The Effects of Exercise Training on Exercise Capacity and Vascular Function after Transcatheter Aortic Valve Implantation—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Intervention
2.4. Exercise Capacity and Muscle Strength
2.5. Vascular Function
2.6. Self-Reported Health Status
2.7. Laboratory Biomarkers
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thaden, J.J.; Nkomo, V.T.; Enriquez-Sarano, M. The global burden of aortic stenosis. Prog. Cardiovasc. Dis. 2014, 56, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Prendergast, B. Aortic-valve stenosis—From patients at risk to severe valve obstruction. N. Engl. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Pohle, K.; Maffert, R.; Ropers, D.; Moshage, W.; Stilianakis, N.; Daniel, W.G.; Achenbach, S. Progression of aortic valve calcification: Association with coronary atherosclerosis and cardiovascular risk factors. Circulation 2001, 104, 1927–1932. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Pascual, C.; Paredes-Galan, E.; Ferrero-Martinez, A.I.; Baz-Alonso, J.A.; Duran-Munoz, D.; Gonzalez-Babarro, E.; Sanmartín, M.; Parajes, T.; Torres-Torres, I.; Piñón-Esteban, M.; et al. The frailty syndrome and mortality among very old patients with symptomatic severe aortic stenosis under different treatments. Int. J. Cardiol. 2016, 224, 125–131. [Google Scholar] [CrossRef]
- Poggianti, E.; Venneri, L.; Chubuchny, V.; Jambrik, Z.; Baroncini, L.A.; Picano, E. Aortic valve sclerosis is associatedwith systemic endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 41, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Di Francescomarino, S.; Sciartilli, A.; Di Valerio, V.; Di Baldassarre, A.; Gallina, S. The effect of physical exercise on endothelial function. Sports Med. 2009, 39, 797–812. [Google Scholar] [CrossRef]
- Wald, D.S.; Williams, S.; Bangash, F.; Bestwick, J.P. Watchful Waiting in Aortic Stenosis: The Problem of Acute Decompensation. Am J Med. 2018, 131, 173–177. [Google Scholar] [CrossRef]
