Sex Differences in Heart Failure: What Do We Know?
Abstract
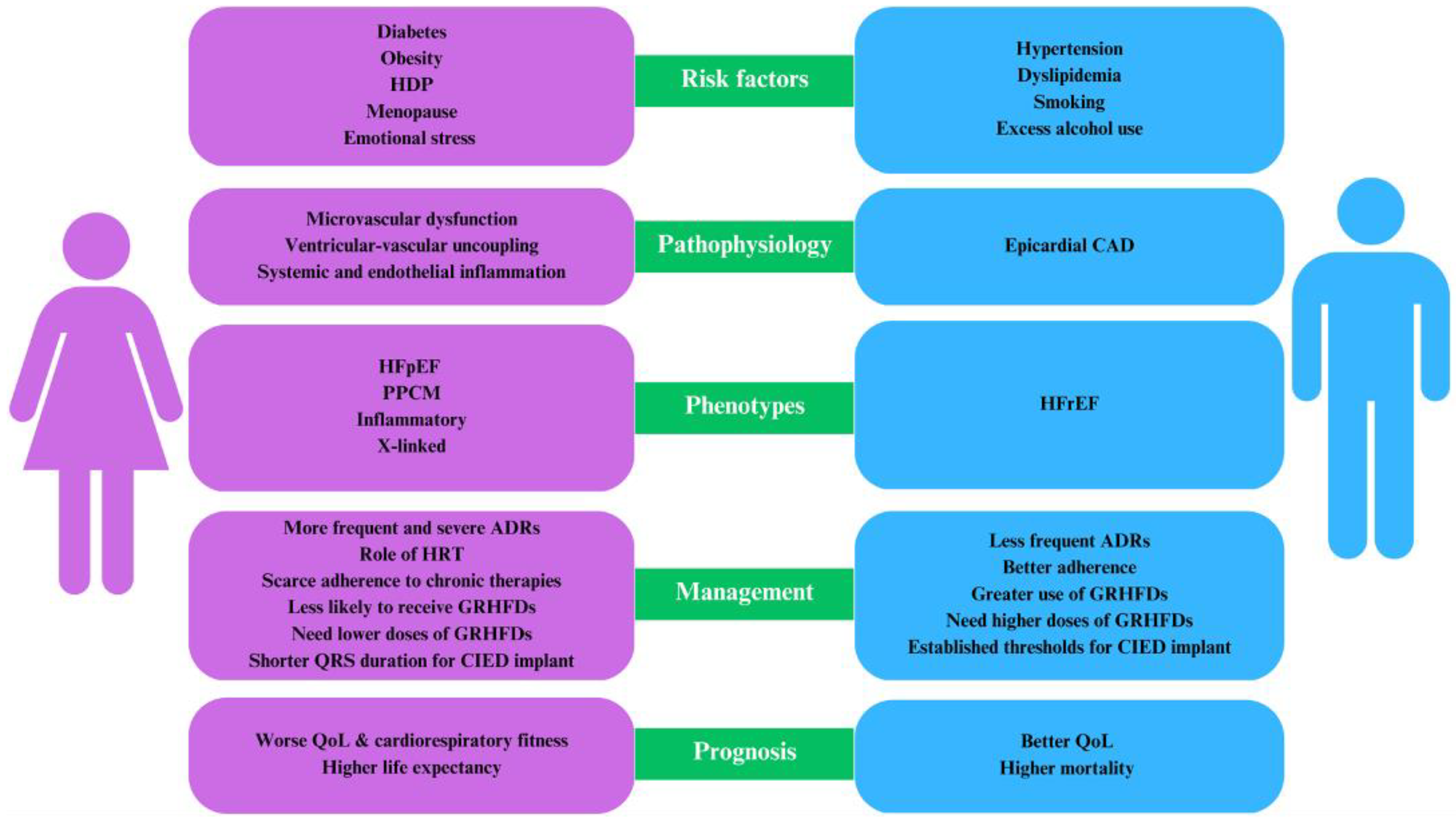
:Highlights
- Women predominantly exhibit HFpEF compared to men.
- Factors exclusive to women, such as adverse pregnancy outcomes and premature menopause, elevate the risk of HF.
- The establishment of sex-specific optimal drug dosages and concrete guidelines for device therapy is essential.
- Concerted multidisciplinary initiatives are crucial to bridge the existing sex disparities in HF management.
