In-Hospital Mortality Outcomes of ST-Segment Elevation Myocardial Infarction: A Cross-Sectional Study from a Tertiary Academic Hospital in Johannesburg, South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Study Setting and Participants
2.2. Data Collection
2.3. Statistical Analysis
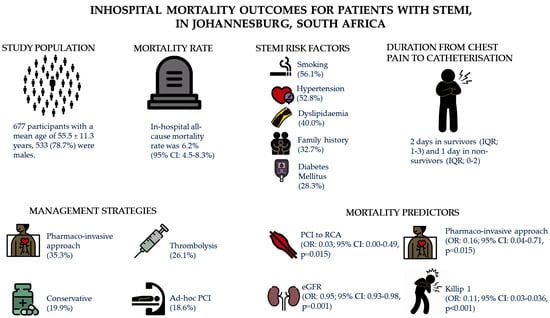
3. Results
3.1. Demographic and Clinical Characteristics of Patients with ST-Segment Elevation Myocardial Infarction
3.2. Management Strategies
3.3. Coronary Angiography Findings, Complications and Predictors of In-Hospital Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belle, L.; Cayla, G.; Cottin, Y.; Coste, P.; Khalife, K.; Labèque, J.-N.; Farah, B.; Perret, T.; Goldstein, P.; Gueugniaud, P.-Y.; et al. French Registry on Acute ST-elevation and non−ST-elevation Myocardial Infarction 2015 (FAST-MI 2015): Design and baseline data. Arch. Cardiovasc. Dis. 2017, 110, 366–378. [Google Scholar] [CrossRef]
- Yao, H.; Ekou, A.; Niamkey, T.; Gan, S.H.; Kouamé, I.; Afassinou, Y.; Ehouman, E.; Touré, C.; Zeller, M.; Cottin, Y.; et al. Acute Coronary Syndromes in Sub-Saharan Africa: A 10-Year Systematic Review. J. Am. Heart Assoc. 2022, 11, e021107. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A. Descriptive Epidemiology of Cardiovascular Risk Factors and Diabetes in Sub-Saharan Africa. Prog. Cardiovasc. Dis. 2013, 56, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Schamroth, C. Management of acute coronary syndrome in South Africa: Insights from the ACCESS (Acute Coronary Events—A Multinational Survey of Current Management Strategies) registry: Cardiovascular topics. Cardiovasc. J. Afr. 2012, 23, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Katus, H.A.; Lindahl, B.; Morrow, D.A.; Clemmensen, P.M.; et al. Third Universal Definition of Myocardial Infarction. Glob. Heart 2012, 7, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Strobe Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Blankenship, J.C.; Gigliotti, O.S.; Feldman, D.N.; Mixon, T.A.; Patel, R.A.; Sorajja, P.; Yakubov, S.J.; Chambers, C.E. Ad Hoc percutaneous coronary intervention: A consensus statement from the society for cardiovascular angiography and interventions. Catheter. Cardiovasc. Interv. 2013, 81, 748–758. [Google Scholar] [CrossRef]
- Hertz, J.T.; Reardon, J.M.; Rodrigues, C.G.; de Andrade, L.; Limkakeng, A.T.; Bloomfield, G.S.; Lynch, C.A. Acute Myocardial Infarction in Sub-Saharan Africa: The Need for Data. PLoS ONE 2014, 9, e96688. [Google Scholar] [CrossRef]
- Seedat, Y.K.; Mayet, F.G.; Latiff, G.H.; Joubert, G. Risk factors and coronary heart disease in Durban blacks—The missing links. South Afr. Med. J. 1992, 82, 251–256. [Google Scholar]
- Moran, A.; Forouzanfar, M.; Sampson, U.; Chugh, S.; Feigin, V.; Mensah, G. The Epidemiology of Cardiovascular Diseases in Sub-Saharan Africa: The Global Burden of Diseases, Injuries and Risk Factors 2010 Study. Prog. Cardiovasc. Dis. 2013, 56, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah, G.; Roth, G.; Sampson, U.; Moran, A.; Feigin, V.; Forouzanfar, M.; Naghavi, M.; Murray, C. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: A systematic analysis of data from the Global Burden of Disease Study 2013: Cardiovascular topic. Cardiovasc. J. Afr. 2015, 26, S6–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkoke, C.; Luchuo, E.B. Coronary heart disease in sub-Saharan Africa: Still rare, misdiagnosed or underdiagnosed? Cardiovasc. Diagn. Ther. 2016, 6, 64–66. [Google Scholar] [CrossRef]