- De Backer, O.; Sondergaard, L. Challenges When Expanding Transcatheter Aortic Valve Implantation to Younger Patients. Front. Cardiovasc. Med. 2018, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Green, P.; Woglom, A.E.; Genereux, P.; Daneault, B.; Paradis, J.M.; Schnell, S.; Hawkey, M.; Maurer, M.S.; Kirtane, A.J.; Kodali, S.; et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: A single-center experience. JACC Cardiovasc. Interv. 2012, 5, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Horn, P.; Stern, D.; Veulemans, V.; Heiss, C.; Zeus, T.; Merx, M.W.; Kelm, M.; Westenfeld, R. Improved endothelial function and decreased levels of endothelium-derived microparticles after transcatheter aortic valve implantation. EuroIntervention 2015, 10, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Anayo, L.; Rogers, P.; Long, L.; Dalby, M.; Taylor, R. Exercise-based cardiac rehabilitation for patients following open surgical aortic valve replacement and transcatheter aortic valve implant: A systematic review and meta-analysis. Open Heart 2019, 6, e000922. [Google Scholar] [PubMed]
- Bellmann, B.; Lin, T.; Greissinger, K.; Rottner, L.; Rillig, A.; Zimmerling, S. The Beneficial Effects of Cardiac Rehabilitation. Cardiol. Ther. 2020, 9, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Eichler, S.; Salzwedel, A.; Reibis, R.; Nothroff, J.; Harnath, A.; Schikora, M.; Butter, C.; Wegscheider, K.; Völler, H. Multicomponent cardiac rehabilitation in patients after transcatheter aortic valve implantation: Predictors of functional and psychocognitive recovery. Eur. J. Prev. Cardiol. 2017, 24, 257–264. [Google Scholar] [CrossRef]
- Butter, C.; Gross, J.; Haase-Fielitz, A.; Sims, H.; Deutsch, C.; Bramlage, P.; Neuss, M. Impact of Rehabilitation on Outcomes after TAVI: A Preliminary Study. J. Clin. Med. 2018, 7, 326. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, G.S.; Melo, R.D.; Deresz, L.F.; Lago, P.D.; Pontes, M.R.; Karsten, M. Cardiac rehabilitation programme after transcatheter aortic valve implantation versus surgical aortic valve replacement: Systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 688–697. [Google Scholar] [CrossRef]
- Russo, N.; Compostella, L.; Tarantini, G.; Setzu, T.; Napodano, M.; Bottio, T.; D’onofrio, A.; Isabella, G.; Gerosa, G.; Iliceto, S.; et al. Cardiac rehabilitation after transcatheter versus surgical prosthetic valve implantation for aortic stenosis in the elderly. Eur. J. Prev. Cardiol. 2014, 21, 1341–1348. [Google Scholar] [CrossRef]