Abstract
1. Introduction
2. Heart Failure: Definition and Phenotypes of Disease
3. Sex-Specific Risk Factors for Heart Failure
4. Sex-Specific Differences in Ischemic and Non-Ischemic Cardiomyopathies
4.1. Ischemic Heart Disease
4.2. Dilated Cardiomyopathy
4.3. Peripartum Cardiomyopathy
4.4. Hypertrophic Cardiomyopathy
4.5. Fabry Disease
4.6. Cardiac Amyloidosis
4.7. Arrhythmogenic Cardiomyopathy
4.8. Inflammatory Cardiomyopathies
5. Heart Failure Management
6. Does a Gender-Related Issue Exist in Heart Failure?
7. Transformative Solutions for Improvement
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, V.; Wachtell, K.; Gerdts, E.; Bella, J.N.; Papademetriou, V.; Tuxen, C.; Nieminen, M.S.; Dahlöf, B.; de Simone, G.; Devereux, R.B. Left Ventricular Function and Hemodynamic Features of Inappropriate Left Ventricular Hypertrophy in Patients with Systemic Hypertension: The LIFE Study. Am. Heart J. 2001, 141, 784–791. [Google Scholar] [CrossRef]
- Alfakih, K.; Plein, S.; Thiele, H.; Jones, T.; Ridgway, J.P.; Sivananthan, M.U. Normal Human Left and Right Ventricular Dimensions for MRI as Assessed by Turbo Gradient Echo and Steady-State Free Precession Imaging Sequences. J. Magn. Reson. Imaging 2003, 17, 323–329. [Google Scholar] [CrossRef]
- Salton, C.J.; Chuang, M.L.; O’Donnell, C.J.; Kupka, M.J.; Larson, M.G.; Kissinger, K.V.; Edelman, R.R.; Levy, D.; Manning, W.J. Gender Differences and Normal Left Ventricular Anatomy in an Adult Population Free of Hypertension. J. Am. Coll. Cardiol. 2002, 39, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.K.; Das, S.R.; Leonard, D.; Peshock, R.M.; Kazi, F.; Abdullah, S.M.; Canham, R.M.; Levine, B.D.; Drazner, M.H. Women Have Higher Left Ventricular Ejection Fractions Than Men Independent of Differences in Left Ventricular Volume: The Dallas Heart Study. Circulation 2006, 113, 1597–1604. [Google Scholar] [CrossRef] [Green Version]
- Suthahar, N.; Lau, E.S.; Blaha, M.J.; Paniagua, S.M.; Larson, M.G.; Psaty, B.M.; Benjamin, E.J.; Allison, M.A.; Bartz, T.M.; Januzzi, J.L.; et al. Sex-Specific Associations of Cardiovascular Risk Factors and Biomarkers With Incident Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 1455–1465. [Google Scholar] [CrossRef]
- Countouris, M.E.; Villanueva, F.S.; Berlacher, K.L.; Cavalcante, J.L.; Parks, W.T.; Catov, J.M. Association of Hypertensive Disorders of Pregnancy With Left Ventricular Remodeling Later in Life. J. Am. Coll. Cardiol. 2021, 77, 1057–1068. [Google Scholar] [CrossRef]
- Williams, D.; Stout, M.J.; Rosenbloom, J.I.; Olsen, M.A.; Joynt Maddox, K.E.; Deych, E.; Davila-Roman, V.G.; Lindley, K.J. Preeclampsia Predicts Risk of Hospitalization for Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2021, 78, 2281–2290. [Google Scholar] [CrossRef]
- Hall, P.S.; Nah, G.; Howard, B.V.; Lewis, C.E.; Allison, M.A.; Sarto, G.E.; Waring, M.E.; Jacobson, L.T.; Manson, J.E.; Klein, L.; et al. Reproductive Factors and Incidence of Heart Failure Hospitalization in the Women’s Health Initiative. J. Am. Coll. Cardiol. 2017, 69, 2517–2526. [Google Scholar] [CrossRef]
- Santema, B.T.; Ouwerkerk, W.; Tromp, J.; Sama, I.E.; Ravera, A.; Regitz-Zagrosek, V.; Hillege, H.; Samani, N.J.; Zannad, F.; Dickstein, K.; et al. Identifying Optimal Doses of Heart Failure Medications in Men Compared with Women: A Prospective, Observational, Cohort Study. Lancet 2019, 394, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Dual Angiotensin Receptor and Neprilysin Inhibition as an Alternative to Angiotensin-converting Enzyme Inhibition in Patients with Chronic Systolic Heart Failure: Rationale for and Design of the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF). Eur. J. Heart Fail. 2013, 15, 1062–1073. [Google Scholar] [CrossRef] [Green Version]
- Merrill, M.; Sweitzer, N.K.; Lindenfeld, J.; Kao, D.P. Sex Differences in Outcomes and Responses to Spironolactone in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2019, 7, 228–238. [Google Scholar] [CrossRef]
