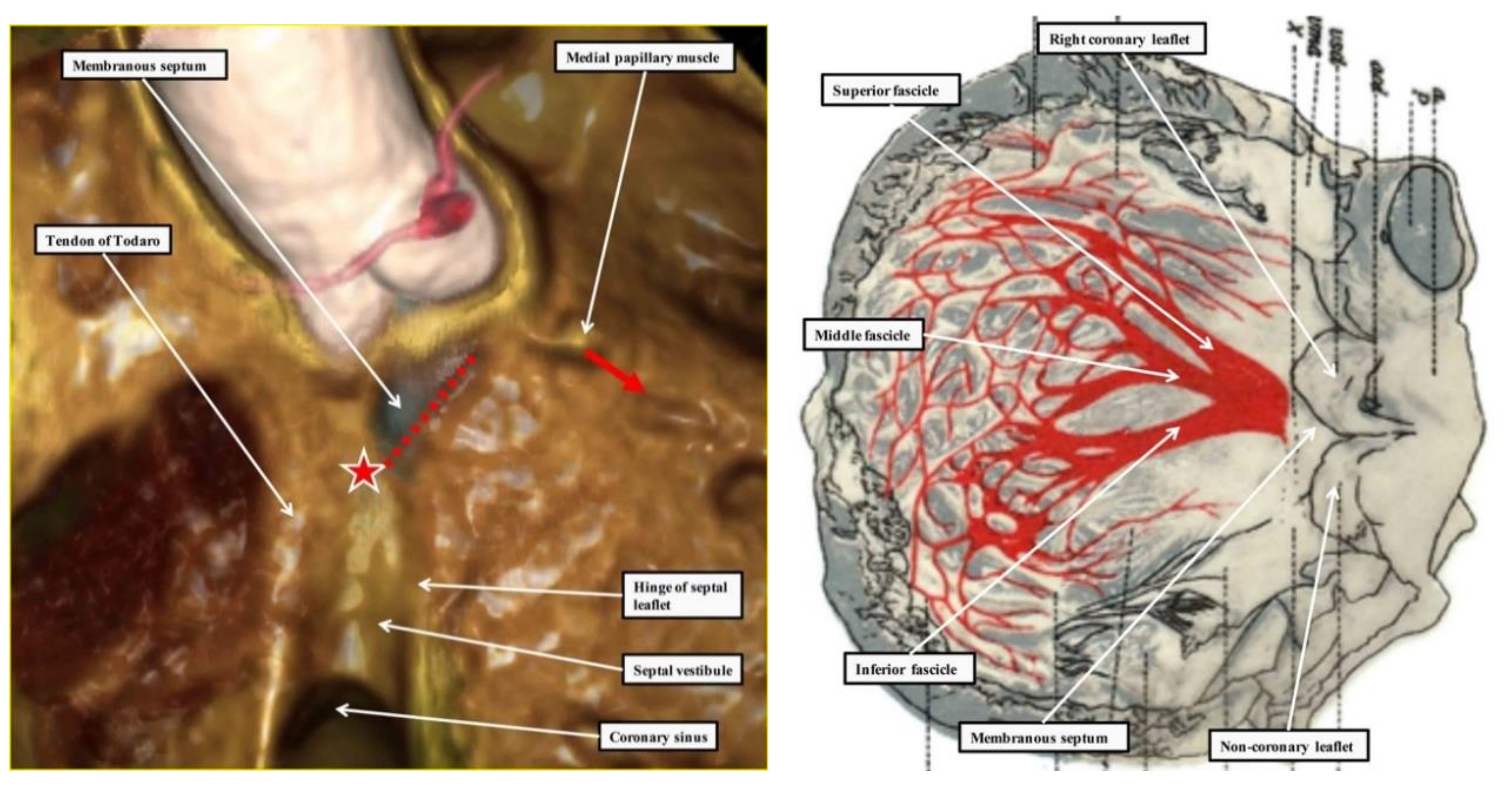
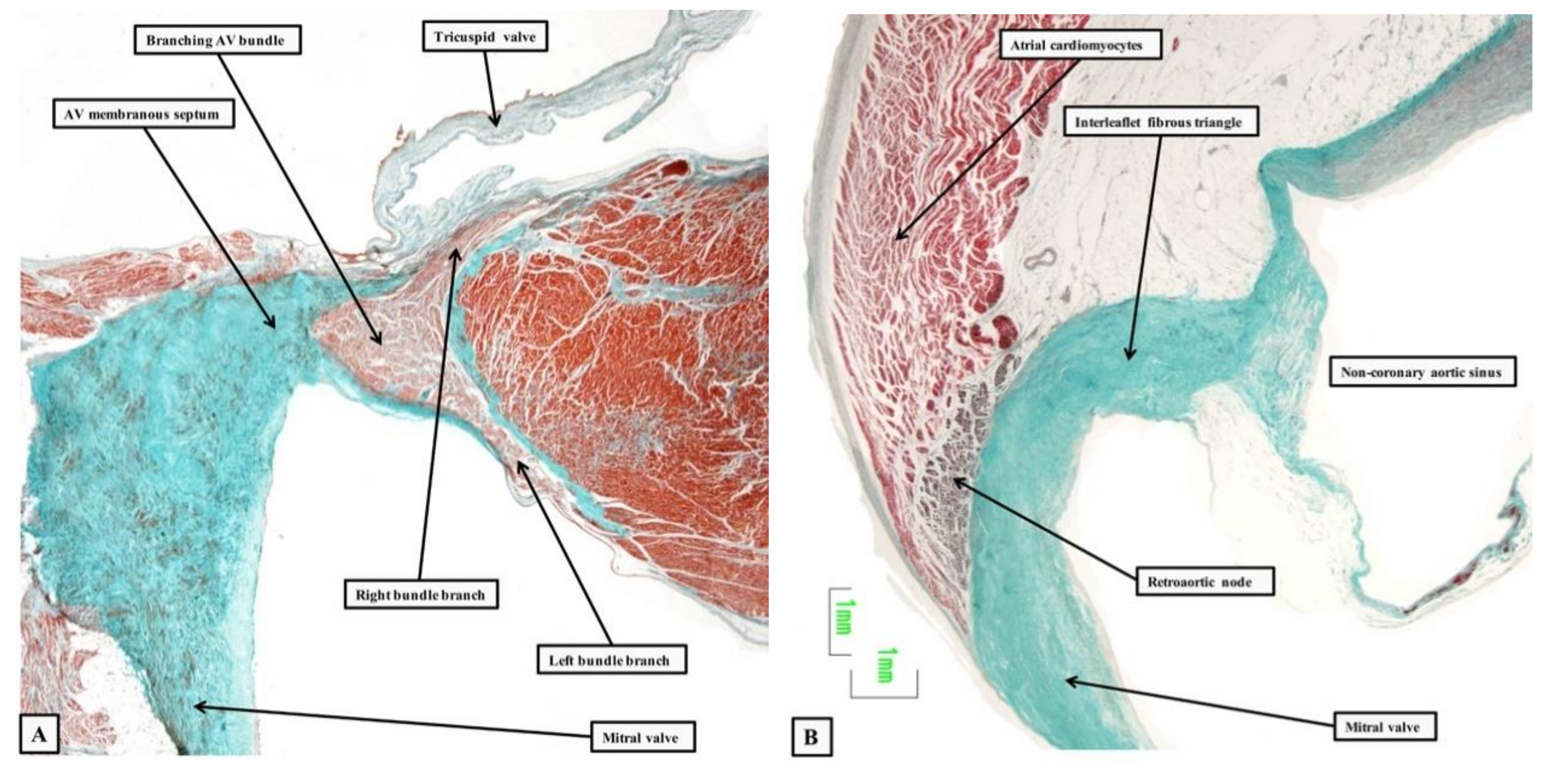
2.1. The Structure of the Normal Atrioventricular Junctions
2.2. Location of the Atrioventricular Node
2.3. Penetration and Branching of the Conduction Axis
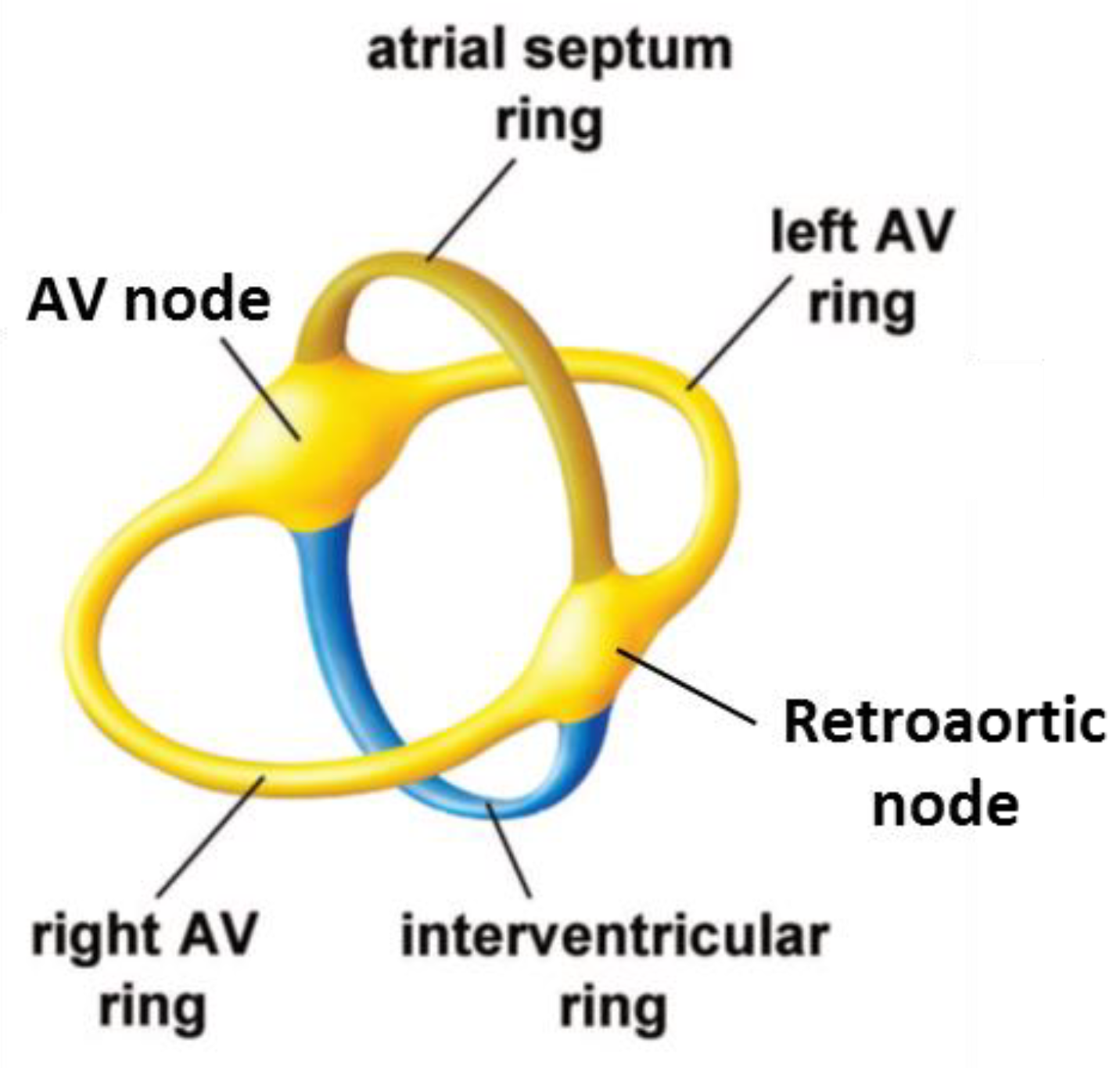
2.4. The Retroaortic Node
3. Development of the Cardiac Chambers
4. The Development of the Atrioventricular Conduction Axis
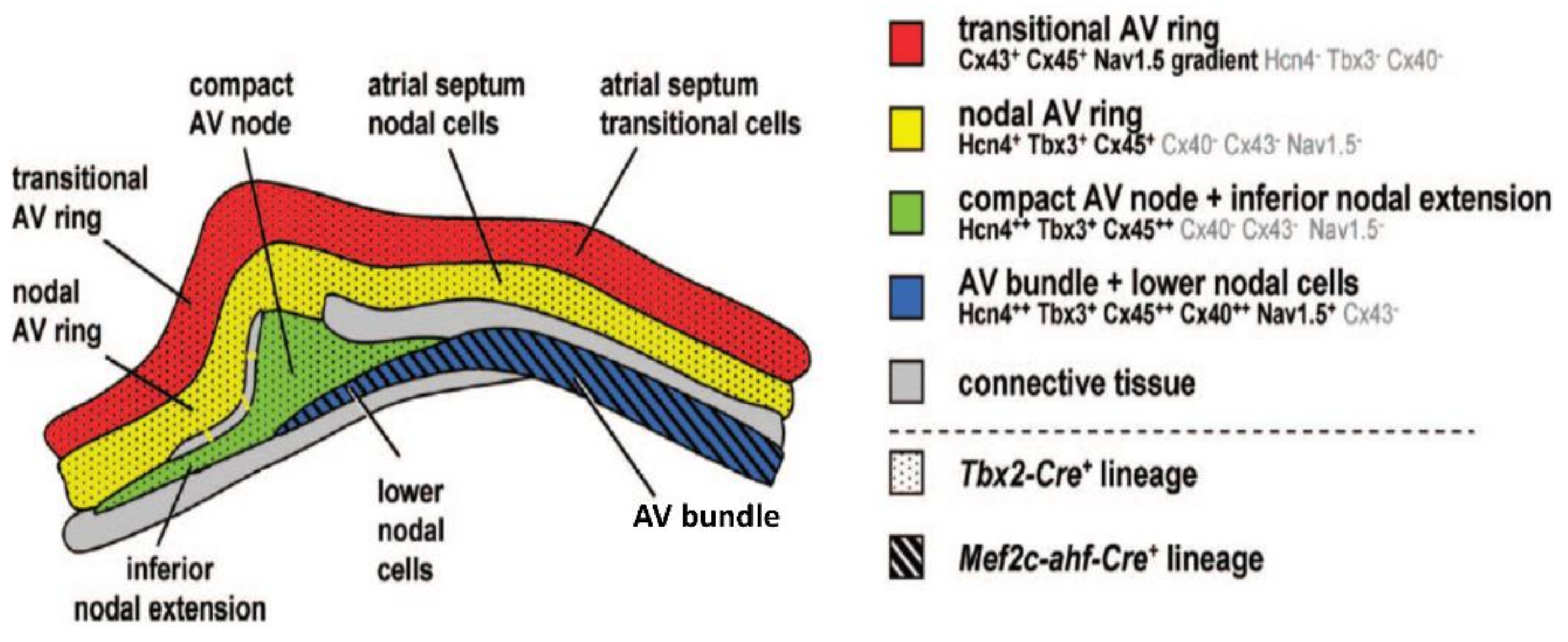
5. Molecular Characterization of the Atrioventricular Conduction Axis
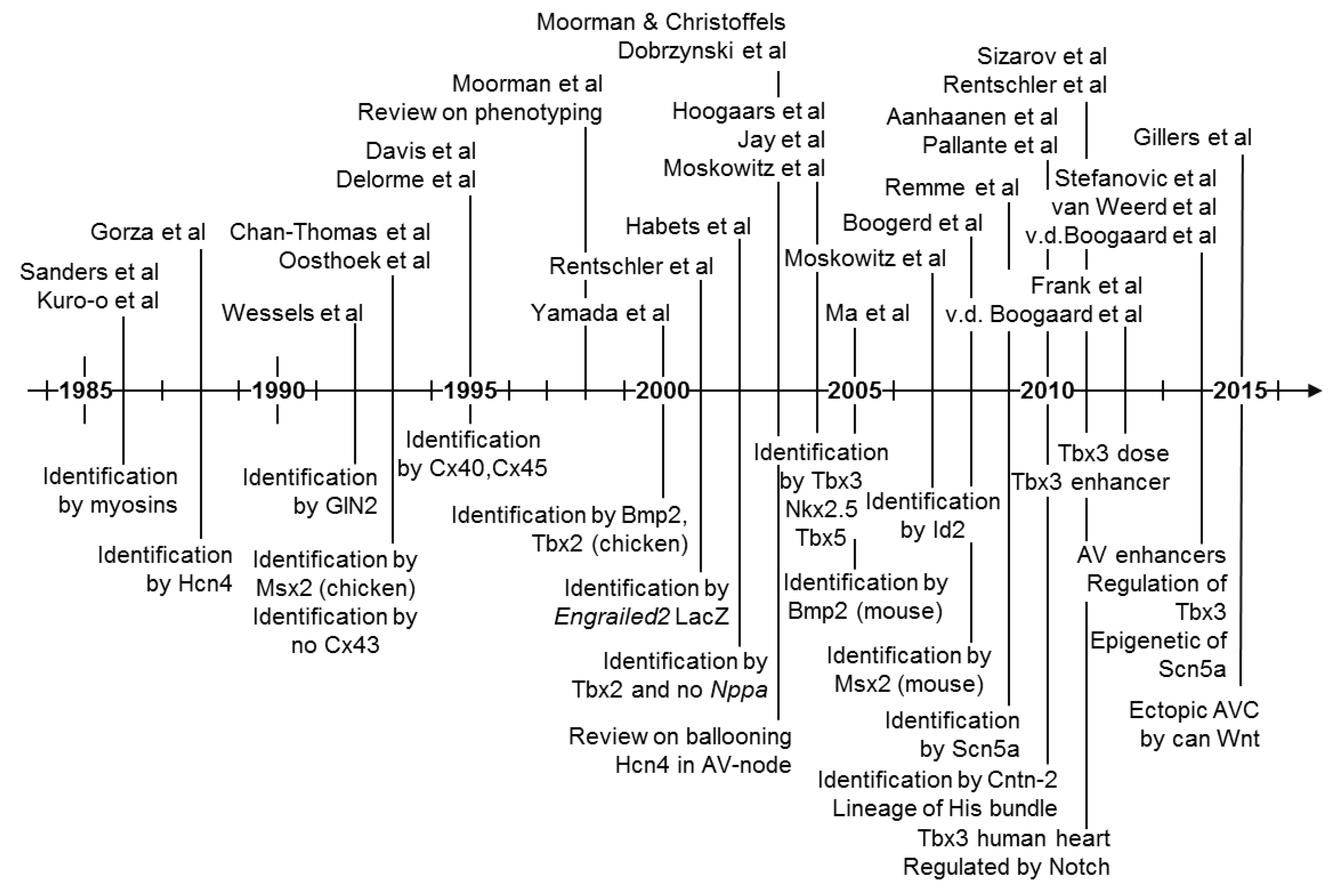
6. Evolutionary Origin of the Atrioventricular Conduction Axis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tawara, S. Das Reizleitungssystem des Säugetierherzens; Gustav Fischer: Jena, Germany, 1906. [Google Scholar]
- Anderson, R.H.; Ho, S.Y. The Morphology of the Specialized Atrioventricular Junctional Area: The Evolution of Understanding. Pacing Clin. Electrophysiol. 2022, 25, 957–966. [Google Scholar] [CrossRef]
- Silverman, M.E.; Grove, D.; Upshaw, C.B., Jr. Why does the heart beat? The discovery of the electrical system of the heart. Circulation 2006, 113, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Flack, M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J. Anat. Physiol. 1907, 41, 172–189. [Google Scholar] [PubMed]
- Keith, A. An Autobiography; Watts & Co: London, UK, 1950; p. 255. [Google Scholar]
- His, W. Die Tätigkeit des embryonalen Herzens und deren Bedeutung für die Lehre von der Herzbewegung beim Menschen. Arb Med. Klin. (Leipzig) 1893, 14–49. [Google Scholar]
- Kent, A.F. Researches on the Structure and Function of the Mammalian Heart. J. Physiol. 1983, 14, 233–254. [Google Scholar] [CrossRef]
- Gaskell, W.H. On the Innervation of the Heart, with especial reference to the Heart of the Tortoise. J. Physiol. 1883, 4, 43–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessels, A.; Vermeulen, J.L.M.; Verbeek, F.J.; Viragh, S.Z.; Kalman, F.; Lamers, W.H.; Moorman, A.F.M. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat. Rec. 1992, 232, 97–111. [Google Scholar] [PubMed]
- Yanni, J.; Boyett, M.R.; Anderson, R.H.; Dobrzynski, H. The extent of the specialized atrioventricular ring tissues. Heart Rhythm 2009, 6, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Mori, S. Wilhelm His Junior and his bundle. J. Electrocardiol. 2006, 49, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Boukens, B.J.; Crossley, D.A., II; Conner, J.; Mohan, R.A.; van Duijvenboden, K.; Postma, A.V.; Gloschat, C.R.; Elsey, R.M.; Sedmera, D.; et al. Specialized impulse conduction pathway in the alligator heart. eLife 2008, 7, e32120. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.M.; de Jong, F.; Denyn, M.M.; Lamers, W.H. Development of the Cardiac Conduction System. Circ. Res. 1998, 82, 629–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, R.S.; Boyett, M.R.; Hart, G.; Nikolaidou, T.; Cai, X.; Corno, A.F.; Alphonso, N.; Jeffery, N.; Jarvis, J.C. Contrast enhanced micro-computed tomography resolves the 3-dimensional morphology of the cardiac conduction system in mammalian hearts. PLoS ONE 2012, 7, e35299. [Google Scholar] [CrossRef]
