The Current State of Research on Sirtuin-Mediated Autophagy in Cardiovascular Diseases
Abstract
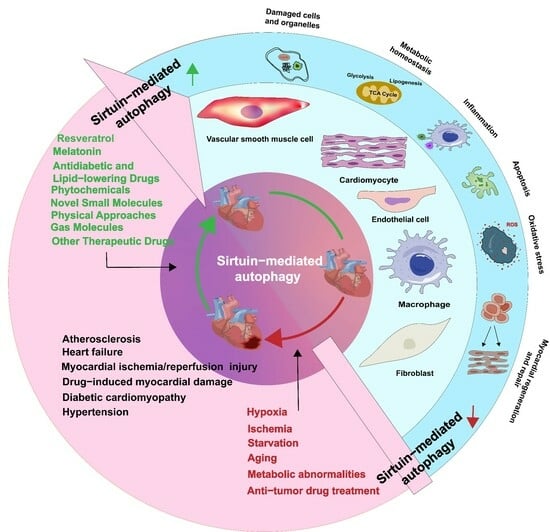
:1. Introduction
2. Regulation of Cardiovascular Autophagy by Various Sirtuins
2.1. Sirtuins in the Nucleus
2.2. Sirtuins in the Mitochondria
2.3. Sirtuins in the Cytoplasm
3. Role of Sirtuin-Induced Autophagy in Cardiovascular Diseases
3.1. Atherosclerosis
3.2. Myocardial Ischemia/Reperfusion Injury
3.3. Diabetic Cardiomyopathy
3.4. Drug-Induced Cardiac Injury
3.5. Heart Failure
3.6. Hypertension
3.7. Cardiogenesis and Cardiac Maintenance
4. Advances in Sirtuin/Autophagy-Based Therapies
4.1. Resveratrol
4.2. Melatonin
4.3. Antidiabetic and Lipid-Lowering Drugs
4.4. Phytochemicals
4.5. Novel Small Molecules
4.6. Gas Molecules
4.7. Physical Approaches
4.8. Other Therapeutic Drugs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| ATG5 | autophagy-related 5 |
| ATG7 | autophagy-related 7 |
| ATG12 | autophagy-related 12 |
| BNIP3 | BCL2 interacting protein 3 |
| CPS1 | carbamoyl-phosphate synthase 1 |
| EX-527 | SIRT1 inhibitor |
| JNK | c-Jun amino-terminal kinase |
| Nkx3.2 | NK3 homeobox 2 |
| PARK2 | parkin RBR E3 ubiquitin protein ligase |
| PCSK6 | proprotein convertase subtilisin/kexin type 6 |
| PINK | PTEN induced putative kinase |
| 17β-E2 | 17beta-estradiol |
| 3MA | 3-methyladenine; |
| 4EBP1 | 4E binding protein 1 |
| AK-7 | SIRT2 inhibitor; |
| AKT | protein kinase B |
| AMPK | adenosine 5′-monophosphate (AMP)-activated protein kinase |
| Ang II | angiotensin II |
| AS | atherosclerosis |
| ATG12 | autophagy related 12 |
| ATG5 | autophagy related 5 |
| Bnip | BCL2 interacting protein |
| cAMP | 3′-5′-cyclic adenosine monophosphate |
| CHMP2B | CHarged Multivesicular body Protein 2B |
| circRNA | circular RNA |
| CREB | cAMP-response element binding protein |
| DCM | diabetic cardiomyopathy; |
| DOX | doxorubicin; |
| eEF2 | eukaryotic elongation factor 2 |
| eEF2k | eukaryotic elongation factor 2 kinase |
| EndoMT | endothelial-to-mesenchymal transition |
| EPC | endothelial progenitor cell |
| ERK | extracellular regulated kinase |
| ERS | endoplasmic reticulum stress |
| EX-527 | SIRT1 inhibitor |
| FAT10 | human leukocyte antigen-F adjacent transcript 10 |
| FF | fenofibrate |
| FGF21 | fibroblast growth factor 21 |
| FOXM1 | the forkhead box M1 |
| FOXO1 | the forkhead box-1 |
| FOXO3a | the forkhead box O3a |
| GATA5 | GATA-binding protein 5 |
| GLS | glutaminase |
| GTSP1 | glutathione S-transferase P1 |
| H/R | hypoxia–reoxygenation |
| Hes-1 | hairy and Enhancer of split homolog-1 |
| HUVECs | human umbilical vein endothelial cells |
| IGF2 | insulin-like growth factor 2 |
| IL-1B | interleukin-1B |
| LC3 | microtubule-associated protein light chain 3 |
| LDHB | lactate dehydrogenase B |
| LKB1 | liver kinase B1 |
| LncRNA PVT1 | Long noncoding RNA PVT1 |
| LncRNA | long noncoding RNA |
| MDA | malondialdehyde |
| MDL800 | SIRT6 activator |
| MFN2 | mitofusin 2 |
| MI | myocardial infarction |
| MI/R | myocardial ischemia/reperfusion |
| MnSOD2 | manganese superoxide dismutase 2 |
| Mst1 | macrophage stimulating 1 |
| MT2 | melatonin membrane receptor 2 |
| MTHFR | methylenetetrahydrofolate-reductase |
| mTOR | mammalian target of rapamycin |
| NAD+ | nicotinamide adenine dinucleotide |
| Nampt | nicotinamide phosphoribosyl transferase |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| NLRX1 | nucleotide-binding oligomerization domain-like receptor family member X1 |
| NRF2 | the nuclear factor E2-related factor 2 |
| OPA1 | optic atrophy 1 |
| ox-LDL | oxidative modification of low-density lipoprotein |
| P70S6K | 70 kDa ribosomal protein S6 kinase |
| PARK2 | parkin RBR E3 ubiquitin protein ligase |
| PGC-1α | peroxisome proliferator-activated receptor γ coactivator 1-α |
| PI3K | phosphoinositide 3-kinase |
| PICK1 | protein interacting with C kinase 1 |
| PINK | PTEN induced putative kinase |
| ROS | reactive oxygen species |
| RSV | resveratrol |
| SCFD1 | sec1 family domain containing 1 |
| SIRTs | silent information regulators |
| SMAD4 | mothers against decapentaplegic homolog 4 |
| SQSTM1 | sequestosome |
| STAT3 | signal transducer and activator of transcription 3 |
| SUMO1 | small ubiquitin-like modifier 1 |
| TFEB | transcription factor EB |
| TGFBR1 | type1 transforming growth factor beta receptor |
| TLR9 | the Toll-like receptor 9 |
| TMBIM6 | transmembrane Bax inhibitor Motif Containing 6 |
| TNF-α | tumor necrosing factor alpha |
| ULK1 | unc-51-like autophagy activating kinase 1 |
| vWf | von Willebrand facto |
| YAP | yes-associated protein |
References
- Rine, J.; Herskowitz, I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics 1987, 116, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ge, J.; Li, H. Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Aventaggiato, M.; Vernucci, E.; Barreca, F.; Russo, M.A.; Tafani, M. Sirtuins’ control of autophagy and mitophagy in cancer. Pharmacol. Ther. 2021, 221, 107748. [Google Scholar] [CrossRef]
- Sciarretta, S.; Hariharan, N.; Monden, Y.; Zablocki, D.; Sadoshima, J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr. Cardiol. 2011, 32, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Verma, S.; Seranova, E.; Sarkar, S.; Kumar, D. Selective Autophagy and Xenophagy in Infection and Disease. Front. Cell Dev. Biol. 2018, 6, 147. [Google Scholar] [CrossRef]
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017, 120, 1812–1824. [Google Scholar] [CrossRef]