- Dewan, P.; Rørth, R.; Jhund, P.S.; Shen, L.; Raparelli, V.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Køber, L.; et al. Differential Impact of Heart Failure With Reduced Ejection Fraction on Men and Women. J. Am. Coll. Cardiol. 2019, 73, 29–40. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Elgendy, I.Y.; Saad, M.; Elgendy, A.Y.; Barakat, A.F.; Mentias, A.; Abuzaid, A.; Bavry, A.A. Does Gender Influence the Cardiovascular Benefits Observed with Sodium Glucose Co-Transporter-2 (SGLT-2) Inhibitors? A Meta-Regression Analysis. Cardiol. Ther. 2017, 6, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Rodenburg, E.M.; Stricker, B.H.; Visser, L.E. Sex Differences in Cardiovascular Drug-Induced Adverse Reactions Causing Hospital Admissions: Cardiovascular Drugs, Sex and Adverse Drug Reactions. Br. J. Clin. Pharmacol. 2012, 74, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Zusterzeel, R.; Selzman, K.A.; Sanders, W.E.; Caños, D.A.; O’Callaghan, K.M.; Carpenter, J.L.; Piña, I.L.; Strauss, D.G. Cardiac Resynchronization Therapy in Women: US Food and Drug Administration Meta-Analysis of Patient-Level Data. JAMA Intern. Med. 2014, 174, 1340. [Google Scholar] [CrossRef] [Green Version]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V. Sex and Gender Differences in Heart Failure. Int. J. Heart Fail. 2020, 2, 157. [Google Scholar] [CrossRef]
- Agarwala, A.; Michos, E.D.; Samad, Z.; Ballantyne, C.M.; Virani, S.S. The Use of Sex-Specific Factors in the Assessment of Women’s Cardiovascular Risk. Circulation 2020, 141, 592–599. [Google Scholar] [CrossRef]
- Gender in Cardiovascular Diseases: Impact on Clinical Manifestations, Management, and Outcomes. Eur. Heart J. 2016, 37, 24–34. [CrossRef] [Green Version]
- Levinsson, A.; Dubé, M.-P.; Tardif, J.-C.; de Denus, S. Sex, Drugs, and Heart Failure: A Sex-Sensitive Review of the Evidence Base behind Current Heart Failure Clinical Guidelines: Sex, Drugs, and Heart Failure. ESC Heart Fail. 2018, 5, 745–754. [Google Scholar] [CrossRef]
- Melloni, C.; Berger, J.S.; Wang, T.Y.; Gunes, F.; Stebbins, A.; Pieper, K.S.; Dolor, R.J.; Douglas, P.S.; Mark, D.B.; Newby, L.K. Representation of Women in Randomized Clinical Trials of Cardiovascular Disease Prevention. Circ Cardiovasc. Qual. Outcomes 2010, 3, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.D.; Peters, E.; Wassef, A.; Desmarais, P.; Rémillard-Labrosse, D.; Tremblay-Gravel, M. Evolution of Age and Female Representation in the Most-Cited Randomized Controlled Trials of Cardiology of the Last 20 Years. Circ Cardiovasc. Qual. Outcomes 2018, 11, e004713. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Luchner, A. Gender-Specific Differences of Cardiac Remodeling in Subjects with Left Ventricular Dysfunction: A Population-Based Study. Cardiovasc. Res. 2002, 53, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Devereux, R.B.; Roman, M.J.; Paranicas, M.; Lee, E.T.; Welty, T.K.; Fabsitz, R.R.; Robbins, D.; Rhoades, E.R.; Rodeheffer, R.J.; Cowan, L.D.; et al. A Population-Based Assessment of Left Ventricular Systolic Dysfunction in Middle-Aged and Older Adults: The Strong Heart Study. Am. Heart J. 2001, 141, 439–446. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.E.; Aung, N.; Sanghvi, M.M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.M.; et al. Reference Ranges for Cardiac Structure and Function Using Cardiovascular Magnetic Resonance (CMR) in Caucasians from the UK Biobank Population Cohort. J. Cardiovasc. Magn. Reson. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Taegtmeyer, H. Metabolic Crosstalk in Heart Failure. J. Am. Coll. Cardiol. 2011, 58, 1126–1127. [Google Scholar] [CrossRef] [Green Version]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of Diabetes in Congestive Heart Failure: The Framingham Study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Henry, R.M.A.; Kamp, O.; Kostense, P.J.; Spijkerman, A.M.W.; Dekker, J.M.; Van Eijck, R.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D.A. Left Ventricular Mass Increases With Deteriorating Glucose Tolerance, Especially in Women: Independence of Increased Arterial Stiffness or Decreased Flow-Mediated Dilation. Diabetes Care 2004, 27, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Rutter, M.K.; Parise, H.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Meigs, J.B.; Nesto, R.W.; Wilson, P.W.F.; Vasan, R.S. Impact of Glucose Intolerance and Insulin Resistance on Cardiac Structure and Function: Sex-Related Differences in the Framingham Heart Study. Circulation 2003, 107, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Hales, C.M. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief, no. 360; National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Packer, M.; Kitzman, D.W. Obesity-Related Heart Failure With a Preserved Ejection Fraction. JACC: Heart Fail. 2018, 6, 633–639. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Moscucci, F.; Sciomer, S.; Maffei, S.; Nasi, M.; Pinti, M.; Bucciarelli, V.; Dei Cas, A.; Parati, G.; Ciccone, M.M.; et al. Cardiovascular Prevention in Women: An Update by the Italian Society of Cardiology Working Group on ‘Prevention, Hypertension and Peripheral Disease. J. Cardiovasc. Med. 2023, 24, e147–e155. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Selleri, V.; Zanini, G.; Nasi, M.; Pinti, M.; Stefanelli, C.; Fedele, F.; Gallina, S. Physical Activity and Diet in Older Women: A Narrative Review. JCM 2022, 12, 81. [Google Scholar] [CrossRef]