- Pressler, A.; Christle, J.W.; Lechner, B.; Grabs, V.; Haller, B.; Hettich, I.; Jochheim, D.; Mehilli, J.; Lange, R.; Bleiziffer, S.; et al. Exercise training improves exercise capacity and quality of life after transcatheter aortic valve implantation: A randomized pilot trial. Am. Heart J. 2016, 182, 44–53. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Leon, M.B.; Piazza, N.; Nikolsky, E.; Blackstone, E.H.; Cutlip, D.E.; Kappetein, A.P.; Krucoff, M.W.; Mack, M.; Mehran, R.; Miller, C.; et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the Valve Academic Research Consortium. Eur. Heart J. 2011, 32, 205–217. [Google Scholar] [CrossRef]
- Ellert, U.; Kurth, B.M. Methodological views on the SF-36 summary scores based on the adult German population. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2004, 47, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- EuroQol. EQ-5D, an Instrument to Describe and Value Health. 2022. Available online: https://euroqol.org/euroqol/ (accessed on 10 November 2022).
- Rupel, V.; Ogorevc, M. The EQ-5D health states value set for Slovenia. Slov. J. Public Health 2012, 51, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Prevolnik Rupel, V.; Ogorevc, M. EQ-5D-5L Slovenian population norms. Health Qual. Life Outcomes 2020, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Novak, T.; Vizintin Cuderman, T.; Krevel, B.; Tasic, J.; Rajkovic, U.; Fras, Z.; Jug, B. Exercise capacity improvement after cardiac rehabilitation following myocardial infarction and its association with long-term cardiovascular events. Eur. J. Cardiovasc. Nurs. 2022, 21, 76–84. [Google Scholar] [CrossRef]
- Bhattal, G.K.; Park, K.E.; Winchester, D.E. Home-Based Cardiac Rehabilitation (HBCR) In Post-TAVR Patients: A Prospective, Single-Center, Cohort, Pilot Study. Cardiol. Ther. 2020, 9, 541–548. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [Green Version]
- Vasic, D.; Novakovic, M.; Bozic Mijovski, M.; Barbic Zagar, B.; Jug, B. Short-Term Water- and Land-Based Exercise Training Comparably Improve Exercise Capacity and Vascular Function in Patients After a Recent Coronary Event: A Pilot Randomized Controlled Trial. Front. Physiol. 2019, 10, 903. [Google Scholar] [CrossRef] [Green Version]