- Conti, V.; Forte, M.; Corbi, G.; Russomanno, G.; Formisano, L.; Landolfi, A.; Izzo, V.; Filippelli, A.; Vecchione, C.; Carrizzo, A. Sirtuins: Possible Clinical Implications in Cardio and Cerebrovascular Diseases. Curr. Drug Targets 2017, 18, 473–484. [Google Scholar] [CrossRef]
- Baeken, M.W. Sirtuins and their influence on autophagy. J. Cell. Biochem. 2023. In Press. [Google Scholar] [CrossRef]
- Lee, I.H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Packer, M. Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: Implications for understanding the effects of current and future treatments for heart failure. Eur. Heart J. 2020, 41, 3856–3861. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Bennett, M.R. Sirtuins in atherosclerosis: Guardians of healthspan and therapeutic targets. Nat. Rev. Cardiol. 2022, 19, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shi, B.; Ma, M.; Wu, X.; Lin, X. The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J. Cell. Physiol. 2019, 234, 5488–5495. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, F.; Zhang, Y.; Sun, J.; Gao, F.; Shi, G. Resveratrol, novel application by preconditioning to attenuate myocardial ischemia/reperfusion injury in mice through regulate AMPK pathway and autophagy level. J. Cell. Mol. Med. 2022, 26, 4216–4229. [Google Scholar] [CrossRef] [PubMed]
- Balarastaghi, S.; Barangi, S.; Hosseinzadeh, H.; Imenshahidi, M.; Moosavi, Z.; Razavi, B.M.; Karimi, G. Melatonin improves arsenic-induced hypertension through the inactivation of the Sirt1/autophagy pathway in rat. Biomed. Pharmacother. 2022, 151, 113135. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. α-Linolenic acid but not linolenic acid protects against hypertension: Critical role of SIRT3 and autophagic flux. Cell Death Dis. 2020, 11, 83. [Google Scholar] [CrossRef]
- Qiu, Z.; Ming, H.; Zhang, Y.; Yu, Y.; Lei, S.; Xia, Z.Y. The Protective Role of Bmal1-Regulated Autophagy Mediated by HDAC3/SIRT1 Pathway in Myocardial Ischemia/Reperfusion Injury of Diabetic Rats. Cardiovasc. Drugs Ther. 2022, 36, 229–243. [Google Scholar] [CrossRef]
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A.M. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: A review of the protective role of melatonin. J. Pineal Res. 2014, 57, 367–380. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, K.; Gao, C.; Ma, W.; Liu, M.; Guo, X.; Bao, G.; Han, B.; Hu, H.; Zhao, Z. Activation of FMS-like tyrosine kinase 3 protects against isoprenaline-induced cardiac hypertrophy by improving autophagy and mitochondrial dynamics. FASEB J. 2022, 36, e22672. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, K.; Wu, H.; Zhang, X.; Ye, W.; Tan, X.; Xiong, Y. Lin28a attenuates cerebral ischemia/reperfusion injury through regulating Sirt3-induced autophagy. Brain Res. Bull. 2021, 170, 39–48. [Google Scholar] [CrossRef]
- Hao, Y.; Lu, Q.; Yang, G.; Ma, A. Lin28a protects against postinfarction myocardial remodeling and dysfunction through Sirt1 activation and autophagy enhancement. Biochem. Biophys. Res. Commun. 2016, 479, 833–840. [Google Scholar] [CrossRef]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; Depinho, R.A.; Sadoshima, J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res. 2010, 107, 1470–1482. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ikeda, Y.; Uchikado, Y.; Akasaki, Y.; Sadoshima, J.; Ohishi, M. Estrogen Plays a Crucial Role in Rab9-Dependent Mitochondrial Autophagy, Delaying Arterial Senescence. J. Am. Heart Assoc. 2021, 10, e019310. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Wang, J.; Zhong, L.; Chen, X.; Zhang, R.; Wang, H. Glucose restriction delays senescence and promotes proliferation of HUVECs via the AMPK/SIRT1-FOXA3-Beclin1 pathway. Exp. Gerontol. 2020, 139, 111053. [Google Scholar] [CrossRef]
- Li, C.; Guo, Z.; Liu, F.; An, P.; Wang, M.; Yang, D.; Tang, Q. PCSK6 attenuates cardiac dysfunction in doxorubicin-induced cardiotoxicity by regulating autophagy. Free Radic. Biol. Med. 2023, 203, 114–128. [Google Scholar] [CrossRef]
- Ning, S.; Li, Z.; Ji, Z.; Fan, D.; Wang, K.; Wang, Q.; Hua, L.; Zhang, J.; Meng, X.; Yuan, Y. MicroRNA-494 suppresses hypoxia/reoxygenation-induced cardiomyocyte apoptosis and autophagy via the PI3K/AKT/mTOR signaling pathway by targeting SIRT1. Mol. Med. Rep. 2020, 22, 5231–5242. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, F.; Niu, Q.; Jiao, M.; Han, X.; Zhang, K.; Ma, W.; Mi, S.; Guo, S.; Zhao, Z. Downregulation of miR-128 Ameliorates Ang II-Induced Cardiac Remodeling via SIRT1/PIK3R1 Multiple Targets. Oxid. Med. Cell. Longev. 2021, 2021, 8889195. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, K.; Li, P.; Wu, Z. Down-regulating miR-217-5p Protects Cardiomyocytes against Ischemia/Reperfusion Injury by Restoring Mitochondrial Function via Targeting SIRT1. Inflammation 2021, 44, 383–396. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, J.H.; Cao, S.D.; Gao, C.X.; He, Y.; Wang, Y.; Wan, H.T.; Jin, B. Effect of main ingredients of Danhong Injection against oxidative stress induced autophagy injury via miR-19a/SIRT1 pathway in endothelial cells. Phytomedicine 2021, 83, 153480. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Yang, M.; Wu, C.; Lan, R.; Wang, W.; Li, Y. Circ-SIRT1 inhibits cardiac hypertrophy via activating SIRT1 to promote autophagy. Cell Death Dis. 2021, 12, 1069. [Google Scholar] [CrossRef]