- Rangel, I.; Gonçalves, A.; de Sousa, C.; Leite, S.; Campelo, M.; Martins, E.; Amorim, S.; Moura, B.; Silva Cardoso, J.; Maciel, M.J. Iron Deficiency Status Irrespective of Anemia: A Predictor of Unfavorable Outcome in Chronic Heart Failure Patients. Cardiology 2014, 128, 320–326. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Damy, T.; Terbah, M.; Kerebel, S.; Baguet, J.-P.; Hanon, O.; Zannad, F.; Laperche, T.; Leclercq, C.; Concas, V.; et al. High Prevalence of Iron Deficiency in Patients with Acute Decompensated Heart Failure. Eur. J. Heart Fail. 2014, 16, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Le Jemtel, T.H.; Arain, S. Mediators of Anemia in Chronic Heart Failure. Heart Fail. Clin. 2010, 6, 289–293. [Google Scholar] [CrossRef]
- Agarwal, A.; Shah, R. Anemia Associated with Chronic Heart Failure: Current Concepts. CIA 2013, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Silverberg, D.S.; Wexler, D.; Iaina, A.; Schwartz, D. The Role of Anemia in the Progression of Congestive Heart Failure: Is There a Place for Erythropoietin and Intravenous Iron? Transfus. Altern. Transfus. Med. 2008, 6, 26–37. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen Signaling and Cardiovascular Disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Salerni, S.; Di Francescomarino, S.; Cadeddu, C.; Acquistapace, F.; Maffei, S.; Gallina, S. The Different Role of Sex Hormones on Female Cardiovascular Physiology and Function: Not Only Oestrogens. Eur. J. Clin. Investig. 2015, 45, 634–645. [Google Scholar] [CrossRef]
- Medina-Inojosa, J.R.; Vinnakota, S.; Garcia, M.; Arciniegas Calle, M.; Mulvagh, S.L.; Lopez-Jimenez, F.; Bhagra, A. Role of Stress and Psychosocial Determinants on Women’s Cardiovascular Risk and Disease Development. J. Women’s Health 2019, 28, 483–489. [Google Scholar] [CrossRef]
- Shaw, L.J.; Bairey Merz, C.N.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights From the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006, 47, S4–S20. [Google Scholar] [CrossRef] [Green Version]
- Wellons, M.; Ouyang, P.; Schreiner, P.J.; Herrington, D.M.; Vaidya, D. Early Menopause Predicts Future Coronary Heart Disease and Stroke: The Multi-Ethnic Study of Atherosclerosis. Menopause 2012, 19, 1081–1087. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.C.; Libby, P. Cardiovascular Disease in Patients with Chronic Inflammation: Mechanisms Underlying Premature Cardiovascular Events in Rheumatologic Conditions. Eur. Heart J. 2015, 36, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Lubiszewska, B.; Kruk, M.; Broda, G.; Ksiezycka, E.; Piotrowski, W.; Kurjata, P.; Zieliński, T.; Ploski, R. The Impact of Early Menopause on Risk of Coronary Artery Disease (PREmature Coronary Artery Disease in Women—PRECADIW Case-Control Study). Eur. J. Prev. Cardiol. 2012, 19, 95–101. [Google Scholar] [CrossRef]
- Tooher, J.; Thornton, C.; Makris, A.; Ogle, R.; Korda, A.; Hennessy, A. All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension 2017, 70, 798–803. [Google Scholar] [CrossRef]
- Khan, N.A. Sex Differences in Acute Coronary Syndrome Symptom Presentation in Young Patients. JAMA Intern. Med. 2013, 173, 1863–1871. [Google Scholar] [CrossRef]
- Canto, J.G. Symptom Presentation of Women With Acute Coronary Syndromes: Myth vs Reality. Arch. Intern. Med. 2007, 167, 2405. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, N.R.; Sampson, B.A.; Abrecht, C.R.; Siegfried, J.S.; Hochman, J.S.; Reynolds, H.R. Women Have Less Severe and Extensive Coronary Atherosclerosis in Fatal Cases of Ischemic Heart Disease: An Autopsy Study. Am. Heart J. 2011, 161, 681–688. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Shaw, L.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Pepine, C.J.; Mankad, S.; Sharaf, B.L.; et al. Insights From the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006, 47, S21–S29. [Google Scholar] [CrossRef] [Green Version]
- Pepine, C.J.; Ferdinand, K.C.; Shaw, L.J.; Light-McGroary, K.A.; Shah, R.U.; Gulati, M.; Duvernoy, C.; Walsh, M.N.; Bairey Merz, C.N. Emergence of Nonobstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2015, 66, 1918–1933. [Google Scholar] [CrossRef]
- Colella, T.J.; Gravely, S.; Marzolini, S.; Grace, S.L.; Francis, J.A.; Oh, P.; Scott, L.B. Sex Bias in Referral of Women to Outpatient Cardiac Rehabilitation? A Meta-Analysis. Eur. J. Prev. Cardiol. 2015, 22, 423–441. [Google Scholar] [CrossRef]
