N-acetylglucosamine-1-Phosphate Transferase Suppresses Lysosomal Hydrolases in Dysfunctional Osteoclasts: A Potential Mechanism for Vascular Calcification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Carotid Arteries
2.2. Immunohistochemistry and Immunofluorescence for Human Tissues
2.3. CD14-Immunoreactive Monocyte Isolation and Osteoclast Differentiation
2.4. siRNA Transfection Experiments
2.5. Real-Time PCR Analysis
2.6. Immunofluorescence for Human Cells
2.7. TRAP Staining and Quantification
2.8. Resorption Assay for Osteoclast Functional Analysis
2.9. CTSK Activity Assay
2.10. Statistics
3. Results
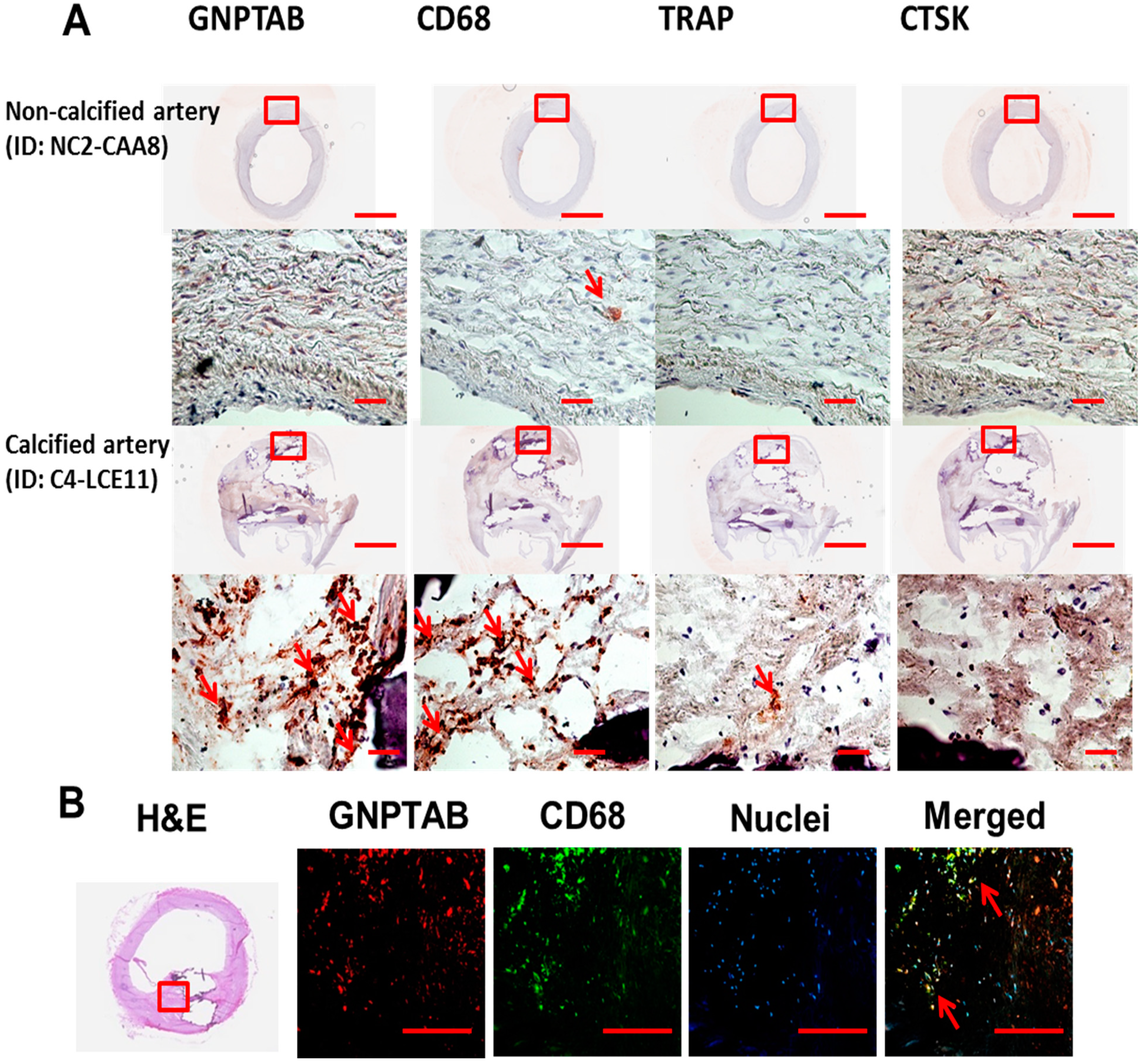
3.1. Human Calcified Arteries Contain Enriched GNPTAB around Calcified Areas but Low Levels of Osteoclast Lysosomal Hydrolases
| Human Arteries ID | GNPTAB | CD68 | TRAP | CTSK | |
|---|---|---|---|---|---|
| Non-Calcified Human Carotid Arteries (n = 3) | |||||
| NC1-CAA5 | - | + | - | + | |
| NC2-CAA8 | - | + | - | + | |
| NC3-CAA11 | - | + | - | + | |
| Calcified Human Carotid Arteries (n = 6) | |||||
| C1-CAA2b | +++ | ++++ | - | + | |
| C2-LCE1 | ++ | ++++ | + | + | |
| C3-LCE4 | ++++ | ++++ | + | - | |
| C4-LCE11 | ++++ | ++++ | + | - | |
| C5-RCE7 | + | ++ | - | - | |
| C6-RCE10 | ++ | ++++ | + | - | |

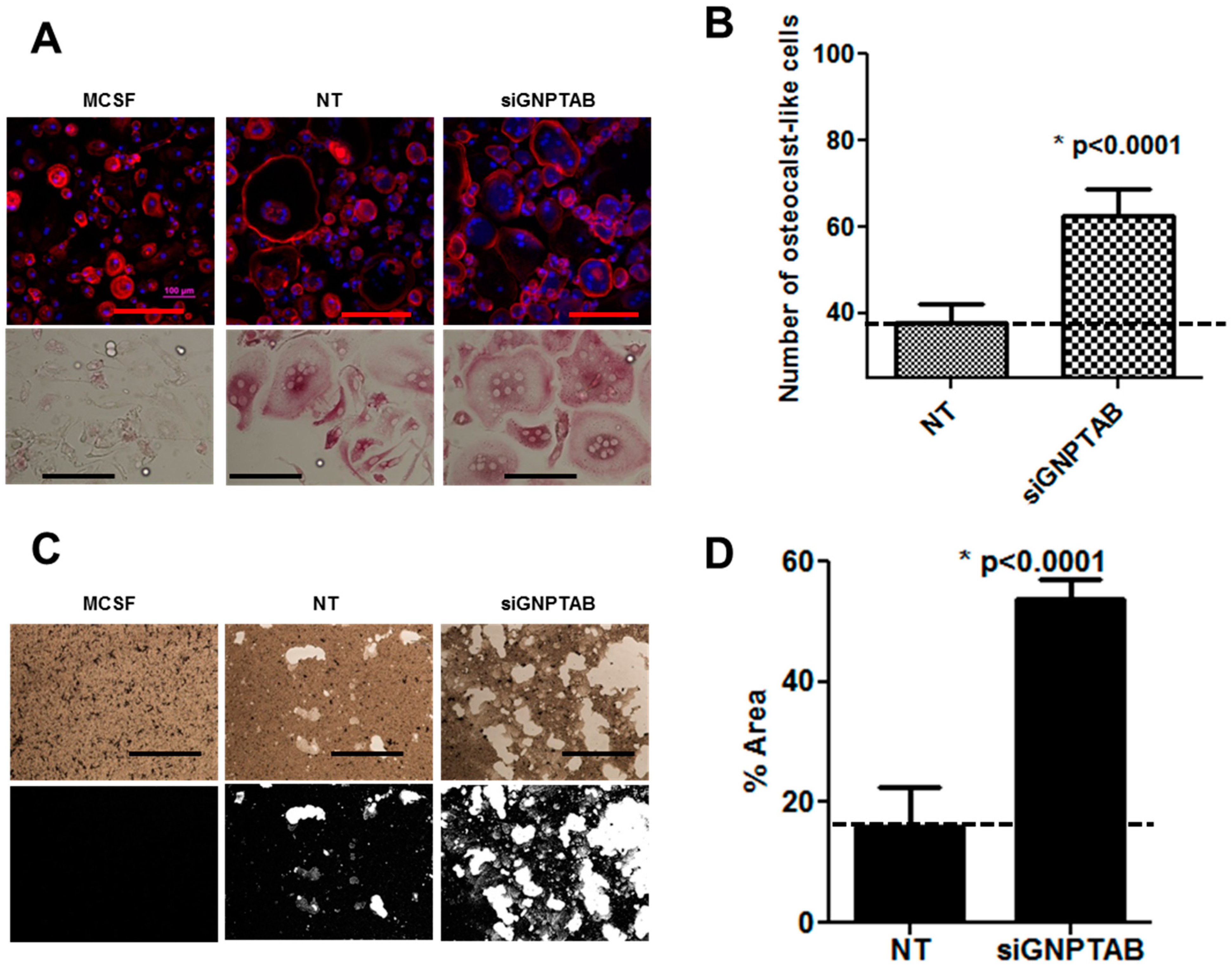