- Novakovic, M.; Prokselj, K.; Rajkovic, U.; Vizintin Cuderman, T.; Jansa Trontelj, K.; Fras, Z.; Jug, B. Exercise training in adults with repaired tetralogy of Fallot: A randomized controlled pilot study of continuous versus interval training. Int. J. Cardiol. 2018, 255, 37–44. [Google Scholar] [CrossRef]
- Trsan, J.; Kosuta, D.; Rajkovic, U.; Fras, Z.; Jug, B.; Novakovic, M. Vascular Function in Patients After Myocardial Infarction: The Importance of Physical Activity. Front. Physiol. 2021, 12, 763043. [Google Scholar] [CrossRef]
- Montero, D.; Walther, G.; Benamo, E.; Perez-Martin, A.; Vinet, A. Effects of exercise training on arterial function in type 2 diabetes mellitus: A systematic review and meta-analysis. Sports Med. 2013, 43, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.J.; Smart, N.A. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int. J. Cardiol. 2017, 231, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janić, M.; Lunder, M.; Šabovič, M. Arterial Stiffness and Cardiovascular Therapy. BioMed Res. Int. 2014, 2014, 621437. [Google Scholar] [CrossRef] [Green Version]
- Izawa, K.; Hirano, Y.; Yamada, S.; Oka, K.; Omiya, K.; Iijima, S. Improvement in physiological outcomes and health-related quality of life following cardiac rehabilitation in patients with acute myocardial infarction. Circ. J. 2004, 68, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.S.; Walker, S.; Smart, N.A.; Piepoli, M.F.; Warren, F.C.; Ciani, O.; Whellan, D.; O’Connor, C.; Keteyian, S.J.; Coats, A.; et al. Impact of Exercise Rehabilitation on Exercise Capacity and Quality-of-Life in Heart Failure: Individual Participant Meta-Analysis. J. Am. Coll. Cardiol. 2019, 73, 1430–1443. [Google Scholar] [CrossRef]
- Poudel, A.N.; Zhu, S.; Cooper, N.; Roderick, P.; Alwan, N.; Tarrant, C.; Ziauddeen, N.; Yao, G.L. Impact of Covid-19 on health-related quality of life of patients: A structured review. PLoS ONE 2021, 16, e0259164. [Google Scholar] [CrossRef]
- Kulnik, S.T.; Sareban, M.; Höppchen, I.; Droese, S.; Egger, A.; Gutenberg, J.; Mayr, B.; Reich, B.; Wurhofer, D.; Niebauer, J. Outpatient Cardiac Rehabilitation Closure and Home-Based Exercise Training During the First COVID-19 Lockdown in Austria: A Mixed-Methods Study. Front. Psychol. 2022, 13, 817912. [Google Scholar] [CrossRef]