- Massing, G.K.; James, T.N. Anatomical configuration of the His bundle and bundle branches in the human heart. Circulation 1976, 53, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, H.; Becker, A.E. Dead-end tract of the conduction axis. Int. J. Cardiol. 1985, 7, 13–20. [Google Scholar] [CrossRef]
- Anderson, R.H.; Davies, M.J.; Becker, A.E. Atrioventricular ring specialized tissue in the normal heart. Eur. J. Cardiol. 1974, 2, 219. [Google Scholar]
- Anderson, R.H.; Becker, A.E.; Arnold, R.; Wilkinson, J.L. The conducting tissues in congenitally corrected transposition. Circulation 1974, 50, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Milo, S.; Ho, S.Y.; Macartney, F.J.; Wilkinson, J.L.; Becker, A.E.; Wenink, A.C.; De Groot, A.C.G.; Anderson, R.H. Straddling and overriding atrioventricular valves: Morphology and classification. Am. J. Cardiol. 1979, 44, 1122–1134. [Google Scholar] [CrossRef]
- Bohora, S.; Lokhandwala, Y.; Sternick, E.B.; Anderson, R.H.; Wellens, H.J.J. Reappraisal and new observations on atrial tachycardia ablated from the non-coronary aortic sinus of Valsalva: Authors’ reply. Europace 2018, 20, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.; Tessari, A.; Moorman, A.F.; De Boer, P.A.; Hagoort, J.; Soufan, A.T.; Campione, M.; Christoffels, V.M. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc. Res. 2004, 62, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Wang, T.; Christoffels, V.M.; Moorman, A.F. Evolution and development of the building plan of the vertebrate heart. Biochim. Biophys. Acta 2013, 1833, 783–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorman, A.F.; Vermeulen, J.L.; Koban, M.U.; Schwartz, K.; Lamers, W.H.; Boheler, K.R. Patterns of expression of sarcoplasmic reticulum Ca2+-ATPase and phospholamban mRNAs during rat heart development. Circ. Res. 1995, 76, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.; Christoffels, V.M. Cardiac chamber formation: Development, genes, and evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef] [PubMed]
- Van Eif, V.W.W.; Devalla, H.D.; Boink, G.J.J.; Christoffels, V.M. Transcriptional regulation of the cardiac conduction system. Nat. Rev. Cardiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Reckova, M.; DeAlmeida, A.; Coppen, S.R.; Kubalak, S.W.; Gourdie, R.G.; Thompson, R.P. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 274, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soufan, A.T.; Van den Berg, G.; Ruijter, J.M.; De Boer, P.A.; Van den Hoff, M.J.; Moorman, A.F. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ. Res. 2006, 99, 545–552. [Google Scholar] [CrossRef] [PubMed]