- Kakou-Guikahue, M.; N’guetta, R.; Anzouan-Kacou, J.-B.; Kramoh, E.; N’dori, R.; Ba, S.A.; Diao, M.; Sarr, M.; Kane, A.; Kane, A.; et al. Optimizing the management of acute coronary syndromes in sub-Saharan Africa: A statement from the AFRICARDIO 2015 Consensus Team. Arch. Cardiovasc. Dis. 2016, 109, 376–383. [Google Scholar] [CrossRef]
- Atun, R.; I Davies, J.; Gale, E.A.M.; Bärnighausen, T.; Beran, D.; Kengne, A.P.; Levitt, N.S.; Mangugu, F.W.; Nyirenda, M.J.; Ogle, G.D.; et al. Diabetes in sub-Saharan Africa: From clinical care to health policy. Lancet Diabetes Endocrinol. 2017, 5, 622–667. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, M.E.; Wit, F.W.N.M.; Roos, M.T.L.; Brewster, L.M.; Akande, T.M.; de Beer, I.H.; Mfinanga, S.G.; Kahwa, A.M.; Gatongi, P.; Van Rooy, G.; et al. Hypertension in Sub-Saharan Africa: Cross-Sectional Surveys in Four Rural and Urban Communities. PLoS ONE 2012, 7, e32638. [Google Scholar] [CrossRef] [Green Version]
- Noubiap, J.J.; Bigna, J.J.; Nansseu, J.R.; Nyaga, U.F.; Balti, E.V.; Echouffo-Tcheugui, J.B.; Kengne, A.P. Prevalence of dyslipidaemia among adults in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e998–e1007. [Google Scholar] [CrossRef] [Green Version]
- Guthold, R.; Louazani, S.A.; Riley, L.M.; Cowan, M.J.; Bovet, P.; Damasceno, A.; Sambo, B.H.; Tesfaye, F.; Armstrong, T.P. Physical Activity in 22 African Countries: Results from the World Health Organization STEPwise Approach to Chronic Disease Risk Factor Surveillance. Am. J. Prev. Med. 2011, 41, 52–60. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Ebireri, J.; Aderemi, A.V.; Omoregbe, N.; Adeloye, D. Interventions addressing risk factors of ischaemic heart disease in sub-Saharan Africa: A systematic review. BMJ Open 2016, 6, e011881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wekesah, F.M.; Kyobutungi, C.; E Grobbee, D.; Klipstein-Grobusch, K. Understanding of and perceptions towards cardiovascular diseases and their risk factors: A qualitative study among residents of urban informal settings in Nairobi. BMJ Open 2019, 9, e026852. [Google Scholar] [CrossRef] [Green Version]
- Mohanan, P.P.; Mathew, R.; Harikrishnan, S.; Krishnan, M.N.; Zachariah, G.; Joseph, J.; Eapen, K.; Abraham, M.; Menon, J.; Thomas, M.; et al. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: Results from the Kerala ACS Registry. Eur. Heart J. 2013, 34, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, X.; Wang, Q.; Hu, S.; Wang, Y.; A Masoudi, F.; A Spertus, J.; Krumholz, H.M.; Jiang, L. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): A retrospective analysis of hospital data. Lancet 2015, 385, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, K.; Tanaka, A.; Yoshioka, N.; Morita, Y.; Yoshida, R.; Kanzaki, Y.; Watanabe, N.; Yamauchi, R.; Komeyama, S.; Sugiyama, H.; et al. In-hospital mortality among consecutive patients with ST-Elevation myocardial infarction in modern primary percutaneous intervention era ~ Insights from 15-year data of single-center hospital-based registry. PLoS ONE 2021, 16, e0252503. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Lange, S.A.; Wittlinger, T.; Lehnert, G.; Rigopoulos, A.G.; Noutsias, M. In-hospital mortality after acute STEMI in patients undergoing primary PCI. Herz 2017, 43, 741–745. [Google Scholar] [CrossRef] [PubMed]