- Campbell, D.; Davison, J. The Impact of COVID-19 on the Health-Related Quality of Life of Individuals Living in Scottish Communities with High Infection Rates. Eur. J. Environ. Public. Health 2022, 6, em0099. [Google Scholar] [CrossRef]
- Ferreira, L.N.; Pereira, L.N.; da Fe Bras, M.; Ilchuk, K. Quality of life under the COVID-19 quarantine. Qual. Life Res. 2021, 30, 1389–1405. [Google Scholar] [CrossRef]
- Ping, W.; Zheng, J.; Niu, X.; Guo, C.; Zhang, J.; Yang, H.; Shi, Y. Evaluation of health-related quality of life using EQ-5D in China during the COVID-19 pandemic. PLoS ONE 2020, 15, e0234850. [Google Scholar] [CrossRef] [PubMed]
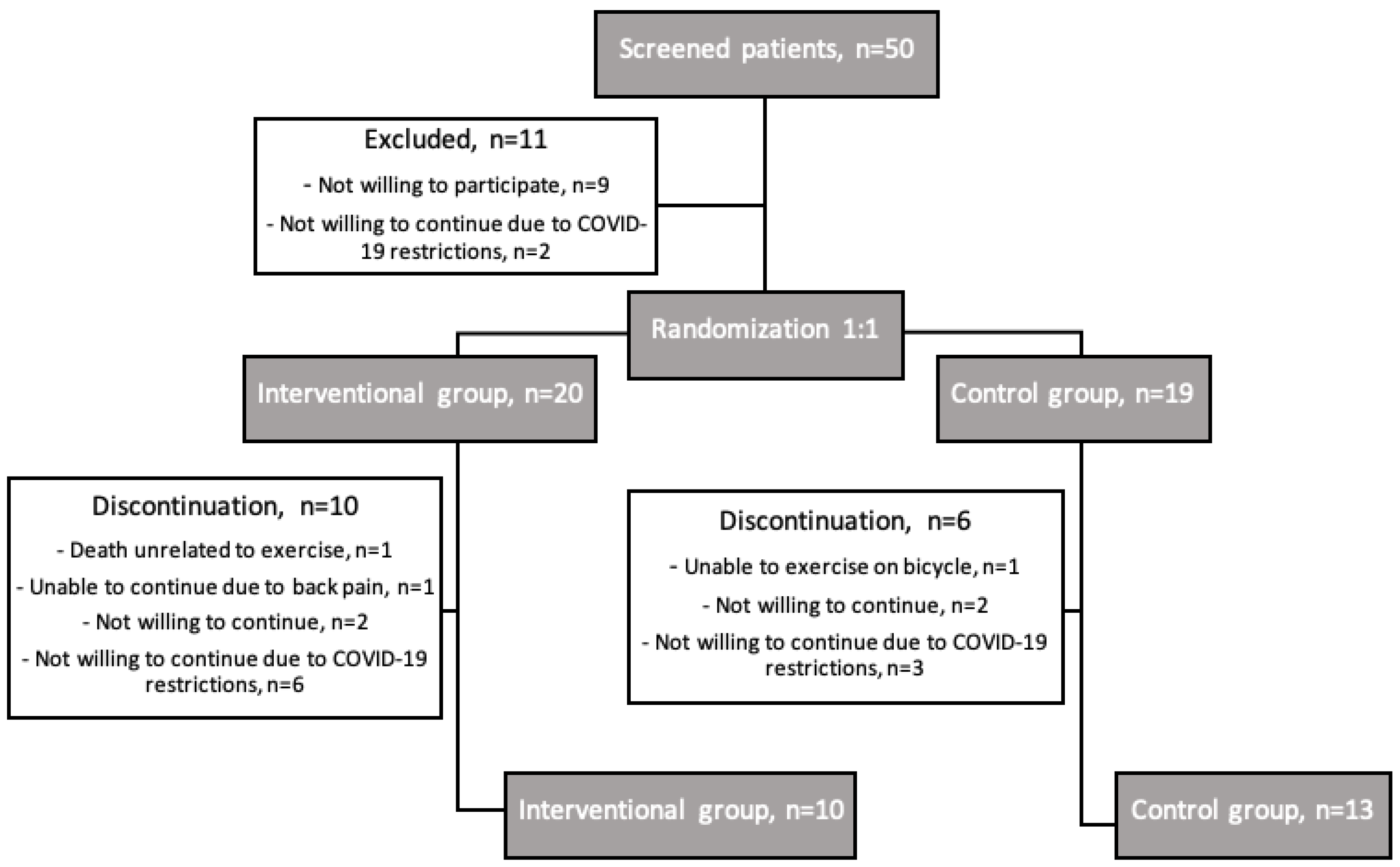
| Baseline Characteristics: | Interventional Group | Control Group | p |
|---|---|---|---|
| Participants number | 10 (44) | 13 (56) | |
| Age, years | 81 (5) | 82 (4) | 0.617 |
| Gender—female | 7 (70) | 7 (54) | 0.363 |
| BMI, kg/m2 | 30.9 (4.9) | 26.3 (5.2) | 0.043 |
| NYHA Class | |||
| I | 1 (10) | 0.178 | |
| II | 8 (80) | 13 (100) | |
| III | 1 (10) | ||
| 6MWT, m | 346 (77) | 313 (68) | 0.297 |
| Hand grip test, kg | 22 (3) | 24 (6) | 0.419 |
| Systolic blood pressure, mmHg | 158 (22) | 163 (27) | 0.568 |
| Diastolic blood pressure, mmHg | 82 (14) | 75 (9) | 0.138 |
| Atrial fibrillation | 3 (30) | 3 (23) | 0.537 |
| Diabetes mellitus | 3 (30) | 4 (31) | 0.663 |
| Hypertension | 9 (90) | 12 (92) | 0.692 |
| Hyperlipidaemia | 7 (70) | 11 (85) | 0.367 |
| Coronary artery disease | 4 (40) | 9 (70) | 0.164 |
| History of acute myocardial infarction | 1 (10) | 3 (23) | 0.404 |
| Peripheral artery disease | 1 (10) | 1 (7) | 0.692 |
| History of cerebrovascular insult | 1 (10) | 1 (7) | 0.692 |
| Chronic obstructive pulmonary disease | 3 (30) | 1 (7) | 0.200 |
| Cardiac implantable device | 4 (40) | 3 (23) | 0.337 |
| Echocardiography: | |||
| LVEF, % | 57 (13) | 55 (13) | 0.676 |
| Pulmonary artery systolic pressure, mmHg | 35 (14) | 40 (8) | 0.247 |
| Laboratory: | |||
| Creatinine, mmol/L | 95 (28) | 80 (23) | 0.178 |
| eGFR, mL/min/10.73 m2 | 64 (23) | 70 (14) | 0.315 |
| Haemoglobin level, g/L | 135 (19) | 134 (8) | 0.901 |
| HbA1c level, % | 6.1 (1) | 6.3 (0.9) | 0.648 |
| NT-proBNP, ng/L | 573 (407–1025) | 599 (222–2333) | 0.973 |