3.2. GNPTAB Silencing Increases Osteoclastogenesis and Resorption Pits Formed by Functional Osteoclast-Like Cells

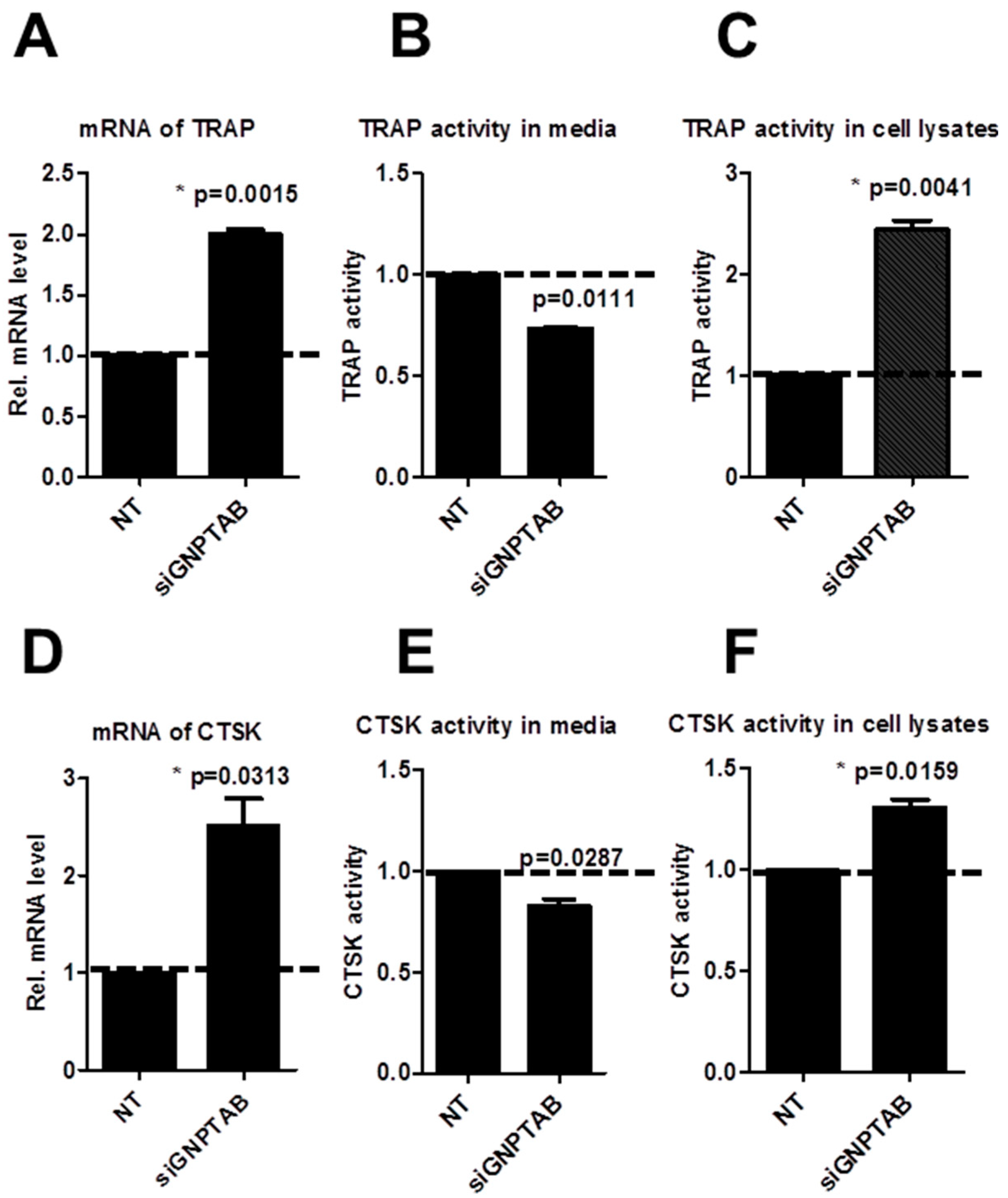
3.3. GNPTAB Silencing Increased Secretion of Lysosomal Hydrolases
4. Discussion
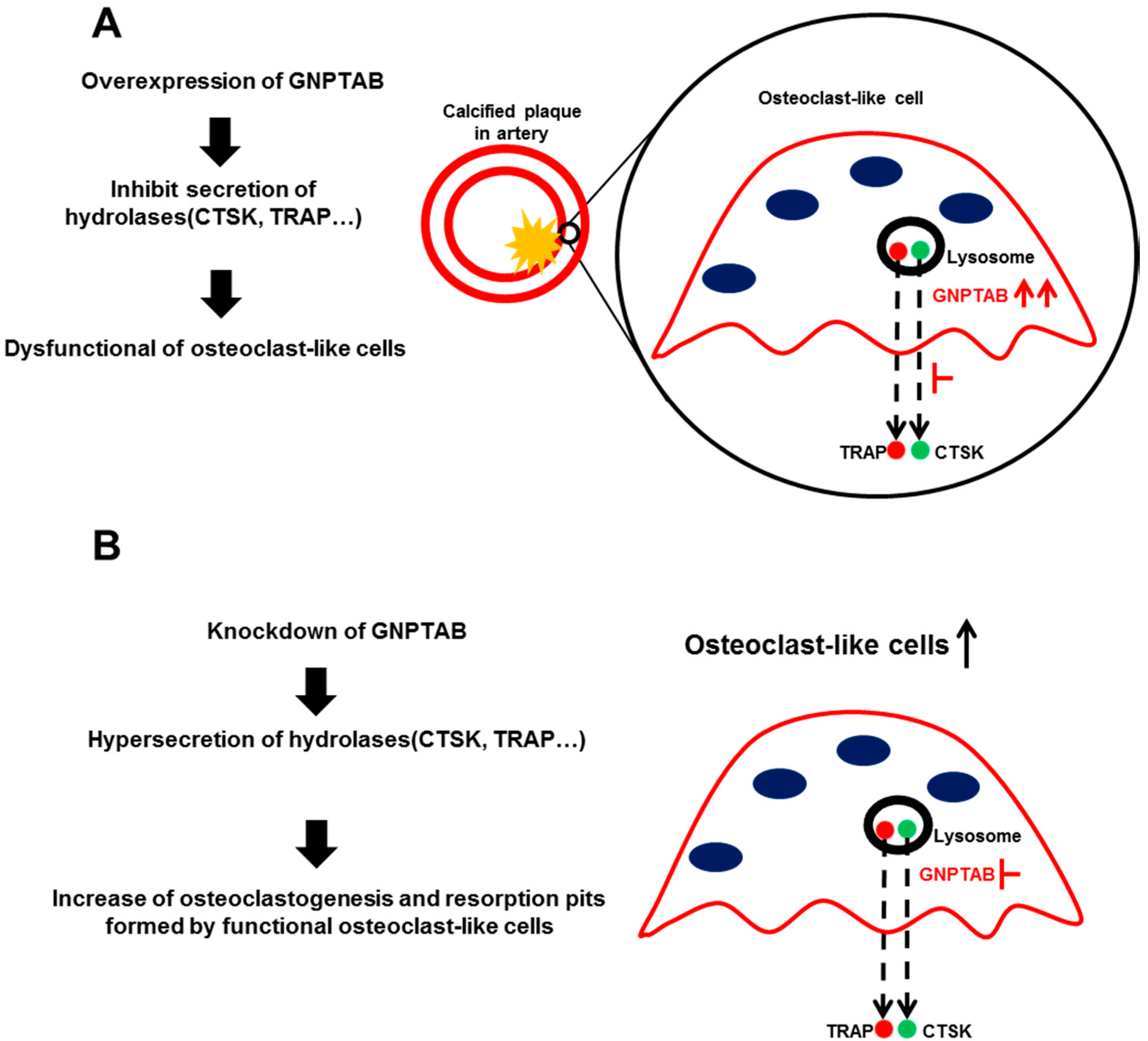
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giachelli, C.M.; Bae, N.; Almeida, M.; Denhardt, D.T.; Alpers, C.E.; Schwartz, S.M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J. Clin. Investig. 1993, 92, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.J.; Schoen, F.J.; Levy, J.T.; Nelson, A.C.; Howard, S.L.; Oshry, L.J. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am. J. Pathol. 1983, 113, 143–155. [Google Scholar] [PubMed]
- Boström, K.; Watson, K.; Horn, S.; Wortham, C.; Herman, I.; Demer, L. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Investig. 1993, 91, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Boström, K.; Demer, L.L. Regulatory mechanisms in vascular calcification. Crit. Rev. Eukaryot. Gene Expr. 1999, 10, 151–158. [Google Scholar]
- Simpson, C.L.; Lindley, S.; Eisenberg, C.; Basalyga, D.M.; Starcher, B.C.; Simionescu, D.T.; Vyavahare, N.R. Toward cell therapy for vascular calcification: Osteoclast-mediated demineralization of calcified elastin. Cardiovasc. Pathol. 