- N’guetta, R.; Ekou, A.; Yao, H.; Anzouan-Kacou, J.; Gérardin, B.; Pillière, R.; Adoh, A.; Seka, R. Percutaneous coronary intervention in the management of acute coronary syndromes in Ivory Coast: Challenges and outcomes. Ann. Cardiol. Angeiol. 2018, 67, 244–249. [Google Scholar] [CrossRef]
- Bogale, K.; Mekonnen, D.; Nedi, T.; Woldu, M.A. Treatment Outcomes of Patients with Acute Coronary Syndrome Admitted to Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Clin. Med. Insights Cardiol. 2019, 13, 1179546819839417. [Google Scholar] [CrossRef]
- Stassen, W.; Wallis, L.; Lambert, C.; Castren, M.; Kurland, L. Percutaneous coronary intervention still not accessible for many South Africans. Afr. J. Emerg. Med. 2017, 7, 105–107. [Google Scholar] [CrossRef]
- Varwani, M.H.; Jeilan, M.; Ngunga, M.; Barasa, A. Outcomes in patients with acute coronary syndrome in a referral hospital in sub-Saharan Africa. Cardiovasc. J. Afr. 2019, 30, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Nyaaba, G.N.; Stronks, K.; Aikins, A.D.-G.; Kengne, A.P.; Agyemang, C. Tracing Africa’s progress towards implementing the Non-Communicable Diseases Global action plan 2013–2020: A synthesis of WHO country profile reports. BMC Public Health 2017, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.S.; Islam, S.; Rosengren, A.; Franzosi, M.G.; Steyn, K.; Yusufali, A.H.; Keltai, M.; Diaz, R.; Rangarajan, S.; Yusuf, S. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur. Heart J. 2008, 29, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Larsson, B.; Bengtsson, C.; Björntorp, P.; Lapidus, L.; Sjöstrom, L.; Svardsudd, K.; Tlbblin, G.; Wedel, H.; Welin, L.; Wilhelmsen, L. Is Abdominal Body Fat Distribution a Major Explanation for the Sex Difference in the Incidence of Myocardial Infarction? The study of men born in 1913 and the study of women, Goteborg, Sweden. Am. J. Epidemiol. 1992, 135, 266–273. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Statistics South Africa. Mid-Year Population Estimates. 2022. Available online: www.statssa.gov.za (accessed on 8 August 2023).
- Koga, S.; Honda, S.; Maemura, K.; Nishihira, K.; Kojima, S.; Takegami, M.; Asaumi, Y.; Yamashita, J.; Saji, M.; Kosuge, M.; et al. Effect of Infarction-Related Artery Location on Clinical Outcome of Patients with Acute Myocardial Infarction in the Contemporary Era of Percutaneous Coronary Intervention―Subanalysis from the Prospective Japan Acute Myocardial Infarction Registry (JAMIR). Circ. J. 2022, 86, 651–659. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of Hospital Mortality in the Global Registry of Acute Coronary Events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.T.; Pereira, M.; Araujo, C.; Malmivaara, A.; Ferrieres, J.; Degano, I.R.; Kirchberger, I.; Farmakis, D.; Garel, P.; Torre, M.; et al. Heart rate at admission is a predictor of in-hospital mortality in patients with acute coronary syndromes: Results from 58 European hospitals: The European Hospital Benchmarking by Outcomes in acute coronary syndrome Processes study. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 149–157. [Google Scholar] [CrossRef]