- Yang, J.; Lin, X.; Wang, L.; Sun, T.; Zhao, Q.; Ma, Q.; Zhou, Y. LncRNA MALAT1 Enhances ox-LDL-Induced Autophagy through the SIRT1/MAPK/NF-κB Pathway in Macrophages. Curr. Vasc. Pharmacol. 2020, 18, 652–662. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Sun, Y.Y.; Xing, Y.F.; Wang, Y.B.; Lu, X.T.; Bai, W.W.; Liu, X.Q.; Zhao, Y.X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell. Mol. Med. 2014, 18, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Cardioprotective Effects of Sirtuin-1 and Its Downstream Effectors: Potential Role in Mediating the Heart Failure Benefits of SGLT2 (Sodium-Glucose Cotransporter 2) Inhibitors. Circ. Heart Fail. 2020, 13, e007197. [Google Scholar] [CrossRef]
- Zheng, Y.; Kou, J.; Wang, P.; Ye, T.; Wang, Z.; Gao, Z.; Cong, L.; Li, M.; Dong, B.; Yang, W.; et al. Berberine-induced TFEB deacetylation by SIRT1 promotes autophagy in peritoneal macrophages. Aging 2021, 13, 7096–7119. [Google Scholar] [CrossRef]
- Pires Da Silva, J.; Monceaux, K.; Guilbert, A.; Gressette, M.; Piquereau, J.; Novotova, M.; Ventura-Clapier, R.; Garnier, A.; Lemaire, C. SIRT1 Protects the Heart from ER Stress-Induced Injury by Promoting eEF2K/eEF2-Dependent Autophagy. Cells 2020, 9, 426. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Meng, Q.; Wang, S.; Yan, P.; Wang, X.; Luo, D.; Zhou, X.; Ji, R. Quercetin Improves Cardiomyocyte Vulnerability to Hypoxia by Regulating SIRT1/TMBIM6-Related Mitophagy and Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5529913. [Google Scholar] [CrossRef]
- Wan, R.; Yuan, P.; Guo, L.; Shao, J.; Liu, X.; Lai, W.; Kong, Q.; Chen, L.; Ge, J.; Xu, Z.; et al. Ubiquitin-like protein FAT10 suppresses SIRT1-mediated autophagy to protect against ischemic myocardial injury. J. Mol. Cell. Cardiol. 2021, 153, 1–13. [Google Scholar] [CrossRef]
- Yuan, P.; Hu, Q.; He, X.; Long, Y.; Song, X.; Wu, F.; He, Y.; Zhou, X. Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death Dis. 2020, 11, 141. [Google Scholar] [CrossRef]
- Takeda-Watanabe, A.; Kitada, M.; Kanasaki, K.; Koya, D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem. Biophys. Res. Commun. 2012, 427, 191–196. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef]
- Lu, J.; Sun, D.; Liu, Z.; Li, M.; Hong, H.; Liu, C.; Gao, S.; Li, H.; Cai, Y.; Chen, S.; et al. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl. Res. 2016, 172, 96–112.e6. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Yang, M.; Liu, Y.; Song, J.W.; Wang, J.; Chi, H.J.; Liu, X.Y.; Zuo, K.; Yang, X.C.; Zhong, J.C. MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling. Eur. J. Pharmacol. 2020, 883, 173374. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Yang, X.; Bai, X.; Lu, Y.; Zhu, L.; Cheng, W.; Shu, M.; Zhu, Y.; Du, X.; et al. Deacetylation of Caveolin-1 by Sirt6 induces autophagy and retards high glucose-stimulated LDL transcytosis and atherosclerosis formation. Metabolism 2022, 131, 155162. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Jiang, W.; Liu, M.; Wang, Y.; Ma, H.; Mu, N.; Wang, H. SIRT6 Protects Against Myocardial Ischemia-Reperfusion Injury by Attenuating Aging-Related CHMP2B Accumulation. J. Cardiovasc. Transl. Res. 2022, 15, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Wu, J.; Liu, M.; Li, M.; Sun, Y.; Huang, W.; Li, Y.; Zhang, Y.; Tang, W.; et al. Endothelial SIRT6 Is Vital to Prevent Hypertension and Associated Cardiorenal Injury Through Targeting Nkx3.2-GATA5 Signaling. Circ. Res. 2019, 124, 1448–1461. [Google Scholar] [CrossRef]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef]
- Yu, W.; Cui, X.; Wan, Z.; Yu, Y.; Liu, X.; Jin, L. Silencing forkhead box M1 promotes apoptosis and autophagy through SIRT7/mTOR/IGF2 pathway in gastric cancer cells. J. Cell. Biochem. 2018, 119, 9090–9098. [Google Scholar] [CrossRef]
- Wu, S.Y.; Du, Y.C.; Yue, C.F. Sirt7 protects chondrocytes degeneration in osteoarthritis via autophagy activation. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9246–9255. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, C.Y.; Zhang, Y.; Zhao, J.; Han, B.M.; Xia, S.J. SIRT7 depletion inhibits cell proliferation and androgen-induced autophagy by suppressing the AR signaling in prostate cancer. J. Exp. Clin. Cancer Res. 2020, 39, 28. [Google Scholar] [CrossRef]
- Araki, S.; Izumiya, Y.; Rokutanda, T.; Ianni, A.; Hanatani, S.; Kimura, Y.; Onoue, Y.; Senokuchi, T.; Yoshizawa, T.; Yasuda, O.; et al. Sirt7 Contributes to Myocardial Tissue Repair by Maintaining Transforming Growth Factor-β Signaling Pathway. Circulation 2015, 132, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, T.; Fu, F.; Cui, Z.; Lai, Q.; Zhang, Y.; Yu, B.; Liu, F.; Kou, J.; Li, F. Omentin1 ameliorates myocardial ischemia-induced heart failure via SIRT3/FOXO3a-dependent mitochondrial dynamical homeostasis and mitophagy. J. Transl. Med. 2022, 20, 447. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, L. Spinacetin alleviates doxorubicin-induced cardiotoxicity by initiating protective autophagy through SIRT3/AMPK/mTOR pathways. Phytomedicine 2022, 101, 154098. [Google Scholar] [CrossRef]