2007, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.A.; Kizer, N.; Biswas, R.; Alvarez, U.; Strauss-Schoenberger, J.; Rifas, L.; Rittling, S.R.; Denhardt, D.T.; Hruska, K.A. Osteopontin deficiency produces osteoclast dysfunction due to reduced cd44 surface expression. Mol. Biol. Cell 2003, 14, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Teti, A.; Migliaccio, S.; Taranta, A.; Bernardini, S.; De Rossi, G.; Luciani, M.; Iacobini, M.; de Felice, L.; Boldrini, R.; Bosman, C. Mechanisms of osteoclast dysfunction in human osteopetrosis: Abnormal osteoclastogenesis and lack of osteoclast-specific adhesion structures. J. Bone Miner. Res. 1999, 14, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix gla protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, F.; Ruf, N.; Vaingankar, S.; Toliat, M.R.; Suk, A.; Höhne, W.; Schauer, G.; Lehmann, M.; Roscioli, T.; Schnabel, D. Mutations in enpp1 are associated with “idiopathic” infantile arterial calcification. Nat. Genet. 2003, 34, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, A.; McGregor, D.H.; Anderson, H.C. Matrix vesicles in atherosclerotic calcification. Exp. Biol. Med. 1983, 172, 173–177. [Google Scholar] [CrossRef]