- Supervía, M.; Medina-Inojosa, J.R.; Yeung, C.; Lopez-Jimenez, F.; Squires, R.W.; Pérez-Terzic, C.M.; Brewer, L.C.; Leth, S.E.; Thomas, R.J. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clin. Proc. 2017, 92, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Oosenbrug, E.; Marinho, R.P.; Zhang, J.; Marzolini, S.; Colella, T.J.F.; Pakosh, M.; Grace, S.L. Sex Differences in Cardiac Rehabilitation Adherence: A Meta-Analysis. Can. J. Cardiol. 2016, 32, 1316–1324. [Google Scholar] [CrossRef]
- Samayoa, L.; Grace, S.L.; Gravely, S.; Scott, L.B.; Marzolini, S.; Colella, T.J.F. Sex Differences in Cardiac Rehabilitation Enrollment: A Meta-Analysis. Can. J. Cardiol. 2014, 30, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Roswell, R.O.; Kunkes, J.; Chen, A.Y.; Chiswell, K.; Iqbal, S.; Roe, M.T.; Bangalore, S. Impact of Sex and Contact-to-Device Time on Clinical Outcomes in Acute ST-Segment Elevation Myocardial Infarction—Findings From the National Cardiovascular Data Registry. J. Am. Heart Assoc. 2017, 6, e004521. [Google Scholar] [CrossRef] [Green Version]
- Stember, A. Sex Differences in Reperfusion in Young Patients With ST-Segment-Elevation Myocardial Infarction: Results from the VIRGO Study. J. Emerg. Med. 2015, 49, 121–122. [Google Scholar] [CrossRef]
- Codd, M.B.; Sugrue, D.D.; Gersh, B.J.; Melton, L.J. Epidemiology of Idiopathic Dilated and Hypertrophic Cardiomyopathy. A Population-Based Study in Olmsted County, Minnesota, 1975–1984. Circulation 1989, 80, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Halliday, B.P.; Gulati, A.; Ali, A.; Newsome, S.; Lota, A.; Tayal, U.; Vassiliou, V.S.; Arzanauskaite, M.; Izgi, C.; Krishnathasan, K.; et al. Sex- and Age-Based Differences in the Natural History and Outcome of Dilated Cardiomyopathy: Sex- and Age-Based Differences in DCM. Eur. J. Heart Fail. 2018, 20, 1392–1400. [Google Scholar] [CrossRef] [Green Version]
- Seidelmann, S.B.; Laur, O.; Hwa, J.; Depasquale, E.; Bellumkonda, L.; Sugeng, L.; Pomianowski, P.; Testani, J.; Chen, M.; McKenna, W.; et al. Familial Dilated Cardiomyopathy Diagnosis Is Commonly Overlooked at the Time of Transplant Listing. J. Heart Lung Transplant. 2017, 35, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Ware, S.M.; Wilkinson, J.D.; Tariq, M.; Schubert, J.A.; Sridhar, A.; Colan, S.D.; Shi, L.; Canter, C.E.; Hsu, D.T.; Webber, S.A.; et al. Genetic Causes of Cardiomyopathy in Children: First Results From the Pediatric Cardiomyopathy Genes Study. J. Am. Heart Aaaoc. 2021, 10, e017731. [Google Scholar] [CrossRef] [PubMed]
- Piano, M. Effects of Alcohol on the Cardiovascular System in Women. ARCR 2020, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Mohammed, M.; Vuddanda, V.; Ansari, M.W.; Masoomi, R.; Gupta, K. National Trends, Gender, Management, and Outcomes of Patients Hospitalized for Myocarditis. Am. J. Cardiol. 2019, 124, 131–136. [Google Scholar] [CrossRef]
- Ku, L.; Feiger, J.; Taylor, M.; Mestroni, L. Familial Dilated Cardiomyopathy. Circulation 2003, 108, e118–e121. [Google Scholar] [CrossRef]
- Kamisago, M.; Sharma, P.; Woolf, P.K. Mutations in Sarcomere Protein Genes as a Cause of Dilated Cardiomyopathy. N. Engl. J. Med. 2000, 343, 1688–1696. [Google Scholar] [CrossRef]
- Lakdawala, N.K.; Dellefave, L.; Sparks, E.; Cirino, A.; Depalma, S.; Funke, B.; Colan, S.D.; Watkins, H.; Robinson, P.; Redwood, C.S.; et al. Familial Dilated Cardiomyopathy Caused by an Alpha-Tropomyosin Mutation: The Distinctive Natural History of Sarcomeric DCM. J. Card. Fail. 2009, 15, S3. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Heart Rhythm. 2018, 15, e73–e189. [Google Scholar] [CrossRef] [Green Version]
- Ricci, F.; De Innocentiis, C.; Verrengia, E.; Ceriello, L.; Mantini, C.; Pietrangelo, C.; Irsuti, F.; Gabriele, S.; D’Alleva, A.; Khanji, M.Y.; et al. The Role of Multimodality Cardiovascular Imaging in Peripartum Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Geske, J.B.; Ong, K.C.; Siontis, K.C.; Hebl, V.B.; Ackerman, M.J.; Hodge, D.O.; Miller, V.M.; Nishimura, R.A.; Oh, J.K.; Schaff, H.V.; et al. Women with Hypertrophic Cardiomyopathy Have Worse Survival. Eur. Heart J. 2017, 38, 3434–3440. [Google Scholar] [CrossRef] [Green Version]