- De Boer, B.A.; Van den Berg, G.; De Boer, P.A.; Moorman, A.F.; Ruijter, J.M. Growth of the developing mouse heart: An interactive qualitative and quantitative 3D atlas. Dev. Biol. 2012, 368, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sizarov, A.; Ya, J.; De Boer, B.A.; Lamers, W.H.; Christoffels, V.M.; Moorman, A.F. Formation of the building plan of the human heart: Morphogenesis, growth, and differentiation. Circulation 2011, 123, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Luxán, G.; Casanova, J.C.; Martínez-Poveda, B.; Prados, B.; D’amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F.; et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Sylva, M.; Van den Hoff, M.J.; Moorman, A.F. Development of the human heart. Am. J. Med. Genet. A 2014, 164A, 1347–1371. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, I.P.; Pizard, A.; Patel, V.V.; Bruneau, B.G.; Kim, J.B.; Kupershmidt, S.; Roden, D.; Berul, C.I.; Seidman, C.E.; Seidman, J.G. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development 2004, 131, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- Jay, P.Y.; Harris, B.S.; Maguire, C.T.; Buerger, A.; Wakimoto, H.; Tanaka, M.; Kupershmidt, S.; Roden, D.M.; Schultheiss, T.M.; O’Brien, T.X.; et al. Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J. Clin. Investig. 2004, 113, 1130–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habets, P.E.; Moorman, A.F.; Clout, D.E.; Van Roon, M.A.; Lingbeek, M.; Van Lohuizen, M.; Campione, M.; Christoffels, V.M. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: Implications for cardiac chamber formation. Genes Dev. 2002, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hoogaars, W.M.; Barnett, P.; Grieskamp, T.; Rana, M.S.; Buermans, H.; Farin, H.F.; Petry, M.; Heallen, T.; Martin, J.F.; et al. Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation. Cell. Mol. Life Sci. 2012, 69, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.U.; Carter, K.L.; Thomas, K.R.; Burr, R.M.; Bakker, M.L.; Coetzee, W.A.; Tristani-Firouzi, M.; Bamshad, M.J.; Christoffels, V.M.; Moon, A.M. Lethal arrhythmias in Tbx3-deficient mice reveal extreme dosage sensitivity of cardiac conduction system function and homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, E154–E163. [Google Scholar] [CrossRef] [PubMed]
- Aanhaanen, W.T.; Boukens, B.J.; Sizarov, A.; Wakker, V.; de Gier-de Vries, C.; van Ginneken, A.C.; Moorman, A.F.; Coronel, R.; Christoffels, V.M. Defective Tbx2-dependent patterning of the atrioventricular canal myocardium causes accessory pathway formation in mice. J. Clin. Investig. 2011, 121, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Gillers, B.S.; Chiplunkar, A.; Aly, H.; Valenta, T.; Basler, K.; Christoffels, V.M.; Efimov, I.R.; Boukens, B.J.; Rentschler, S. Canonical Wnt Signaling Regulates Atrioventricular Junction Programming and Electrophysiological Properties. Circ. Res. 2015, 116, 398–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R.A.; Mommersteeg, M.T.; Domínguez, J.N.; Choquet, C.; Wakker, V.; De Gier-de Vries, C.; Boink, G.J.; Boukens, B.J.; Miquerol, L.; Verkerk, A.O.; et al. Embryonic Tbx3+ cardiomyocytes form the mature cardiac conduction system by progressive fate restriction. Development 2018. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.; Houweling, A.C.; De Boer, P.A.; Christoffels, V.M. Sensitive nonradioactive detection of mRNA in tissue sections: Novel application of the whole-mount in situ hybridization protocol. J. Histochem. Cytochem. 2001, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Jong, F.; Opthof, T.A.A.M.W.; Wilde, A.A.M.; Janse, M.J.; Charles, R.; Lamers, W.H.; Moorman, A.F.M. Persisting zones of slow impulse conduction in developing chicken hearts. Circ. Res. 1992, 71, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Arguello, C.; Alanis, J.; Pantoja, O.; Valenzuela, B. Electrophysiological and ultrastructural study of the atrioventricular canal during the development of the chick embryo. J. Mol. Cell. Cardiol. 1986, 18, 499–510. [Google Scholar] [CrossRef]
- Dobrzynski, H.; Anderson, R.H.; Atkinson, A.; Borbas, Z.; D’souza, A.; Fraser, J.F.; Inada, S.; Logantha, S.J.; Monfredi, O.; Morris, G.M.; et al. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol. Ther. 2013, 139, 260–288. [Google Scholar] [CrossRef] [PubMed]
- Bogue, J.Y. The Electrocardiogram of the Developing Chick. J. Exp. Biol. 1993, 10, 286–292. [Google Scholar]
- Bogue, J.Y. The Heart Rate of the Developing Chick. J. Exp. Biol. 1932, 9, 351–358. [Google Scholar]
- Van Mierop, L.H. Location of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am. J. Physiol. 1967, 212, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentschler, S.; Vaidya, D.M.; Tamaddon, H.; Degenhardt, K.; Sassoon, D.; Morley, G.E.; Jalife, J.; Fishman, G.I. Visualization and functional characterization of the developing murine cardiac conduction system. Development 2001, 128, 1785–1792. [Google Scholar] [PubMed]
- Chuck, E.T.; Meyers, K.; France, D.; Creazzo, T.L.; Morley, G.E. Transitions in ventricular activation revealed by two-dimensional optical mapping. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 280, 990–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankova, B.; Benes, J., Jr.; Krejci, E.; Dupays, L.; Theveniau-Ruissy, M.; Miquerol, L.; Sedmera, D. The effect of connexin40 deficiency on ventricular conduction system function during development. Cardiovasc. Res. 2012, 95, 469–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, K.; Goudy, J.; Henley, T.; Bressan, M. Optical Electrophysiology in the Developing Heart. J. Cardiovasc. Dev. Dis. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Durrer, D.; Van Dam, R.T.; Freud, G.E.; Janse, M.J.; Meijler, F.L.; Arzbaecher, R.C. Total excitation of the isolated human heart. Circulation 1970, 41, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Van Rijen, H.V.; Van Veen, T.A.; Van Kempen, M.J.; Wilms-Schopman, F.J.; Potse, M.; Krueger, O.; Willecke, K.; Opthof, T.; Jongsma, H.J.; De Bakker, J.M. Impaired conduction in the bundle branches of mouse hearts lacking the gap junction protein connexin40. Circulation 2001, 103, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Meysen, S.; Mangoni, M.; Bois, P.; Van Rijen, H.V.; Abran, P.; Jongsma, H.; Nargeot, J.; Gros, D. Architectural and functional asymmetry of the His-Purkinje system of the murine heart. Cardiovasc. Res. 2004, 63, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Habets, P.E.; Franco, D.; Campione, M.; De Jong, F.; Lamers, W.H.; Bao, Z.Z.; Palmer, S.; Biben, C.; Harvey, R.P.; et al. Chamber formation and morphogenesis in the developing mammalian heart. Dev. Biol. 2000, 223, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Devalla, H.D.; Anderson, R.H.; Passier, R.; Christoffels, V.M.; Moorman, A.F. Molecular Analysis of the Patterning of the Conduction Tissues in the Developing Human Heart. Circ. Arrhythm. Electrophysiol. 2011, 4, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D. Function and form in the developing cardiovascular system. Cardiovasc. Res. 2011, 91, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aanhaanen, W.T.; Mommersteeg, M.T.; Norden, J.; Wakker, V.; De Gier-de Vries, C.; Anderson, R.H.; Kispert, A.; Moorman, A.F.; Christoffels, V.M. Developmental origin, growth, and three-dimensional architecture of the atrioventricular conduction axis of the mouse heart. Circ. Res. 2010, 107, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Van den Hoff, M.J.; Adamo, R.F.; Phelps, A.L.; Lockhart, M.M.; Sauls, K.; Briggs, L.E.; Norris, R.A.; Van Wijk, B.; Perez-Pomares, J.M.; et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012, 366, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.H.; Durrer, D.; Janse, M.J.; Van Capelle, F.J.; Billete, J.; Becker, A.E.; Durrer, D. A combined morphological and electrophysiological study of the atrioventricular node of the rabbit heart. Circ. Res. 1974, 35, 909–922. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.A.; De Bakker, J.M.; Vermeulen, J.T.; Moorman, A.F.; Loh, P.; Thibault, B.; Vermeulen, J.L.; Becker, A.E.; Janse, M.J. Atrioventricular junctional tissue. Discrepancy between histological and electrophysiological characteristics. Circulation 1996, 94, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.; De Groot, I.J.M.; Geerts, W.J.C.; De Jong, F.; Van Horssen, A.A.; Los, J.A.; Moorman, A.F.M. The local expression of adult chicken heart myosins during development. II. Ventricular conducting tissue. Anat. Embryol. 1986, 174, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Tsuchimochi, H.; Ueda, S.; Takaku, F.; Yazaki, Y. Distribution of cardiac myosin isozymes in human conduction system. Immunohistochemical study using monoclonal antibodies. J. Clin. Investig. 1986, 77, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Gorza, L.; Schiaffino, S.; Vitadello, M. Heart conduction system: A neural crest derivative? Brain Res. 1988, 457, 360–366. [Google Scholar] [CrossRef]