- Giachelli, C.M. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 2004, 15, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Bini, A.; Mann, K.G.; Kudryk, B.J.; Schoen, F.J. Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Vaananen, H.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Knott, L.; Avery, N.C.; Cox, T.M.; Evans, M.J.; Hayman, A.R. Altered collagen in tartrate-resistant acid phosphatase (trap)-deficient mice: A role for trap in bone collagen metabolism. Calcif. Tissue Int. 2007, 80, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Gowen, M.; Lazner, F.; Dodds, R.; Kapadia, R.; Feild, J.; Tavaria, M.; Bertoncello, I.; Drake, F.; Zavarselk, S.; Tellis, I. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J. Bone Miner. Res. 1999, 14, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Schraer, H.; Gay, C.V. Ultrastructural immunocytochemical localization of carbonic anhydrase in normal and calcitonin-treated chick osteoclasts. Anat. Rec. 1982, 204, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Silverton, S.F.; Dodgson, S.J.; Fallon, M.D.; Forster, R. Carbonic anhydrase activity of chick osteoclasts is increased by parathyroid hormone. Am. J. Physiol. Endocrinol. Metab. 1987, 253, E670–E674. [Google Scholar]
- Min, H.; Morony, S.; Sarosi, I.; Dunstan, C.R.; Capparelli, C.; Scully, S.; Van, G.; Kaufman, S.; Kostenuik, P.J.; Lacey, D.L. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med. 2000, 192, 463–474. [Google Scholar] [CrossRef]