- Olivotto, I.; Maron, M.S.; Adabag, A.S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-Related Differences in the Clinical Presentation and Outcome of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowin, E.J.; Maron, M.S.; Wells, S.; Patel, P.P.; Koethe, B.C.; Maron, B.J. Impact of Sex on Clinical Course and Survival in the Contemporary Treatment Era for Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e012041. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, L.; Marchi, A.; Fumagalli, C.; Maurizi, N.; Oddo, A.; Pieri, F.; Girolami, F.; Rowin, E.; Mazzarotto, F.; Cicoira, M.; et al. Sex-Related Differences in Exercise Performance and Outcome of Patients with Hypertrophic Cardiomyopathy. Eur. J. Prev. Cardiol. 2020, 27, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Anastasiou, Z.; O’Mahony, C.; Guttman, O.P.; Gimeno, J.R.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Garcia-Pavia, P.; et al. Mortality Among Referral Patients With Hypertrophic Cardiomyopathy vs the General European Population. JAMA Cardiol. 2020, 5, 73. [Google Scholar] [CrossRef]
- Ricci, F.; Banihashemi, B.; Pirouzifard, M.; Sundquist, J.; Sundquist, K.; Sutton, R.; Fedorowski, A.; Zöller, B. Familial Risk of Dilated and Hypertrophic Cardiomyopathy: A National Family Study in Sweden. ESC Heart Fail. 2023, 10, 121–132. [Google Scholar] [CrossRef]
- Eng, C.M.; Fletcher, J.; Wilcox, W.R.; Waldek, S.; Scott, C.R.; Sillence, D.O.; Breunig, F.; Charrow, J.; Germain, D.P.; Nicholls, K.; et al. Fabry Disease: Baseline Medical Characteristics of a Cohort of 1765 Males and Females in the Fabry Registry. J. Inher Metab. Dis. 2007, 30, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, W.R.; Oliveira, J.P.; Hopkin, R.J.; Ortiz, A.; Banikazemi, M.; Feldt-Rasmussen, U.; Sims, K.; Waldek, S.; Pastores, G.M.; Lee, P.; et al. Females with Fabry Disease Frequently Have Major Organ Involvement: Lessons from the Fabry Registry. Mol. Genet. Metab. 2008, 93, 112–128. [Google Scholar] [CrossRef]
- Galanos, J.; Nicholls, K.; Grigg, L.; Kiers, L.; Crawford, A.; Becker, G. Clinical Features of Fabry’s Disease in Australian Patients: Fabry’s Disease in Australia. Intern. Med. J. 2002, 32, 575–584. [Google Scholar] [CrossRef]
- Brunjes, D.L.; Castano, A.; Clemons, A.; Rubin, J.; Maurer, M.S. Transthyretin Cardiac Amyloidosis in Older Americans. J. Card. Fail. 2016, 22, 996–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The Role of Androgens and Estrogens on Healthy Aging and Longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, H.; Ando, Y.; Ueda, M.; Kawagashira, Y.; Iijima, M.; Fujitake, J.; Hayashi, M.; Yamamoto, M.; Mukai, E.; Nakamura, T.; et al. Distinct Characteristics of Amyloid Deposits in Early- and Late-Onset Transthyretin Val30Met Familial Amyloid Polyneuropathy. J. Neurol. Sci. 2009, 287, 178–184. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Försti, A.; Sundquist, J.; Sundquist, K. Incidence and Survival in Non-Hereditary Amyloidosis in Sweden. BMC Public. Health 2012, 12, 974. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, M.; Argirò, A.; Allinovi, M.; Tassetti, L.; Zocchi, C.; Gabriele, M.; Andrei, V.; Fumagalli, C.; Di Mario, C.; Tomberli, A.; et al. Sex-Related Differences in Clinical Presentation and All-Cause Mortality in Patients with Cardiac Transthyretin Amyloidosis and Light Chain Amyloidosis. Int. J. Cardiol. 2022, 351, 71–77. [Google Scholar] [CrossRef]
- Batra, J.; Rosenblum, H.; Defilippis, E.M.; Griffin, J.M.; Saith, S.E.; Gamino, D.; Teruya, S.; Santos, J.D.L.; Helmke, S.; Burkhoff, D.; et al. Sex Differences in the Phenotype of Transthyretin Cardiac Amyloidosis Due to Val122Ile Mutation: Insights from Noninvasive Pressure–Volume Analysis. J. Card. Fail. 2021, 27, 67–74. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Haland, T.F.; Leren, I.S.; Saberniak, J.; Edvardsen, T. Arrhythmogenic Right Ventricular Cardiomyopathy, Clinical Manifestations, and Diagnosis. Europace 2016, 18, 965–972. [Google Scholar] [CrossRef]
- Corrado, D.; Thiene, G. Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: Clinical Impact of Molecular Genetic Studies. Circulation 2006, 113, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Cadrin-Tourigny, J.; Bosman, L.P.; Nozza, A.; Wang, W.; Tadros, R.; Bhonsale, A.; Bourfiss, M.; Fortier, A.; Lie, Ø.H.; Saguner, A.M.; et al. A New Prediction Model for Ventricular Arrhythmias in Arrhythmogenic Right Ventricular Cardiomyopathy. Eur. Heart J. 2022, 43, e1–e9. [Google Scholar] [CrossRef]
- Rootwelt-Norberg, C.; Lie, Ø.H.; Chivulescu, M.; Castrini, A.I.; Sarvari, S.I.; Lyseggen, E.; Almaas, V.M.; Bogsrud, M.P.; Edvardsen, T.; Haugaa, K.H. Sex Differences in Disease Progression and Arrhythmic Risk in Patients with Arrhythmogenic Cardiomyopathy. EP Eur. 2021, 23, 1084–1091. [Google Scholar] [CrossRef]