| Uric acid, mmol/L | 366 (94) | 334 (81) | 0.401 |
| Total cholesterol, mmol/L | 5.2 (0.9) | 4.4 (1.4) | 0.137 |
| HDL cholesterol, mmol/L | 1.4 (0.4) | 1.4 (0.4) | 0.964 |
| LDL cholesterol, mmol/L | 3 (0.8) | 2.3 (1.1) | 0.107 |
| Triglycerides, mmol/L | 1.9 (1.2) | 1.7 (0.8) | 0.662 |
| Myoglobin, mgl/L | 61.9 (28.6) | 61.9 (20.2) | 0.872 |
| CK, mkat/L | 1.6 (0.7) | 1.5 (0.8) | 0.762 |
| Medications: | |||
| Aspirin | 8 (80) | 12 (92) | 0.398 |
| Oral anticoagulant | 3 (30) | 3 (23) | 0.537 |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 8 (80) | 11 (85) | 0.596 |
| Angiotensin receptor neprilysin inhibitor | 1 (10) | 0 | 0.435 |
| Calcium channel blocker | 2 (20) | 4 (31) | 0.463 |
| Beta-blocker | 7 (70) | 13 (100) | 0.068 |
| Mineralocorticoid receptor antagonist | 3 (30) | 2 (15) | 0.367 |
| Furosemide | 3 (30) | 6 (46) | 0.363 |
| Statin | 6 (60) | 10 (77) | 0.337 |
| TAVI: | |||
| Days after TAVI | 101 (18) | 118 (18) | 0.081 |
| Device used: | |||
| Medtronic CoreValve Evolute R | 4 (40) | 5 (39) | 0.157 |
| Edwards Sapien 3 | 6 (60) | 4 (31) | |
| Abbott Portico | 0 | 4 (31) | |
| Valve-in-valve | 2 (20) | 1 (8) | 0.398 |
| Exercise Testing: | Interventional Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Before, n = 10 | Final, n = 10 | p | Before, n = 13 | Final, n = 13 | p | |
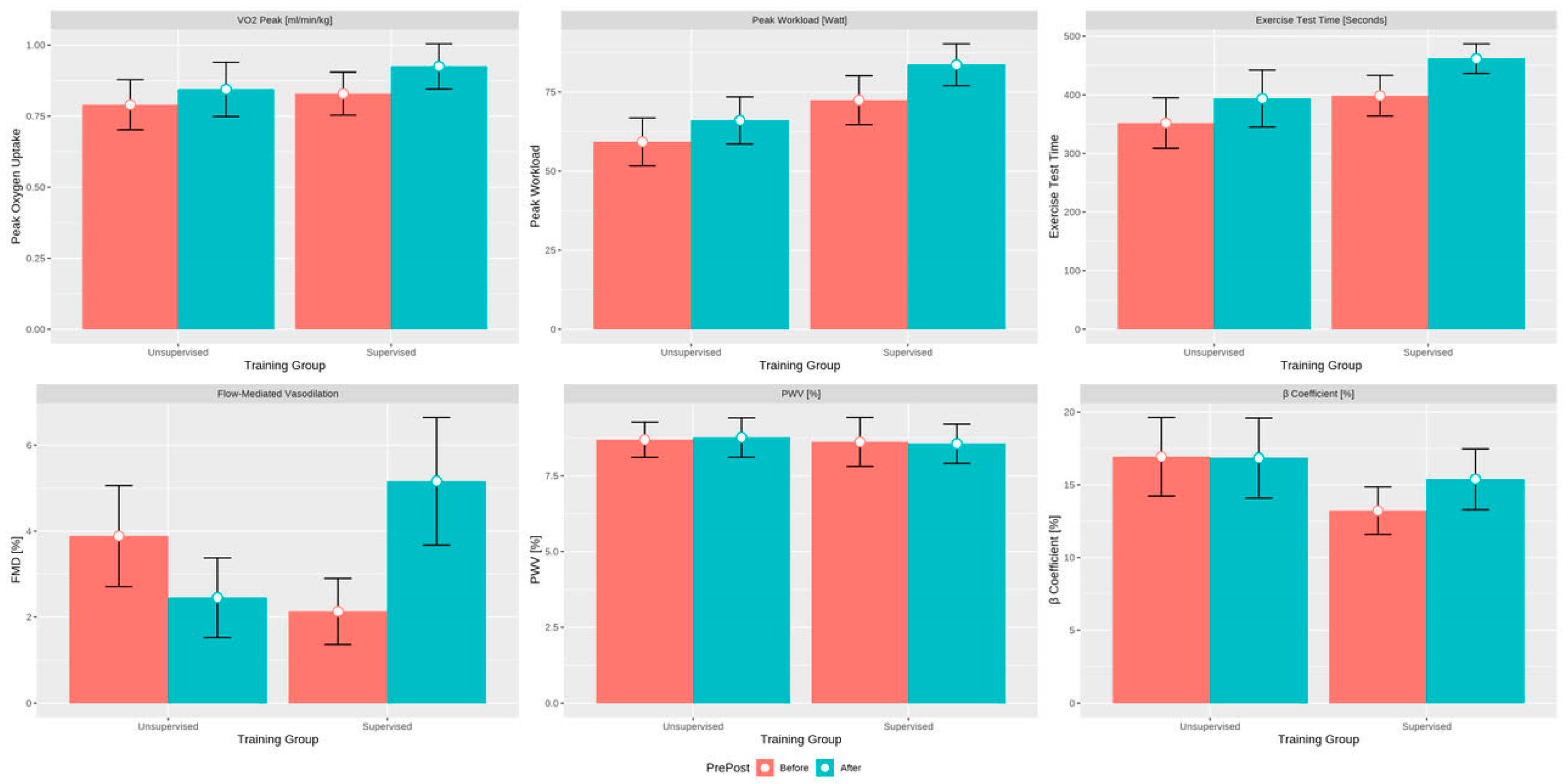
| Exercise time, s | 418 (388–434) | 466 (427–503) | 0.01 | 356 (274–431) | 369 (321–427) | 0.092 |
| Workload, W | 75 (65–89) | 82 (74–93) | 0.01 | 59 (46–72) | 66 (55–75) | 0.11 |
| VO2 peak, mL/min/kg | 0.87 (0.66–0.98) | 0.89 (0.80–1.08) | 0.049 | 0.76 (0.64–0.91) | 0.91 (0.72–0.98) | 0.065 |
| Vascular Function: | ||||||
| FMD, % | 2.10 (0.95–2.57) | 4.55 (3.35–5.78) | 0.02 | 3.00 (2.10–4.00) | 2.30 (0.90–3.00) | 0.069 |
| Exercise Testing: | Effect Size (Time) | p | Effect Size (Intervention) | p | Effect Size (Intervention × Time Interaction) | p |
|---|---|---|---|---|---|---|
| Exercise time, s | 47.3 (5; 89.6) | 0.029 | 46.7 (−28.3; 121.7) | 0.222 | 15.9 (−47.1; 78.9) | 0.620 |
| Workload, W | 8.2 (0.6; 15.8) | 0.034 | 9.1 (−5.6; 23.9) | 0.225 | 4.1 (−7.2; 15.3) | 0.478 |
| VO2 peak, mL/min/kg | 0.09 (0.01; 0.16) | 0.020 | −0.01 (−0.18; 0.16) | 0.925 | 0.01(−0.09; 0.12) | 0.795 |