- Doherty, T.M.; Uzui, H.; Fitzpatrick, L.A.; Tripathi, P.V.; Dunstan, C.R.; Asotra, K.; Rajavashisth, T.B. Rationale for the role of osteoclast-like cells in arterial calcification. FASEB J. 2002, 16, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.; Mentaverri, R.; Mozar, A.; Brazier, M.; Kamel, S. The pathophysiology of vascular calcification: Are osteoclast-like cells the missing link? Diabetes Metab. 2008, 34, 16–20. [Google Scholar] [CrossRef]
- Coutinho, M.F.; Prata, M.J.; Alves, S. Mannose-6-phosphate pathway: A review on its role in lysosomal function and dysfunction. Mol. Genet. Metab. 2012, 105, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Braulke, T.; Bonifacino, J.S. Sorting of lysosomal proteins. Biochim. Biophys. Acta 2009, 1793, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Van Meel, E.; Boonen, M.; Zhao, H.; Oorschot, V.; Ross, F.P.; Kornfeld, S.; Klumperman, J. Disruption of the man-6-p targeting pathway in mice impairs osteoclast secretory lysosome biogenesis. Traffic 2011, 12, 912–924. [Google Scholar]
- Kollmann, K.; Damme, M.; Markmann, S.; Morelle, W.; Schweizer, M.; Hermans-Borgmeyer, I.; Röchert, A.; Pohl, S.; Lübke, T.; Michalski, J.