- Akdis, D.; Saguner, A.M.; Shah, K.; Wei, C.; Medeiros-Domingo, A.; von Eckardstein, A.; Lüscher, T.F.; Brunckhorst, C.; Chen, H.S.V.; Duru, F. Sex Hormones Affect Outcome in Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: From a Stem Cell Derived Cardiomyocyte-Based Model to Clinical Biomarkers of Disease Outcome. Eur. Heart J. 2017, 38, 1498–1508. [Google Scholar] [CrossRef] [Green Version]
- Lie, Ø.H.; Dejgaard, L.A.; Saberniak, J.; Rootwelt, C.; Stokke, M.K.; Edvardsen, T.; Haugaa, K.H. Harmful Effects of Exercise Intensity and Exercise Duration in Patients With Arrhythmogenic Cardiomyopathy. JACC Clin. Electrophysiol. 2018, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Hollan, I.; Meroni, P.L.; Ahearn, J.M.; Cohen Tervaert, J.W.; Curran, S.; Goodyear, C.S.; Hestad, K.A.; Kahaleh, B.; Riggio, M.; Shields, K.; et al. Cardiovascular Disease in Autoimmune Rheumatic Diseases. Autoimmun. Rev. 2013, 12, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Nicola, P.J.; Maradit-Kremers, H.; Roger, V.L.; Jacobsen, S.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. The Risk of Congestive Heart Failure in Rheumatoid Arthritis: A Population-Based Study over 46 Years. Arthritis Rheum. 2005, 52, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; Crowson, C.S.; Gabriel, S.E. Cardiovascular Comorbidity in Rheumatic Diseases. Heart Fail. Clin. 2014, 10, 339–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maradit-Kremers, H.; Nicola, P.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. Cardiovascular Death in Rheumatoid Arthritis: A Population-Based Study. Arthritis Rheum. 2005, 52, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1294. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I. Pharmacogenomics, Pharmacokinetics and Pharmacodynamics: Interaction with Biological Differences between Men and Women. Br. J. Pharmacol. 2014, 171, 580–594. [Google Scholar] [CrossRef] [Green Version]
- Jochmann, N.; Stangl, K.; Garbe, E.; Baumann, G.; Stangl, V. Female-Specific Aspects in the Pharmacotherapy of Chronic Cardiovascular Diseases. Eur. Heart J. 2005, 26, 1585–1595. [Google Scholar] [CrossRef]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender Differences in the Effects of Cardiovascular Drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex Differences in Drug Disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolarz, A.J.; Rusch, N.J. Gender Differences in Cardiovascular Drugs. Cardiovasc. Drugs Ther. 2015, 29, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Cadeddu, C.; Franconi, F.; Cassisa, L.; Campesi, I.; Pepe, A.; Cugusi, L.; Maffei, S.; Gallina, S.; Sciomer, S.; Mercuro, G. Arterial Hypertension in the Female World: Pathophysiology and Therapy. J. Cardiovasc. Med. 2016, 17, 229–236. [Google Scholar] [CrossRef]
- Jackson, A.M.; Jhund, P.S.; Solomon, S.D.; McMurray, J.J.V. Response by Jackson et al. to Letter Regarding Article, “Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF”. Circulation 2020, 142, 338–351. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional Variation in Patients and Outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, T.J.; Mellor, H.R.; Roberts, R.R.A. Gender Differences in Drug Toxicity. Trends Pharmacol. Sci. 2010, 31, 108–114. [Google Scholar] [CrossRef]
- Crousillat, D.R.; Ibrahim, N.E. Sex Differences in the Management of Advanced Heart Failure. Curr. Treat. Options Cardio Med. 2018, 20, 88. [Google Scholar] [CrossRef]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of Patient Sex and Gender on Medication Use, Adherence, and Prescribing Alignment with Guidelines. J. Women’s Health 2014, 23, 112–119. [Google Scholar] [CrossRef]
- Leporini, C.; De Sarro, G.; Russo, E. Adherence to Therapy and Adverse Drug Reactions: Is There a Link? Expert. Opin. Drug. Saf. 2014, 13, 41–55. [Google Scholar] [CrossRef]
- Lei, L.; Mao, Y. Hormone Treatments in Congestive Heart Failure. J. Int. Med. Res. 2018, 46, 2063–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study Cohort/ First Author Name | Country | Study Design | Year of Publication | Women, n (%) | Key Messages |
|---|---|---|---|---|---|
| The LIFE study [2] | Denmark, Finland, Iceland, Norway, Sweden, the United Kingdom, and the United States | Retrospective cohort study | 2001 | 391 (41%) | Female sex is an independent predictor of higher systolic LV function in hypertensive patients with electrocardiographic LV hypertrophy. |
| Alfakih et al. [3] | United Kingdom | Retrospective cohort study | 2003 | 30 (50%) | Sex differences in the LV and RV volumes measured by CMR were statistically significant apart from the LVEF. |