- Fan, X.; He, Y.; Wu, G.; Chen, H.; Cheng, X.; Zhan, Y.; An, C.; Chen, T.; Wang, X. Sirt3 activates autophagy to prevent DOX-induced senescence by inactivating PI3K/AKT/mTOR pathway in A549 cells. Biochim. Biophys. Acta. Mol. Cell Res. 2023, 1870, 119411. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, Y.; Ding, X.; Gao, B. The role of Sirt3 in the changes of skeletal muscle mitophagy induced by hypoxic training. Gen. Physiol. Biophys. 2022, 41, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Y.; Gao, Y.; Wang, Z.; Ma, J. Melatonin Attenuates Anoxia/Reoxygenation Injury by Inhibiting Excessive Mitophagy Through the MT2/SIRT3/FoxO3a Signaling Pathway in H9c2 Cells. Drug Des. Dev. Ther. 2020, 14, 2047–2060. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Chen, T.; Zhang, C.; Li, J.; Liu, L.; Qi, Y.; Zheng, X.; Zhang, C.; Bu, P. Sirt3 promotes sensitivity to sunitinib-induced cardiotoxicity via inhibition of GTSP1/JNK/autophagy pathway in vivo and in vitro. Arch. Toxicol. 2019, 93, 3249–3260. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, J.; Wang, Z.Q.; Li, Y. Sirt3 promotes the autophagy of HK-2 human proximal tubular epithelial cells via the inhibition of Notch-1/Hes-1 signaling. Mol. Med. Rep. 2021, 24, 634. [Google Scholar] [CrossRef]
- Li, R.; Xin, T.; Li, D.; Wang, C.; Zhu, H.; Zhou, H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018, 18, 229–243. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, J.; Wang, S.; Cheng, Z.; Hu, J.; Wang, T.; Man, W.; Yin, T.; Guo, W.; Gao, E.; et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J. Pineal Res. 2017, 63, e12418. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, Y.; Huang, G.; Huang, J.; Gao, M.; Sun, M.; Xia, H.; Pare, R.; Li, J.; Ruan, Y. 17β-estradiol suppresses H2O2-induced senescence in human umbilical vein endothelial cells by inducing autophagy through the PVT1/miR-31/SIRT3 axis. J. Steroid Biochem. Mol. Biol. 2023, 227, 106244. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Huang, J.; Song, S.; Wang, Y.; Zeng, Y.; Wu, S.; Ruan, Y. 17β-estradiol inhibits H2O2-induced senescence in HUVEC cells through upregulating SIRT3 expression and promoting autophagy. Biogerontology 2020, 21, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chen, C.C.; Lin, M.H.; Su, H.T.; Ho, M.Y.; Yeh, J.K.; Tsai, M.L.; Hsieh, I.C.; Wen, M.S. TLR9 Binding to Beclin 1 and Mitochondrial SIRT3 by a Sodium-Glucose Co-Transporter 2 Inhibitor Protects the Heart from Doxorubicin Toxicity. Biology 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, C.; Wang, X.; Bai, J.; He, S.; Zhang, J.; Xin, W.; Li, Y.; Jiang, Y.; Li, J.; et al. MicroRNA-874-5p regulates autophagy and proliferation in pulmonary artery smooth muscle cells by targeting Sirtuin 3. Eur. J. Pharmacol. 2020, 888, 173485. [Google Scholar] [CrossRef]
- Lang, A.; Anand, R.; Altinoluk-Hambüchen, S.; Ezzahoini, H.; Stefanski, A.; Iram, A.; Bergmann, L.; Urbach, J.; Böhler, P.; Hänsel, J.; et al. SIRT4 interacts with OPA1 and regulates mitochondrial quality control and mitophagy. Aging 2017, 9, 2163–2189. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Yang, Y.; Zou, P.; Xia, Z.; Li, J. SIRT4 Suppresses Doxorubicin-Induced Cardiotoxicity by Regulating the AKT/mTOR/Autophagy Pathway. Toxicology 2022, 469, 153119. [Google Scholar] [CrossRef]
- Huang, H.; Ouyang, Q.; Mei, K.; Liu, T.; Sun, Q.; Liu, W.; Liu, R. Acetylation of SCFD1 regulates SNARE complex formation and autophagosome-lysosome fusion. Autophagy 2023, 19, 189–203. [Google Scholar] [CrossRef]
- Nakagawa, T.; Lomb, D.J.; Haigis, M.C.; Guarente, L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 2009, 137, 560–570. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef]
- Shi, L.; Yan, H.; An, S.; Shen, M.; Jia, W.; Zhang, R.; Zhao, L.; Huang, G.; Liu, J. SIRT5-mediated deacetylation of LDHB promotes autophagy and tumorigenesis in colorectal cancer. Mol. Oncol. 2019, 13, 358–375. [Google Scholar] [CrossRef]
- Ng, F.; Tang, B.L. Sirtuins’ modulation of autophagy. J. Cell. Physiol. 2013, 228, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, P.; Xie, X.; Hu, F.; Jiang, L.; Hu, R.; Ding, F.; Xiao, H.; Zhang, H. Down Regulation of SIRT2 Reduced ASS Induced NSCLC Apoptosis Through the Release of Autophagy Components via Exosomes. Front. Cell Dev. Biol. 2020, 8, 601953. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.F.; Wang, N.Y.; Wang, X.M.; Liang, S.T.; Zheng, W.; Lu, Y.B.; Zhao, X.; Hao, D.L.; Zhang, Z.Q.; et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Lynn, E.G.; McLeod, C.J.; Gordon, J.P.; Bao, J.; Sack, M.N. SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells. FEBS Lett. 2008, 582, 2857–2862. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Gandhirajan, A.; Kibler, C.; Wang, X.; Vachharajani, V. Sirtuin 2 Dysregulates Autophagy in High-Fat-Exposed Immune-Tolerant Macrophages. Cells 2021, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Park, S.H.; Imbesi, M.; Nathan, W.J.; Zou, X.; Zhu, Y.; Jiang, H.; Parisiadou, L.; Gius, D. Loss of NAD-Dependent Protein Deacetylase Sirtuin-2 Alters Mitochondrial Protein Acetylation and Dysregulates Mitophagy. Antioxid. Redox Signal. 2017, 26, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lu, S.; Gao, Y.; Yang, K.; Wu, D.; Xu, X.; Sun, G.; Sun, X. Araloside C attenuates atherosclerosis by modulating macrophage polarization via Sirt1-mediated autophagy. Aging 2020, 12, 1704–1724. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezabakhsh, A.; Pezeshkian, M.; Rahbarghazi, R.; Nouri, M. Distinct role of autophagy on angiogenesis: Highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res. Ther. 2018, 9, 305. [Google Scholar] [CrossRef]