| VO2 at AT, mL/min/kg | 0.01 (−1.19; 1.2) | 0.992 | −1.07 (−3.39; 1.26) | 0.369 | 0.18 (−1.56; 1.91) | 0.842 |
| VE/VCO2 | −0.24 (−3.36; 2,88) | 0.881 | −0.6 (−4.38; 3.18) | 0.757 | 0.3 (−4.62; 5.21) | 0.906 |
| O2 pulse, mL/beat | −2.32 (−5.95; 1.31) | 0.211 | −1.28 (−5.01; 2.44) | 0.500 | 3.31 (−2.02; 8.63) | 0.223 |
| 6MWT, m | 1.8 (−22.8; 26.4) | 0.886 | 32.4 (−21.4; 86.2) | 0.238 | 24.7 (−11.9; 61.3) | 0.186 |
| Muscular Strength: | ||||||
| Hand grip test, kg | −0.15 (−2.24; 1.94) | 0.885 | −1.31 (−5.17; 2.55) | 0.507 | 2.48 (−0.68; 5.63) | 0.124 |
| Vascular Function: | ||||||
| FMD, % | −1.45 (−2.87; −0.04) | 0.044 | −1.77 (−3.6; 0.07) | 0.059 | 4.49 (2.35; 6.63) | <0.001 |
| Β—coefficient | −0.22 (−2.5; 2.07) | 0.854 | −3.86 (−8.01; 0.29) | 0.068 | 2.42 (−1.05; 5.88) | 0.172 |
| AI | −1.78 (−6.02; 2.47) | 0.412 | 1.53 (−4.5; 7.55) | 0.620 | 1.63 (−4.76; 8.03) | 0.617 |
| PWV, m/s | 0.12 (−0.64; 0.86) | 0.763 | −0.17 (−1.37; 1.04) | 0.788 | −0.14 (−1.27; 0.99) | 0.813 |
| Health Status: | ||||||
| EQ-5D-5L | −0.8 (−0.18; 0.03) | 0.171 | −0.09 (−0.18; 0.005) | 0.064 | 0.15 (−0.01; 0.31) | 0.066 |
| SF-36 physical component | −61.8 (−275.9; 152.3) | 0.571 | −175.9 (−376.3; 24.4) | 0.085 | 248.2 (−67; 563.5) | 0.123 |
| SF-36 mental component | 3.4 (−7.3; 14.1) | 0.532 | 2.4 (−13.3; 18.1) | 0.765 | −0.41 (−16.4; 15.5) | 0.960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitez, L.; Bunc, M.; Jug, B. The Effects of Exercise Training on Exercise Capacity and Vascular Function after Transcatheter Aortic Valve Implantation—A Pilot Study. J. Cardiovasc. Dev. Dis. 2023, 10, 343. https://doi.org/10.3390/jcdd10080343
Vitez L, Bunc M, Jug B. The Effects of Exercise Training on Exercise Capacity and Vascular Function after Transcatheter Aortic Valve Implantation—A Pilot Study. Journal of Cardiovascular Development and Disease. 2023; 10(8):343. https://doi.org/10.3390/jcdd10080343
Chicago/Turabian StyleVitez, Luka, Matjaž Bunc, and Borut Jug. 2023. "The Effects of Exercise Training on Exercise Capacity and Vascular Function after Transcatheter Aortic Valve Implantation—A Pilot Study" Journal of Cardiovascular Development and Disease 10, no. 8: 343. https://doi.org/10.3390/jcdd10080343