- McNamara, R.L.; Kennedy, K.F.; Cohen, D.J.; Diercks, D.B.; Moscucci, M.; Ramee, S.; Wang, T.Y.; Connolly, T.; Spertus, J.A. Predicting In-Hospital Mortality in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 626–635. [Google Scholar] [CrossRef]
- A Hashmi, K.; Adnan, F.; Ahmed, O.; Yaqeen, S.R.; Ali, J.; Irfan, M.; Edhi, M.M.; A Hashmi, A. Risk Assessment of Patients After ST-Segment Elevation Myocardial Infarction by Killip Classification: An Institutional Experience. Cureus 2020, 12. [Google Scholar] [CrossRef]
- NACB Writing Group Members; Morrow, D.A.; Cannon, C.P.; Jesse, R.L.; Newby, L.K.; Ravkilde, J.; Storrow, A.B.; Wu, A.H.; Christenson, R.H. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical Characteristics and Utilization of Biochemical Markers in Acute Coronary Syndromes. Circulation 2007, 115, e356–e375. [Google Scholar] [CrossRef]
- Khullar, N.; Buckley, A.J.; O’connor, C.; Ibrahim, A.; Ibrahim, A.; Ahern, C.; Cahill, C.; Arnous, S.; Kiernan, T.J. Peak troponin T in STEMI: A predictor of all-cause mortality and left ventricular function. Open Heart 2022, 9, e001863. [Google Scholar] [CrossRef]
- Wanamaker, B.L.; Seth, M.M.; Sukul, D.; Dixon, S.R.; Bhatt, D.L.; Madder, R.D.; Rumsfeld, J.S.; Gurm, H.S. Relationship Between Troponin on Presentation and In-Hospital Mortality in Patients With ST-Segment–Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e013551. [Google Scholar] [CrossRef]
- Shouval, R.; Hadanny, A.; Shlomo, N.; Iakobishvili, Z.; Unger, R.; Zahger, D.; Alcalai, R.; Atar, S.; Gottlieb, S.; Matetzky, S.; et al. Machine learning for prediction of 30-day mortality after ST elevation myocardial infraction: An Acute Coronary Syndrome Israeli Survey data mining study. Int. J. Cardiol. 2017, 246, 7–13. [Google Scholar] [CrossRef]
- Danchin, N.; Puymirat, E.; Steg, P.G.; Goldstein, P.; Schiele, F.; Belle, L.; Cottin, Y.; Fajadet, J.; Khalife, K.; Coste, P.; et al. Five-Year Survival in Patients With ST-Segment–Elevation Myocardial Infarction According to Modalities of Reperfusion Therapy: The French Registry on Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) 2005 Cohort. Circulation 2014, 129, 1629–1636. [Google Scholar] [CrossRef]
- Meel, R.; Gonçalves, R. Time to fibrinolytics for acute myocardial infarction: Reasons for delays at Steve Biko Academic Hospital, Pretoria, South Africa. S. Afr. Med. J. 2015, 106, 92. [Google Scholar] [CrossRef] [Green Version]



| Variables | All | All-Cause Mortality | ||
|---|---|---|---|---|
| Patients | No | Yes | ||
| n = 677 | (n = 635) (93.8%) | (n = 42) (6.2%) | p-Value | |
| Age, years | 55.54 ± 11.39 | 55.21 ± 11.11 | 60.54 ± 14.11 | <0.001 |
| Male | 533 (78.73) | 503 (79.21) | 30 (71.43) | 0.233 |
| Co-morbidities | ||||
| Hypertension | 358 (52.88) | 335 (52.76) | 23 (54.76) | 0.801 |
| Diabetes Mellitus | 192 (28.36) | 179 (28.19) | 13 (30.95) | 0.700 |
| Dyslipidaemia | 271 (40.03) | 255 (40.16) | 16 (38.10) | 0.792 |
| Obesity | 153 (22.60) | 144 (22.68) | 9 (21.43) | 0.851 |
| Chronic kidney disease | 17 (2.51) | 17 (2.68) | 0 (0.00) | 0.283 |
| Previous CAD | 21 (3.10) | 20 (3.15) | 1 (2.38) | 0.781 |
| Smoking | ||||
| Current smoker | 380 (56.13) | 362 (57.01) | 18 (42.86) | 0.073 |
| Ex-smoker | 55 (8.12) | 49 (7.72) | 6 (14.29) | 0.131 |
| Vital signs | ||||