- Wang, C.; Mao, C.; Lou, Y.; Xu, J.; Wang, Q.; Zhang, Z.; Tang, Q.; Zhang, X.; Xu, H.; Feng, Y. Monotropein promotes angiogenesis and inhibits oxidative stress-induced autophagy in endothelial progenitor cells to accelerate wound healing. J. Cell. Mol. Med. 2018, 22, 1583–1600. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Song, B.; Ye, X.; Li, Z.; Lu, C. Autophagy-Sirtuin1(SIRT1) Alleviated the Coronary Atherosclerosis (AS)in Mice through Regulating the Proliferation and Migration of Endothelial Progenitor Cells (EPCs) via wnt/β-catenin/GSK3β Signaling Pathway. J. Nutr. Health Aging 2022, 26, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, Y.; Jiang, M.; Wang, F.; Gong, G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4132. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, G.; Pang, Q.; Yu, C.; Xiong, J.; Zhu, J.; Chen, F. SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J. 2017, 284, 1324–1337. [Google Scholar] [CrossRef]
- Wang, T.; Sun, C.; Hu, L.; Gao, E.; Li, C.; Wang, H.; Sun, D. Sirt6 stabilizes atherosclerosis plaques by promoting macrophage autophagy and reducing contact with endothelial cells. Biochem. Cell Biol. 2020, 98, 120–129. [Google Scholar] [CrossRef]
- Zi, Y.; Yi-An, Y.; Bing, J.; Yan, L.; Jing, T.; Chun-Yu, G.; Fan, P.; Hao, L.; Jia-Ni, T.; Han-Jin, H.; et al. Sirt6-induced autophagy restricted TREM-1-mediated pyroptosis in ox-LDL-treated endothelial cells: Relevance to prognostication of patients with acute myocardial infarction. Cell Death Discov. 2019, 5, 88. [Google Scholar] [CrossRef]
- Su, G.; Yang, W.; Wang, S.; Geng, C.; Guan, X. SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem. Biophys. Res. Commun. 2021, 561, 33–39. [Google Scholar] [CrossRef]
- Garlick, P.B.; Davies, M.J.; Hearse, D.J.; Slater, T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987, 61, 757–760. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhang, D.; Yu, P.; Zhang, J.; Yu, S. Research Progress on the Role of Pyroptosis in Myocardial Ischemia-Reperfusion Injury. Cells 2022, 11, 3271. [Google Scholar] [CrossRef]
- Koltai, M.; Tosaki, A.; Hosford, D.; Braquet, P. Ginkgolide B protects isolated hearts against arrhythmias induced by ischemia but not reperfusion. Eur. J. Pharmacol. 1989, 164, 293–302. [Google Scholar] [CrossRef]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef] [PubMed]
- Sinning, C.; Westermann, D.; Clemmensen, P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark. Med. 2017, 11, 11031–11040. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.R.; Pedrozo, Z.; Lavandero, S.; Hill, J.A. Oxidative stress and autophagy in cardiovascular homeostasis. Antioxid. Redox Signal. 2014, 20, 507–518. [Google Scholar] [CrossRef]
- Chen-Scarabelli, C.; Agrawal, P.R.; Saravolatz, L.; Abuniat, C.; Scarabelli, G.; Stephanou, A.; Loomba, L.; Narula, J.; Scarabelli, T.M.; Knight, R. The role and modulation of autophagy in experimental models of myocardial ischemia-reperfusion injury. J. Geriatr. Cardiol. 2014, 11, 338–348. [Google Scholar] [PubMed]
- Lazou, A.; Iliodromitis, E.K.; Cieslak, D.; Voskarides, K.; Mousikos, S.; Bofilis, E.; Kremastinos, D.T. Ischemic but not mechanical preconditioning attenuates ischemia/reperfusion induced myocardial apoptosis in anaesthetized rabbits: The role of Bcl-2 family proteins and ERK1/2. Apoptosis 2006, 11, 2195–2204. [Google Scholar] [CrossRef]
- Yan, H.F.; Tuo, Q.Z.; Yin, Q.Z.; Lei, P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool. Res. 2020, 41, 220–230. [Google Scholar] [CrossRef]
- Luo, G.; Jian, Z.; Zhu, Y.; Zhu, Y.; Chen, B.; Ma, R.; Tang, F.; Xiao, Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019, 43, 2033–2043. [Google Scholar] [CrossRef]
- Zhong, Z.; Luo, X.Y.; Xiang, P.; Ji, H.H.; Wu, X.D.; Chong, A.G.; Hu, X.Y.; Cao, X.L. MRTF-A alleviates myocardial ischemia reperfusion injury by inhibiting the inflammatory response and inducing autophagy. Mol. Cell. Biochem. 2023, 478, 343–359. [Google Scholar] [CrossRef]
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef]
- Blasig, I.E.; Ebert, B.; Hennig, C.; Pali, T.; Tosaki, A. Inverse relationship between ESR spin trapping of oxyradicals and degree of functional recovery during myocardial reperfusion in isolated working rat heart. Cardiovasc. Res. 1990, 24, 263–270. [Google Scholar] [CrossRef]
- Wu, D.; Ji, H.; Du, W.; Ren, L.; Qian, G. Mitophagy alleviates ischemia/reperfusion-induced microvascular damage through improving mitochondrial quality control. Bioengineered 2022, 13, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Kong, F.J.; Dong, Z.; Xin, K.Y.; Wang, X.X.; Sun, A.J.; Zou, Y.Z.; Ge, J.B. Hypertrophic Preconditioning Attenuates Myocardial Ischaemia-Reperfusion Injury by Modulating SIRT3-SOD2-mROS-Dependent Autophagy. Cell Prolif. 2021, 54, e13051. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Lv, C.; Qu, Y.; Yang, H.; Hao, C.; Sun, X.; Hu, X.; Yang, Y.; Tang, Y. Remote Ischemic Conditioning Mediates Cardio-protection After Myocardial Ischemia/Reperfusion Injury by Reducing 4-HNE Levels and Regulating Autophagy via the ALDH2/SIRT3/HIF1α Signaling Pathway. J. Cardiovasc. Transl. Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Li, J.; Lu, K.; Zhang, X.; Wang, T.; Li, Q.; Yu, X.; Han, W.; Sun, L. SIRT3-mediated mitochondrial autophagy in refeeding syndrome-related myocardial injury in sepsis rats. Ann. Transl. Med. 2022, 10, 211. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, L.; Lu, W. Thrombin Aggravates Hypoxia/Reoxygenation Injury of Cardiomyocytes by Activating an Autophagy Pathway-Mediated by SIRT1. Med. Sci. Monit. 2021, 27, e928480. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Mikami, A.; Ogino, A.; Watanabe, T.; Morishita, K.; Okada, H.; Kawasaki, M.; et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015, 11, 1146–1160. [Google Scholar] [CrossRef]
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1973–1983. [Google Scholar] [CrossRef]