| Framingham Heart Study Offspring Cohort [4] | United States | RCT, post-hoc analysis | 2002 | 79 (40%) | Cardiovascular magnetic resonance measures of LV volumes, mass, and linear dimensions differ significantly according to sex and body size. All unadjusted LV parameters were significantly greater in men than in women (p 0.001) but no significant differences in LVEF between men (69%) and women (70%). |
| The Dallas Heart Study [5] | United States | Prospective cohort study | 2006 | 1435 (55%) | Women have higher LVEF than men, reflecting a higher stroke volume for a given EDV. LVEF was higher by 5% in women compared to men. |
| Suthahar et al. [6] | Netherlands, the United States | Retrospective cohort study | 2020 | 12,087 (53%) | Subtle sex-related differences in the prognostic value of individual biomarkers. |
| Countouris et al. [7] | United States | Prospective cohort study | 2021 | 132 (102, (77%) normotensive, 30 (22%) HPD history | Women with HDP are more likely to have evidence of increased LV wall thickness, remodeling, and abnormal diastolic function in the decade after pregnancy and thus require closer surveillance and early and targeted therapies for CVD prevention. |
| Williams et al. [8] | United States | Meta-analysis | 2021 | 2,532,515 (100%) women were included in the study: 2,404,486 (94%) without and 128,029 (5%) with preeclampsia/eclampsia. | Preeclampsia/eclampsia is an independent risk factor for future hospitalizations for HfpEF. |
| Hall et al. [9] | United States | Retrospective cohort study | 2017 | 28,516 (100%) | Premature menopause is associated with a higher risk of incident HF, and null parity is associated with a higher risk for incident HF with preserved ejection fraction. |
| BIOSTAT-CHF study [10] | Europe | Prospective study cohort | 2019 | 1819 (24%) | β-blockers were more frequently used at baseline than were ACE inhibitors or ARBs, and more often in men than in women. |
| PARAGON-HF trial [11] | Europe, North America, Latin America, Asia-Pacific | RCT | 2019 | 4796 (51.7%) | Even when HfpEF is the predominant phenotype in women, they were significantly less likely than men to be treated with a nitrate and an MRA. |
| Merrill et al. [12] | United States, Argentina, Brazil, Canada, Russia, Georgia | RCT, post-hoc analysis | 2019 | 1767 (49.9%) | Women are significantly more likely to be taking calcium channel blockers, whereas men are more likely to be taking β-blockers. |
| Dewan et al. [13] | North America, Latin America, Europe, Russia, Asia-Pacific | RCT, post-hoc analysis | 2019 | 15,415 (21%) | Women are more often treated with digitalis and ARBs, but less likely with an ACEI compared with men. |
| Mahmoud et al. [14] | United States | Meta-analysis | 2017 | 58% | SGLT2i progressive decrement in benefit in women. |
| Rodenburg et al. [15] | Netherlands | Retrospective cohort study | 14,207 (54%) | Serious ADRs occur more in women especially with diuretics and cardiac glycosides. | |
| Zusterzeel et al. [16] | United States | Meta-analysis | 2014 | 4076 (22%) | Women with LBBB benefited from CRT-D at a shorter QRS duration than men with LBBB. |
| Topic | Gaps in Evidence |
|---|---|
| Heart failure classification | |
| LVEF thresholds | Sex-based threshold values in LVEF |
| NPs levels | Relevance of sex and hormonal status in establishing The normal reference range for NPs |
| Sex-specific risk factors | |
| HDPs and APOs | Prognostic role of preeclampsia/eclampsia |
| Premature menopause | HRT and SERMs |
| Cardiomyopathies | Treatment of PPCM and inflammatory cardiomyopathies |
| Heart failure management | |
| Guideline-directed medical therapy | Sex-specific therapeutic options and drug dosages |
| CIED | Sex-specific QRS duration thresholds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arata, A.; Ricci, F.; Khanji, M.Y.; Mantini, C.; Angeli, F.; Aquilani, R.; Di Baldassarre, A.; Renda, G.; Mattioli, A.V.; Nodari, S.; et al. Sex Differences in Heart Failure: What Do We Know? J. Cardiovasc. Dev. Dis. 2023, 10, 277. https://doi.org/10.3390/jcdd10070277
Arata A, Ricci F, Khanji MY, Mantini C, Angeli F, Aquilani R, Di Baldassarre A, Renda G, Mattioli AV, Nodari S, et al. Sex Differences in Heart Failure: What Do We Know? Journal of Cardiovascular Development and Disease. 2023; 10(7):277. https://doi.org/10.3390/jcdd10070277
Chicago/Turabian StyleArata, Allegra, Fabrizio Ricci, Mohammed Y. Khanji, Cesare Mantini, Francesco Angeli, Roberta Aquilani, Angela Di Baldassarre, Giulia Renda, Anna Vittoria Mattioli, Savina Nodari, and et al. 2023. "Sex Differences in Heart Failure: What Do We Know?" Journal of Cardiovascular Development and Disease 10, no. 7: 277. https://doi.org/10.3390/jcdd10070277