- Chan-Thomas, P.S.; Thompson, R.P.; Robert, B.; Yacoub, M.H.; Barton, P.J. Expression of homeobox genes Msx-1 (Hox-7) and Msx-2 (Hox-8) during cardiac development in the chick. Dev. Dyn. 1993, 197, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosthoek, P.W.; Viragh, S.; Lamers, W.H.; Moorman, A.F. Immunohistochemical delineation of the conduction system II: The atrioventricular node and Purkinje fibers. Circ. Res. 1993, 73, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.M.; Rodefeld, M.E.; Green, K.; Beyer, E.C.; Saffitz, J.E. Gap junction protein phenotypes of the human heart and conduction system. J. Cardiovasc. Electrophysiol. 1995, 6, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Delorme, B.; Dahl, E.; Jarry-guichard, T.; Marics, I.; Briand, J.P.; Willecke, K.; Gros, D.; Théveniau-Ruissy, M. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev. Dyn. 1995, 204, 358–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Revelli, J.P.; Eichele, G.; Barron, M.; Schwartz, R.J. Expression of chick Tbx-2, Tbx-3, and Tbx-5 genes during early heart development: Evidence for BMP2 induction of Tbx2. Dev. Biol. 2000, 228, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, H.; Nikolski, V.P.; Sambelashvili, A.T.; Greener, I.D.; Yamamoto, M.; Boyett, M.R.; Efimov, I.R. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circ. Res. 2003, 93, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lu, M.F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskowitz, I.P.; Kim, J.B.; Moore, M.L.; Wolf, C.M.; Peterson, M.A.; Shendure, J.; Nobrega, M.A.; Yokota, Y.; Berul, C.; Izumo, S.; et al. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell 2007, 129, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, K.J.; Wong, L.E.; Christoffels, V.M.; Klarenbeek, M.; Ruijter, J.M.; Moorman, A.F.; Barnett, P. Msx1 and Msx2 are functional interacting partners of T-box factors in the regulation of Connexin43. Cardiovasc. Res. 2008, 78, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remme, C.A.; Verkerk, A.O.; Hoogaars, W.M.H.; Aanhaanen, W.T.J.; Scicluna, B.P.; Annink, C.; Van den Hoff, M.J.B.; Wilde, A.A.M.; Van Veen, T.A.B.; Veldkamp, M.W.; et al. The cardiac sodium channel displays differential distribution in the conduction system and transmural heterogeneity in the murine ventricular myocardium. Basic Res. Cardiol. 2009, 104, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallante, B.A.; Giovannone, S.; Fang-Yu, L.; Zhang, J.; Liu, N.; Kang, G.; Dun, W.; Boyden, P.A.; Fishman, G.I. Contactin-2 expression in the cardiac Purkinje fiber network. Circ. Arrhythm. Electrophysiol. 2010, 3, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Harris, B.S.; Kuznekoff, L.; Jain, R.; Manderfield, L.; Lu, M.M.; Morley, G.E.; Patel, V.V.; Epstein, J.A. Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways. J. Clin. Investig. 2011, 121, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Boogaard, M.; Wong, L.E.; Tessadori, F.; Bakker, M.L.; Dreizehnter, L.K.; Wakker, V.; Bezzina, C.R.; AC‘t Hoen, P.; Bakkers, J.; Barnett, P.; et al. Genetic variation in T-box binding element functionally affects SCN5A/SCN10A enhancer. J. Clin. Investig. 2012, 122, 2519–2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Boogaard, M.; Smemo, S.; Burnicka-Turek, O.; Arnolds, D.E.; van de Werken, H.J.; Klous, P.; McKean, D.; Muehlschlegel, J.D.; Moosmann, J.; Toka, O.; et al. A common genetic variant within SCN10A modulates cardiac SCN5A expression. J. Clin. Investig. 2014, 124, 1844–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanovic, S.; Barnett, P.; Van Duijvenboden, K.; Weber, D.; Gessler, M.; Christoffels, V.M. GATA-dependent regulatory switches establish atrioventricular canal specificity during heart development. Nat. Commun. 2014, 5, 3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.S.; Fishman, G.I. Development and Function of the Cardiac Conduction System in Health and Disease. J. Cardiovasc. Dev. Dis. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Davies, F. The conducting system of the vertebrate heart. Br. Heart J. 1942, 4, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Truex, R.C.; Smythe, M.G. Comparative morphology of the cardiac conduction tissue in animals. Ann. N. Y. Acad. Sci. 1965, 127, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Chiodi, V.; Bortolami, R. The Conducting System of the Vertebrate Heart; Edizioni Calderini: Bologna, Italy, 1967. [Google Scholar]
- Cranefield, P.F. The atrioventricular node and the ventricular conducting system in the nonmammalian vertebrate heart. Ann. N. Y. Acad. Sci. 1965, 127, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Valentinuzzi, M.E.; Hoff, H.E. The sinus venosus-atrial Wenckebach-Luciani phenomenon. J. Electrocardiol. 1972, 5, 1–14. [Google Scholar] [CrossRef]
- Mines, G.R. On dynamic equilibrium in the heart. J. Physiol. 1913, 46, 349–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, B.; Boukens, B.J.; Postma, A.V.; Gunst, Q.D.; van den Hoff, M.J.; Moorman, A.F.; Wang, T.; Christoffels, V.M. Identifying the evolutionary building blocks of the cardiac conduction system. PLoS ONE 2012, 7, e44231. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Spicer, D.E.; Brown, N.A.; Mohun, T.J. The development of septation in the four-chambered heart. Anat. Rec. 2014, 297, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.C.; Tran, V.H.; Spicer, D.E.; Rob, J.M.; Sridharan, S.; Taylor, A.; Anderson, R.H.; Jensen, B. Sequential segmental analysis of the crocodilian heart. J. Anat. 2017, 231, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.W. Comparative cardiac anatomy of the reptilia III. The heart of crocodilians and an hypothesis on the completion of the interventricular septum of crocodilians and birds. J. Morph. 