- Guo, Z.; Tuo, H.; Tang, N.; Liu, F.Y.; Ma, S.Q.; An, P.; Yang, D.; Wang, M.Y.; Fan, D.; Yang, Z.; et al. Neuraminidase 1 deficiency attenuates cardiac dysfunction, oxidative stress, fibrosis, inflammatory via AMPK-SIRT3 pathway in diabetic cardiomyopathy mice. Int. J. Biol. Sci. 2022, 18, 826–840. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Takemura, G.; Kanamori, H.; Takeyama, T.; Watanabe, T.; Morishita, K.; Ogino, A.; Tsujimoto, A.; Goto, K.; Maruyama, R.; et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc. Res. 2012, 96, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Sishi, B.J.; Loos, B.; van Rooyen, J.; Engelbrecht, A.M. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem. Pharmacol. 2013, 85, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, Y.; Zheng, M.; Cao, T.; Wang, G.; Zhang, L.; Ni, R.; Brockman, J.; Zhong, H.; Fan, G.C.; et al. Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity. Clin. Sci. 2019, 133, 1505–1521. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, C.; Xu, S.; Wang, B.; Li, D.; Liu, X.; Mi, Y.; Guo, H.; Jiang, J. SIRT3 attenuates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome via autophagy. Biochem. Pharmacol. 2023, 207, 115354. [Google Scholar] [CrossRef]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation 2016, 133, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.B.; Gao, W.C.; Xie, M.; Li, Z.; Ma, X.; Song, W.; Luo, D.; Huang, Y.; Yang, J.; Zhang, P.; et al. Ang II Promotes Cardiac Autophagy and Hypertrophy via Orai1/STIM1. Front. Pharmacol. 2021, 12, 622774. [Google Scholar] [CrossRef]
- Wang, H.N.; Li, J.L.; Xu, T.; Yao, H.Q.; Chen, G.H.; Hu, J. Effects of Sirt3-autophagy and resveratrol activation on myocardial hypertrophy and energy metabolism. Mol. Med. Rep. 2020, 22, 1342–1350. [Google Scholar] [CrossRef]
- Li, J.; Chen, T.; Xiao, M.; Li, N.; Wang, S.; Su, H.; Guo, X.; Liu, H.; Yan, F.; Yang, Y.; et al. Mouse Sirt3 promotes autophagy in AngII-induced myocardial hypertrophy through the deacetylation of FoxO1. Oncotarget 2016, 7, 86648–86659. [Google Scholar] [CrossRef]
- Gao, J.; Wei, T.; Huang, C.; Sun, M.; Shen, W. Sirtuin 3 governs autophagy-dependent glycolysis during Angiotensin II-induced endothelial-to-mesenchymal transition. FASEB J. 2020, 34, 16645–16661. [Google Scholar] [CrossRef]
- Wei, T.; Huang, G.; Gao, J.; Huang, C.; Sun, M.; Wu, J.; Bu, J.; Shen, W. Sirtuin 3 Deficiency Accelerates Hypertensive Cardiac Remodeling by Impairing Angiogenesis. J. Am. Heart Assoc. 2017, 6, e006114. [Google Scholar] [CrossRef]
- Zhong, X.L.; Miao, H.J.; Fang, Z.M.; Kuken, B.; Song, H.Y.; Zhong, H.; Lu, Y.; Liu, S.M. The effect of SIRT1 gene polymorphisms on ambulatory blood pressure of hypertensive patients in the Kazakh population. Genet. Test. Mol. Biomark. 2015, 19, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004, 556, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef]
- Li, Z.; Margariti, A.; Wu, Y.; Yang, F.; Hu, J.; Zhang, L.; Chen, T. MicroRNA-199a induces differentiation of induced pluripotent stem cells into endothelial cells by targeting sirtuin 1. Mol. Med. Rep. 2015, 12, 3711–3717. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Gurusamy, N.; Das, D.K. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 2011, 1215, 22–33. [Google Scholar] [CrossRef]
- Tanno, M.; Kuno, A.; Horio, Y.; Miura, T. Emerging beneficial roles of sirtuins in heart failure. Basic Res. Cardiol. 2012, 107, 273. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Jin, X.; Liang, X.Y.; Zhou, Y.; Zhang, T.; Xie, Q.; Zhou, X.; Chang, H.; Fu, Y.J.; et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 2013, 9, 2033–2045. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Zhu, W.; Liu, Z.; Liu, H.; Zhou, Y.; Cao, Y.; Liu, C.; Xie, Y. Resveratrol Enhances Autophagic Flux and Promotes Ox-LDL Degradation in HUVECs via Upregulation of SIRT1. Oxid. Med. Cell. Longev. 2016, 2016, 7589813. [Google Scholar] [CrossRef]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Ogino, A.; Takeyama, T.; Kawaguchi, T.; Watanabe, T.; Morishita, K.; Kawasaki, M.; et al. Resveratrol reverses remodeling in hearts with large, old myocardial infarctions through enhanced autophagy-activating AMP kinase pathway. Am. J. Pathol. 2013, 182, 701–713. [Google Scholar] [CrossRef]
- Zheng, M.; Bai, Y.; Sun, X.; Fu, R.; Liu, L.; Liu, M.; Li, Z.; Huang, X. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α Pathway. Molecules 2022, 27, 5545. [Google Scholar] [CrossRef]
- Carrizzo, A.; Iside, C.; Nebbioso, A.; Carafa, V.; Damato, A.; Sciarretta, S.; Frati, G.; Di Nonno, F.; Valenti, V.; Ciccarelli, M.; et al. SIRT1 pharmacological activation rescues vascular dysfunction and prevents thrombosis in MTHFR deficiency. Cell. Mol. Life Sci. 2022, 79, 410. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Yang, Y.; Guo, Y.; Fan, Y.; Zhang, M.; Man, W.; Gao, E.; Hu, W.; Reiter, R.J.; et al. Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. J. Pineal Res. 2017, 62, e12368. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Barangi, S.; Aghaee-Bakhtiari, S.H.; Hosseinzadeh, H.; Moosavi, Z.; Reiter, R.J.; Hayes, A.W.; Mehri, S.; Karimi, G. Melatonin ameliorates arsenic-induced cardiotoxicity through the regulation of the Sirt1/Nrf2 pathway in rats. BioFactors 2023, 49, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.; Dong, X.; Xue, X.D.; Xu, S.; Zhang, X.; Xu, Y.L.; Wang, Z.S.; Wang, Y.; Gao, H.; Liang, Y.X.; et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J. Pineal Res. 2021, 70, e12698. [Google Scholar] [CrossRef]