-C. Lysosomal dysfunction causes neurodegeneration in mucolipidosis ii “knock-in” mice. Brain 2012, 135, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, K.; Pestka, J.M.; Kühn, S.C.; Schöne, E.; Schweizer, M.; Karkmann, K.; Otomo, T.; Catala-Lehnen, P.; Failla, A.V.; Marshall, R.P. Decreased bone formation and increased osteoclastogenesis cause bone loss in mucolipidosis II. EMBO Mol. Med. 2013, 5, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-derived matrix vesicles an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Aikawa, M.; Libby, P.; Figueiredo, J.-L.; Rusanescu, G.; Iwamoto, Y.; Fukuda, D.; Kohler, R.H.; Shi, G.-P.; Jaffer, F.A. Arterial and aortic valve calcification abolished by elastolytic cathepsin s deficiency in chronic renal disease. Circulation 2009, 119, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Alatalo, S.L.; Halleen, J.M.; Hentunen, T.A.; Mönkkönen, J.; Väänänen, H.K. Rapid screening method for osteoclast differentiation in vitro that measures tartrate-resistant acid phosphatase 5b activity secreted into the culture medium. Clin. Chem. 2000, 46, 1751–1754. [Google Scholar] [PubMed]
- Takahashi, N.; Yamana, H.; Yoshiki, S.; Roodman, G.D.; Mundy, G.R.; Jones, S.J.; Boyde, A.; Suda, T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology 1988, 122, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- ImageJ. National Institute of Mental Health: Bethesda, MD, USA, 2010.