1979, 161, 221–240. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Gittenberger-de Groot, A.C.; Vicente-Steijn, R.; Wisse, L.J.; Bartelings, M.M.; Everts, S.; Hoppenbrouwers, T.; Kruithof, B.P.; Jensen, B.; de Bruin, P.W.; et al. Evolution and development of ventricular septation in the amniote heart. PLoS ONE 2014, 9, e106569. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.; Francis, E.T.; King, T.S. The conducting (connecting) system of the crocodilian heart. J. Anat. 1952, 86, 152–161. [Google Scholar] [PubMed]
- Greil, A. Beitrage zur vergelichenden anatomie und entwicklungsgeschichte des herzens und des trauncus arteriosus der wirbelthiere. Morph. Jahrb. 1903, 31, 123–310. [Google Scholar]
- Swett, F.H. The connecting systems of the reptile heart—Alligator. Anat. Rec. 1923, 26, 129–140. [Google Scholar] [CrossRef]
- Bakker, M.L.; Boukens, B.J.; Mommersteeg, M.T.; Brons, J.F.; Wakker, V.; Moorman, A.F.; Christoffels, V.M. Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ. Res. 2008, 102, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Christian, E.; Grigg, G.C. Electrical activation of the ventricular myocardium of the crocodile Crocodylus johnstoni: A combined microscopic and electrophysiological study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 123, 17–23. [Google Scholar] [CrossRef]
- Gregorovicova, M.; Sedmera, D.; Jensen, B. Relative position of the atrioventricular canal determines the electrical activation of developing reptile ventricles. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R. The Heart and Its Conducting System in the Lizard Calotes versicolor (Daudin). Anat. Rec. 1960, 136, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, N.; Matsuura, M.; Onimaru, K.; Tiecke, E.; Kuraku, S.; Kuratani, S.; Tanaka, M. Mechanisms of heart development in the Japanese lamprey, Lethenteron japonicum. Evol. Dev. 2010, 12, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F. Pioneer who Gave Shape to Cardiac Morphology: Antoon FM Moorman, PhD, FESC (vol 126, pg f43, 2012). Circulation 2012, 126. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, R.H.; Mori, S.; Spicer, D.E.; Sanchez-Quintana, D.; Jensen, B. The Anatomy, Development, and Evolution of the Atrioventricular Conduction Axis. J. Cardiovasc. Dev. Dis. 2018, 5, 44. https://doi.org/10.3390/jcdd5030044
Anderson RH, Mori S, Spicer DE, Sanchez-Quintana D, Jensen B. The Anatomy, Development, and Evolution of the Atrioventricular Conduction Axis. Journal of Cardiovascular Development and Disease. 2018; 5(3):44. https://doi.org/10.3390/jcdd5030044
Chicago/Turabian StyleAnderson, Robert H., Shumpei Mori, Diane E. Spicer, Damian Sanchez-Quintana, and Bjarke Jensen. 2018. "The Anatomy, Development, and Evolution of the Atrioventricular Conduction Axis" Journal of Cardiovascular Development and Disease 5, no. 3: 44. https://doi.org/10.3390/jcdd5030044