- Pi, Q.Z.; Wang, X.W.; Jian, Z.L.; Chen, D.; Zhang, C.; Wu, Q.C. Melatonin Alleviates Cardiac Dysfunction Via Increasing Sirt1-Mediated Beclin-1 Deacetylation and Autophagy During Sepsis. Inflammation 2021, 44, 1184–1193. [Google Scholar] [CrossRef]
- Qiao, H.; Ren, H.; Du, H.; Zhang, M.; Xiong, X.; Lv, R. Liraglutide repairs the infarcted heart: The role of the SIRT1/Parkin/mitophagy pathway. Mol. Med. Rep. 2018, 17, 3722–3734. [Google Scholar] [CrossRef]
- Yang, M.; Xi, N.; Gao, M.; Yu, Y. Sitagliptin mitigates hypoxia/reoxygenation (H/R)-induced injury in cardiomyocytes by mediating sirtuin 3 (SIRT3) and autophagy. Bioengineered 2022, 13, 13162–13173. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Wu, C.; Liang, X.; He, L.; Wang, L.; Wang, X. Activation of the sirtuin silent information regulator 1 pathway inhibits pathological myocardial remodeling. Front. Pharmacol. 2023, 14, 1111320. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Liu, D.; Meng, Q.; Yan, P.; Luo, D.; Wang, X.; Zhou, X. Puerarin Attenuates LPS-Induced Inflammatory Responses and Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells through Mitochondrial Quality Control. Oxid. Med. Cell. Longev. 2021, 2021, 6659240. [Google Scholar] [CrossRef]
- Hui, B.; Hou, X.; Liu, R.; Liu, X.H.; Hu, Z. Gypenoside inhibits ox-LDL uptake and foam cell formation through enhancing Sirt1-FOXO1 mediated autophagy flux restoration. Life Sci. 2021, 264, 118721. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Zhang, X. Salidroside protects against ox-LDL-induced endothelial injury by enhancing autophagy mediated by SIRT1-FoxO1 pathway. BMC Complement. Altern. Med. 2019, 19, 111. [Google Scholar] [CrossRef]
- Jin, X.; Chen, M.; Yi, L.; Chang, H.; Zhang, T.; Wang, L.; Ma, W.; Peng, X.; Zhou, Y.; Mi, M. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol. Nutr. Food Res. 2014, 58, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, J.; Zhao, H.; Tang, J.; Shi, G. Paeoniflorin attenuates oxidized low-density lipoprotein-induced apoptosis and adhesion molecule expression by autophagy enhancement in human umbilical vein endothelial cells. J. Cell. Biochem. 2019, 120, 9291–9299. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, D.; Zhou, B.; Liu, Y.; Hao, B.; Yu, S.; Wu, L.; Wang, M.; Song, Z.; Wu, C.; et al. Ginsenoside Rb1 Alleviates Oxidative Low-Density Lipoprotein-Induced Vascular Endothelium Senescence via the SIRT1/Beclin-1/Autophagy Axis. J. Cardiovasc. Pharmacol. 2020, 75, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.R.; Martins, T.R.; Couto, R.; Deus, C.; Pereira, C.V.; Simões, R.F.; Rizvanov, A.A.; Silva, F.; Cunha-Oliveira, T.; Oliveira, P.J.; et al. Berberine-induced cardioprotection and Sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 2904–2923. [Google Scholar] [CrossRef]
- Mao, S.; Chen, P.; Li, T.; Guo, L.; Zhang, M. Tongguan Capsule Mitigates Post-myocardial Infarction Remodeling by Promoting Autophagy and Inhibiting Apoptosis: Role of Sirt1. Front. Physiol. 2018, 9, 589. [Google Scholar] [CrossRef]
- Sun, X.; Han, Y.; Dong, C.; Qu, H.; Yu, Y.; Ju, J.; Bai, Y.; Yang, B. Daming capsule protects against myocardial infarction by promoting mitophagy via the SIRT1/AMPK signaling pathway. Biomed. Pharmacother. 2022, 151, 113162. [Google Scholar] [CrossRef]
- Xue, Y.; Fu, W.; Liu, Y.; Yu, P.; Sun, M.; Li, X.; Yu, X.; Sui, D. Ginsenoside Rb2 alleviates myocardial ischemia/reperfusion injury in rats through SIRT1 activation. J. Food Sci. 2020, 85, 4039–4049. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.X.; Wu, W.Y.; Song, F.; Wu, C.; Li, G.R.; Wang, Y. Cardiac senescence is alleviated by the natural flavone acacetin via enhancing mitophagy. Aging 2021, 13, 16381–16403. [Google Scholar] [CrossRef]
- Guan, S.; Xin, Y.; Ding, Y.; Zhang, Q.; Han, W. Ginsenoside Rg1 Protects against Cardiac Remodeling in Heart Failure via SIRT1/PINK1/Parkin-Mediated Mitophagy. Chem. Biodivers. 2023, 20, e202200730. [Google Scholar] [CrossRef]
- Jiang, Q.; Lu, M.; Li, J.; Zhu, Z. Ginkgolide B Protects Cardiomyocytes from Angiotensin II-Induced Hypertrophy via Regulation of Autophagy through SIRT1-FoxO1. Cardiovasc. Ther. 2021, 2021, 5554569. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Wang, B.; Chen, Y.; Xiao, A.; Zeng, D.; Ou, D.; Yan, S.; Li, W.; Zheng, Q. ZLN005 protects cardiomyocytes against high glucose-induced cytotoxicity by promoting SIRT1 expression and autophagy. Exp. Cell Res. 2016, 345, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, J.; Zhao, F.; Yang, L.; Xu, X.; Shi, Y.; Wei, B. Cardioprotective effect of MLN4924 on ameliorating autophagic flux impairment in myocardial ischemia-reperfusion injury by Sirt1. Redox Biol. 2021, 46, 102114. [Google Scholar] [CrossRef]
- Zhu, L.; Duan, W.; Wu, G.; Zhang, D.; Wang, L.; Chen, D.; Chen, Z.; Yang, B. Protective effect of hydrogen sulfide on endothelial cells through Sirt1-FoxO1-mediated autophagy. Ann. Transl. Med. 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, J.; Li, X.; Liu, H.; Zhao, J.; Liu, M. Hydrogen attenuated oxidized low-density lipoprotein-induced inflammation through the stimulation of autophagy via sirtuin 1. Exp. Ther. Med. 2018, 16, 4042–4048. [Google Scholar] [CrossRef]
- Fan, L.; Chen, D.; Wang, J.; Wu, Y.; Li, D.; Yu, X. Sevoflurane Ameliorates Myocardial Cell Injury by Inducing Autophagy via the Deacetylation of LC3 by SIRT1. Anal. Cell. Pathol. 2017, 2017, 6281285. [Google Scholar] [CrossRef]