- Emmert-Buck, M.R.; Bonner, R.F.; Smith, P.D.; Chuaqui, R.F.; Zhuang, Z.; Goldstein, S.R.; Weiss, R.A.; Liotta, L.A. Laser capture microdissection. Science 1996, 274, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Espina, V.; Wulfkuhle, J.D.; Calvert, V.S.; VanMeter, A.; Zhou, W.; Coukos, G.; Geho, D.H.; Petricoin, E.F.; Liotta, L.A. Laser-capture microdissection. Nat. Protoc. 2006, 1, 586–603. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.C.; Malakellis, M.; Collier, F.M.; Cameron, P.U.; Holloway, W.R.; Gough, T.J.; Gregorio-King, C.; Kirkland, M.A.; Myers, D.E. Induction of osteoclasts from cd14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor kappab ligand (rankl). Clin. Sci. 2000, 99, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.M.; Rothenberg, M.; Kennebunk, M. Corning® Osteo Assay Surface 24 Well Plates with Transwell® Permeable Supports–A Useful Tool For Co-Culture Studies. Corning Life Sciences: Lowell, MA, USA, 2011. [Google Scholar]
- Marks, S., Jr.; Seifert, M. The lifespan of osteoclasts: Experimental studies using the giant granule cytoplasmic marker characteristic of beige mice. Bone 1985, 6, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Bossard, M.J.; Tomaszek, T.A.; Thompson, S.K.; Amegadzie, B.Y.; Hanning, C.R.; Jones, C.; Kurdyla, J.T.; McNulty, D.E.; Drake, F.H.; Gowen, M. Proteolytic activity of human osteoclast cathepsin K expression, purification, activation, and substrate identification. J. Biol. Chem. 1996, 271, 12517–12524. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M.; Räisänen, S.; Salo, J.J.; Reddy, S.V.; Roodman, G.D.; Hentunen, T.A.; Lehenkari, P.P.; Kaija, H.; Vihko, P.; Väänänen, H.K. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J. Biol. Chem. 1999, 274, 22907–22910. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Sleat, D.E.; Lecocq, M.; Hayman, A.R.; Jadot, M.; Lobel, P. Acid phosphatase 5 is responsible for removing the mannose 6-phosphate recognition marker from lysosomal proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 16590–16595. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.R.; Josien, R.; Lee, S.Y.; Sauter, B.; Li, H.-L.; Steinman, R.M.; Choi, Y. Trance (tumor necrosis factor [TNF]-related activation-induced cytokine), a new tnf family member predominantly expressed in T cells, is a dendritic cell–specific survival factor. J. Exp. Med. 1997, 186, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Iwashita, M.; Choi, J.; Aikawa, M.; Aikawa, E. N-acetylglucosamine-1-Phosphate Transferase Suppresses Lysosomal Hydrolases in Dysfunctional Osteoclasts: A Potential Mechanism for Vascular Calcification. J. Cardiovasc. Dev. Dis. 2015, 2, 31-47. https://doi.org/10.3390/jcdd2020031
Lei Y, Iwashita M, Choi J, Aikawa M, Aikawa E. N-acetylglucosamine-1-Phosphate Transferase Suppresses Lysosomal Hydrolases in Dysfunctional Osteoclasts: A Potential Mechanism for Vascular Calcification. Journal of Cardiovascular Development and Disease. 2015; 2(2):31-47. https://doi.org/10.3390/jcdd2020031
Chicago/Turabian StyleLei, Yang, Masaya Iwashita, Jung Choi, Masanori Aikawa, and Elena Aikawa. 2015. "N-acetylglucosamine-1-Phosphate Transferase Suppresses Lysosomal Hydrolases in Dysfunctional Osteoclasts: A Potential Mechanism for Vascular Calcification" Journal of Cardiovascular Development and Disease 2, no. 2: 31-47. https://doi.org/10.3390/jcdd2020031