| Systolic BP (mmHg) | 124.41 ± 26.15 | 125.77 ± 25.09 | 103.46 ± 32.94 | <0.001 |
| Diastolic BP (mmHg) | 78.65 ± 18.49 | 79.36 ± 18.12 | 67.76 ± 20.92 | <0.001 |
| Heart rate | 84.38 ± 22.26 | 83.42 ± 20.37 | 98.83 ± 39.06 | <0.001 |
| NYHA class | ||||
| 1 | 601 (88.77) | 583 (91.81) | 18 (42.86) | <0.001 |
| 2 | 37 (5.47) | 30 (4.72) | 7 (16.67) | 0.001 |
| 3 | 17 (2.51) | 14 (2.20) | 3 (7.14) | 0.048 |
| 4 | 22 (3.25) | 8 (1.26) | 14 (33.33) | <0.001 |
| Killip class | ||||
| 1 | 576 (85.08) | 560 (88.19) | 16 (38.10) | <0.001 |
| 2 | 30 (4.43) | 26 (4.09) | 4 (9.52) | 0.098 |
| 3 | 24 (3.55) | 17 (2.68) | 7 (16.67) | <0.001 |
| 4 | 47 (6.94) | 32 (5.04) | 15 (35.71) | <0.001 |
| Biochemical Parameters | ||||
| Troponin T (ng/L) | 3556 (1495–7189) | 3444 (1389–6893) | 6774 (2732–10,000) | 0.004 |
| CK MB mass (µg/L) | 82.67 (20.17–239.1) | 72.35 (19.38–203.6) | 268 (207.2–268) | 0.013 |
| Haemoglobin (g/dL) | 14.34 ± 2.20 | 14.34 ± 2.18 | 14.32 ± 2.61 | 0.972 |
| Sodium (mmol/L) | 138 ± 9.12 | 139.21 ± 7.87 | 135.29 ± 19.80 | 0.007 |
| Potassium (mmol/L) | 4.2 (3.9–4.6) | 4.2 (3.9–4.6) | 4.45 (4.0–5.2) | 0.021 |
| Urea (mmol/L) | 5.6 (4.4–7.4) | 5.5 (4.4–7.1) | 7.9 (6.0–11.7) | <0.001 |
| Creatinine (µmol/L) | 87 (73–107) | 86 (72.5–104) | 115 (82–149) | <0.001 |
| eGFR (mL/min/1.73 m2) | 78.35 ± 27.21 | 79.78 ± 26.52 | 56.64 ± 28.62 | <0.001 |
| Electrocardiogram findings | ||||
| Sinus rhythm | 610 (90.10) | 581 (91.50) | 29 (69.05) | <0.001 |
| Atrial fibrillation | 12 (1.77) | 10 (1.57) | 2 (4.76) | 0.130 |
| Atrial flutter | 3 (0.44) | 3 (0.47) | 0 (0.00) | 0.655 |
| Ventricular tachycardia | 7 (1.03) | 6 (0.94) | 1 (2.38) | 0.373 |
| Ventricular fibrillation | 5 (0.74) | 3 (0.47) | 2 (4.76) | 0.002 |
| Complete heart block | 22 (3.25) | 16 (2.52) | 6 (14.29) | <0.001 |
| Left bundle branch block | 19 (2.81) | 18 (2.83) | 1 (2.38) | 0.186 |
| Right bundle branch block | 24 (3.55) | 20 (3.15) | 4 (9.52) | 0.186 |
| Management Strategy | All-Cause Mortality | p-Value | |
|---|---|---|---|
| No (n = 635) (93.8%) | Yes (n = 42) (6.2%) | ||
| Pharmaco-invasive | 231(36.4) | 8 (19.0) | 0.023 |
| Thrombolytic therapy only | 166 (26.1) | 11 (26.2) | 0.994 |
| Conservative management | 122 (19.2) | 13 (30.9) | 0.065 |
| Percutaneous coronary intervention only * | 116 (18.3) | 10 (23.8) | 0.371 |
| Variables | All | All-Cause Mortality | ||
|---|---|---|---|---|
| Patients | No | Yes | ||
| n = 677 | (n = 635) (93.8%) | (n = 42) (6.2%) | p-Value | |
| Vessels involved | ||||
| Right coronary artery | 351 (51.85) | 330 (51.97) | 21 (50.00) | 0.850 |
| Left main coronary artery | 14 (2.07) | 12 (1.89) | 2 (4.76) | 0.205 |
| Left circumflex artery | 156 (23.04) | 142 (22.36) | 14 (33.33) | 0.102 |
| Left anterior descending artery | 403 (59.53) | 370 (58.27) | 33 (78.57) | 0.009 |
| Obtuse marginal/Ramus | 29 (4.28) | 27 (4.25) | 2 (4.76) | 0.874 |
| First diagonal branch | 13 (1.92) | 12 (1.89) | 1 (2.38) | 0.822 |
| Distribution of disease on angiography | ||||
| Single vessel disease | 390 (57.61) | 369 (58.11) | 21 (50.00) | 0.303 |
| Double vessel disease | 143 (21.12) | 137 (21.57) | 6 (14.29) | 0.262 |
| Triple vessel disease | 91 (13.44) | 78 (12.28) | 13 (30.95) | 0.001 |
| No lesion * | 20 (2.95) | 20 (3.15) | 0 (0.00) | 0.243 |
| Percutaneous coronary intervention | ||||