- Cong, L.; Gao, Z.; Zheng, Y.; Ye, T.; Wang, Z.; Wang, P.; Li, M.; Dong, B.; Yang, W.; Li, Q.; et al. Electrical stimulation inhibits Val-boroPro-induced pyroptosis in THP-1 macrophages via sirtuin3 activation to promote autophagy and inhibit ROS generation. Aging 2020, 12, 6415–6435. [Google Scholar] [CrossRef]
- Wang, P.; Li, M.; Gao, T.; Fan, J.; Zhang, D.; Zhao, Y.; Zhao, Y.; Wang, Y.; Guo, T.; Gao, X.; et al. Vascular Electrical Stimulation with Wireless, Battery-Free, and Fully Implantable Features Reduces Atherosclerotic Plaque Formation Through Sirt1-Mediated Autophagy. Small 2023, e2300584. [Google Scholar] [CrossRef]
- Jiang, Q.; Hao, R.; Wang, W.; Gao, H.; Wang, C. SIRT1/Atg5/autophagy are involved in the antiatherosclerosis effects of ursolic acid. Mol. Cell. Biochem. 2016, 420, 171–184. [Google Scholar] [CrossRef]
- Wilson, N.; Kataura, T.; Korsgen, M.E.; Sun, C.; Sarkar, S.; Korolchuk, V.I. The autophagy-NAD axis in longevity and disease. Trends Cell Biol. 2023, 33, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Fulmor, I.E.; Parham, C.S.; Antonaccio, M.J. Effects of SQ 26,533 on reperfusion arrhythmias, ST-segment elevation and on infarct size in anesthetized dogs. J. Pharmacol. Exp. Ther. 1986, 237, 1–8. [Google Scholar]
- Martorana, P.A.; Linz, W.; Göbel, H.; Petry, P.; Schölkens, B.A. Effects of nicainoprol on reperfusion arrhythmia in the isolated working rat heart and on ischemia and reperfusion arrhythmia and myocardial infarct size in the anesthetized rat. Eur. J. Pharmacol. 1987, 143, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Hide, E.J.; Piper, J.; Thiemermann, C. Endothelin-1-induced reduction of myocardial infarct size by activation of ATP-sensitive potassium channels in a rabbit model of myocardial ischaemia and reperfusion. Br. J. Pharmacol. 1995, 116, 2597–2602. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef]
- Qin, X.; Liu, B.; Gao, F.; Hu, Y.; Chen, Z.; Xu, J.; Zhang, X. Gluconolactone Alleviates Myocardial Ischemia/Reperfusion Injury and Arrhythmias via Activating PKCε/Extracellular Signal-Regulated Kinase Signaling. Front. Physiol. 2022, 13, 856699. [Google Scholar] [CrossRef]
- Yaoita, H.; Ogawa, K.; Maehara, K.; Maruyama, Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 1998, 97, 276–281. [Google Scholar] [CrossRef]
- Kovacs, P.; Bak, I.; Szendrei, L.; Vecsernyes, M.; Varga, E.; Blasig, I.E.; Tosaki, A. Non-specific caspase inhibition reduces infarct size and improves post-ischaemic recovery in isolated ischaemic/reperfused rat hearts. Naunyn Schmiedeberg’s Arch. Pharmacol. 2001, 364, 501–507. [Google Scholar] [CrossRef]




| Treatment | Mechanism | Cardiovascular Diseases | References |
|---|---|---|---|
| Resveratrol | SIRT1/autophagy SIRT3/autophagy SIRT1/FOXO1/autophagy cAMP/PRKA/AMPK/SIRT1/autophagy | Heart failure; AS; artery thrombosis; MI/R; DCM; MTHFR deficiency | [32,82,125,126,127,128,129,130,131] |
| Melatonin | SIRT1/autophagy SIRT3/FOXO3a/autophagy SIRT3/NRF2 SIRT6/AMPK-PGC-1α-AKT | Heart failure; AS; DCM; MI/R; myocardial damage; hypertension; | [15,56,83,132,133,134,135] |
| Liraglutide | SIRT1/autophagy | MI | [136] |
| Empagliflozin | Beclin1-TLR9-SIRT3 complexes | Drug-induced cardiac injury | [63] |
| Sitagliptin | SIRT3/autophagy | H/R | [137] |
| Fenofibrate | FGF21/SIRT1/autophagy | DCM | [138] |
| Quercetin | SIRT1/TMBIM6 | H/R | [36] |
| Puerarin | SIRT1/autophagy | AS | [139] |
| Gypenoside | SIRT1/FOXO1/autophagy | AS | [140] |
| Delphinidin-3-glucoside | AMPK/SIRT1/autophagy | AS | [141] |
| Berberine | NAD+/SIRT1/TFEB/autophagy PI3K/AKT/mTOR/autophagy SIRT3/autophagy | AS; drug-induced cardiac injury | [34,142] |
| Araloside C | SIRT1/autophagy | AS | [78] |
| Salidroside | SIRT1/FOXO1/autophagy | AS | [143] |
| Paeoniflorin | SIRT1/autophagy | AS | [144] |
| Ginsenoside Rb1 | SIRT1/Beclin1/autophagy | AS | [145] |
| Ginsenoside Rb2 | SIRT1/autophagy | MI/R | [146] |
| Ginsenoside Rg1 | SIRT1/PINK1/Parkin/mitophagy | Heart failure | [147] |
| Spinacetin | SIRT3/AMPK/mTOR autophagy | Drug-induced cardiac injury | [53] |
| Tongguan Capsule | SIRT1/mTOR/P70S6K/4EBP1/autophagy | MI | [148] |
| Daming Capsule | SIRT1/AMPK/NLRX1/autophagy | MI | [149] |
| Acacetin | SIRT1-SIRT6/AMPK/autophagy | Cardiac senescence | [150] |
| Ginkgolide B | SIRT1/FOXO1/autophagy | Heart failure | [151] |
| ZLN005 | SIRT3/autophagy | DCM | [152] |
| MLN4924 | SIRT1/autophagy | MI/R | [153] |
| Sevoflurane | SIRT1/autophagy | MI/R | [154] |
| H2S and H2 | SIRT1/autophagy | AS | [155,156] |
| Electrical stimulation | SIRT1/Atg5 SIRT3/autophagy | AS | [157,158] |
| α-Linolenic acid | SIRT3/autophagy | Hypertension | [16] |
| Ursolic acid | SIRT1/autophagy | AS | [159] |
| Nicotinamide | NAD+/SIRT1/autophagy | Drug-induced Cardiac Injury | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Y.; Ding, H.; Li, D.; Shen, W.; Zhang, X. The Current State of Research on Sirtuin-Mediated Autophagy in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 382. https://doi.org/10.3390/jcdd10090382
Wang Y, Li Y, Ding H, Li D, Shen W, Zhang X. The Current State of Research on Sirtuin-Mediated Autophagy in Cardiovascular Diseases. Journal of Cardiovascular Development and Disease. 2023; 10(9):382. https://doi.org/10.3390/jcdd10090382
Chicago/Turabian StyleWang, Yuqin, Yongnan Li, Hong Ding, Dan Li, Wanxi Shen, and Xiaowei Zhang. 2023. "The Current State of Research on Sirtuin-Mediated Autophagy in Cardiovascular Diseases" Journal of Cardiovascular Development and Disease 10, no. 9: 382. https://doi.org/10.3390/jcdd10090382