| Percutaneous coronary intervention | 365 (53.91) | 347 (54.65) | 18 (42.86) | 0.125 |
| Left main coronary artery | 1 (0.15) | 1 (0.16) | 0 (0.00) | 0.797 |
| Left anterior descending artery | 187 (27.62) | 172 (27.09) | 15 (35.71) | 0.226 |
| Left circumflex artery | 40 (5.91) | 36 (5.67) | 4 (9.52) | 0.305 |
| Right coronary artery | 149 (22.01) | 146 (22.99) | 3 (7.14) | 0.016 |
| Obtuse marginal artery | 1 (0.15) | 1 (0.16) | 0 (0.00) | 0.797 |
| Obtuse marginal/Ramus | 8 (1.18) | 8 (1.26) | 0 (0.00) | 0.464 |
| Variable | Univariable Logistic Regression | Multivariable Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | p-Value | Unadjusted OR (95% CI) | p-Value | Adjusted OR * (95% CI) | p-Value | |
| Age | 1.04 (1.01–1.07) | 0.004 | 1.04 (1.00–1.08) | 0.082 | ||
| Duration between the onset of chest pain and catheterisation (days) | 0.88 (0.75–1.03) | 0.112 | ||||
| Systolic BP (mmHg) | 0.96 (0.95–0.98) | <0.001 | 0.97 (0.94–1.01) | 0.117 | 0.97 (0.93–1.00) | 0.075 |
| Diastolic BP (mmHg) | 0.96 (0.94–0.98) | <0.001 | 1.02 (0.96–1.07) | 0.527 | 1.03 (0.97–1.08) | 0.344 |
| Troponin (ng/L) | 1.00 (1.00–1.00) | 0.007 | 1.00 (1.00–1.00) | 0.838 | 1.00 (1.00–100) | 0.756 |
| Sodium (mmol/L) | 0.98 (0.96–1.00) | 0.030 | 0.99 (0.95–1.02) | 0.414 | 0.99 (0.95–1.02) | 0.463 |
| Potassium (mmol/L) | 1.03 (1.00–1.06) | 0.038 | 1.03 (0.98–1.08) | 0.211 | 1.03 (0.98–1.09) | 0.268 |
| Urea (mmol/L) | 1.04 (1.00–1.07) | 0.014 | 1.03 (0.98–1.08) | 0.196 | 1.03 (0.98–1.08) | 0.228 |
| eGFR (mL/min/1.73 m2) | 0.96 (0.95–0.98) | <0.001 | 0.96 (0.93–0.98) | <0.001 | 0.96 (0.93–0.98) | 0.001 |
| NYHA class 1 | 0.07 (0.03–0.13) | <0.001 | ||||
| NYHA class 2 | 4.03 (1.66–9.83) | 0.002 | ||||
| NYHA class 3 | 3.41 (0.94–12.37) | 0.062 | ||||
| Killip class 1 | 0.08 (0.04–0.16) | <0.001 | 0.13 (0.04–0.39) | <0.001 | 0.11 (0.03–0.36) | <0.001 |
| Killip class 2 | 2.47 (0.82–7.43) | 0.109 | ||||
| Sinus rhythm | 0.21 (0.10–0.42) | <0.001 | ||||
| ECG Heart Rate (bpm) | 1.02 (1.01–1.04) | <0.001 | 1.02 (1.00–1.04) | 0.022 | 1.02 (1.00–1.04) | 0.022 |
| Anterior MI | 1.73 (0.90–3.31) | 0.099 | ||||
| PCI to the RCA | 0.26 (0.08–0.85) | 0.025 | 0.04 (0.00–0.53) | 0.015 | 0.03 (0.00–0.49) | 0.015 |
| Pharmaco-invasive approach | 0.43 (0.20–0.96) | 0.038 | 0.20 (0.05–0.79) | 0.022 | 0.16 (0.04–0.71) | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndaba, L.; Mutyaba, A.; Mpanya, D.; Tsabedze, N. In-Hospital Mortality Outcomes of ST-Segment Elevation Myocardial Infarction: A Cross-Sectional Study from a Tertiary Academic Hospital in Johannesburg, South Africa. J. Cardiovasc. Dev. Dis. 2023, 10, 348. https://doi.org/10.3390/jcdd10080348
Ndaba L, Mutyaba A, Mpanya D, Tsabedze N. In-Hospital Mortality Outcomes of ST-Segment Elevation Myocardial Infarction: A Cross-Sectional Study from a Tertiary Academic Hospital in Johannesburg, South Africa. Journal of Cardiovascular Development and Disease. 2023; 10(8):348. https://doi.org/10.3390/jcdd10080348
Chicago/Turabian StyleNdaba, Lindokuhle, Arthur Mutyaba, Dineo Mpanya, and Nqoba Tsabedze. 2023. "In-Hospital Mortality Outcomes of ST-Segment Elevation Myocardial Infarction: A Cross-Sectional Study from a Tertiary Academic Hospital in Johannesburg, South Africa" Journal of Cardiovascular Development and Disease 10, no. 8: 348. https://doi.org/10.3390/jcdd10080348