Myocardial Blush Grade Predicts Postoperative Atrial Fibrillation following Mitral Valve Replacement: A Novel Perspective
Abstract
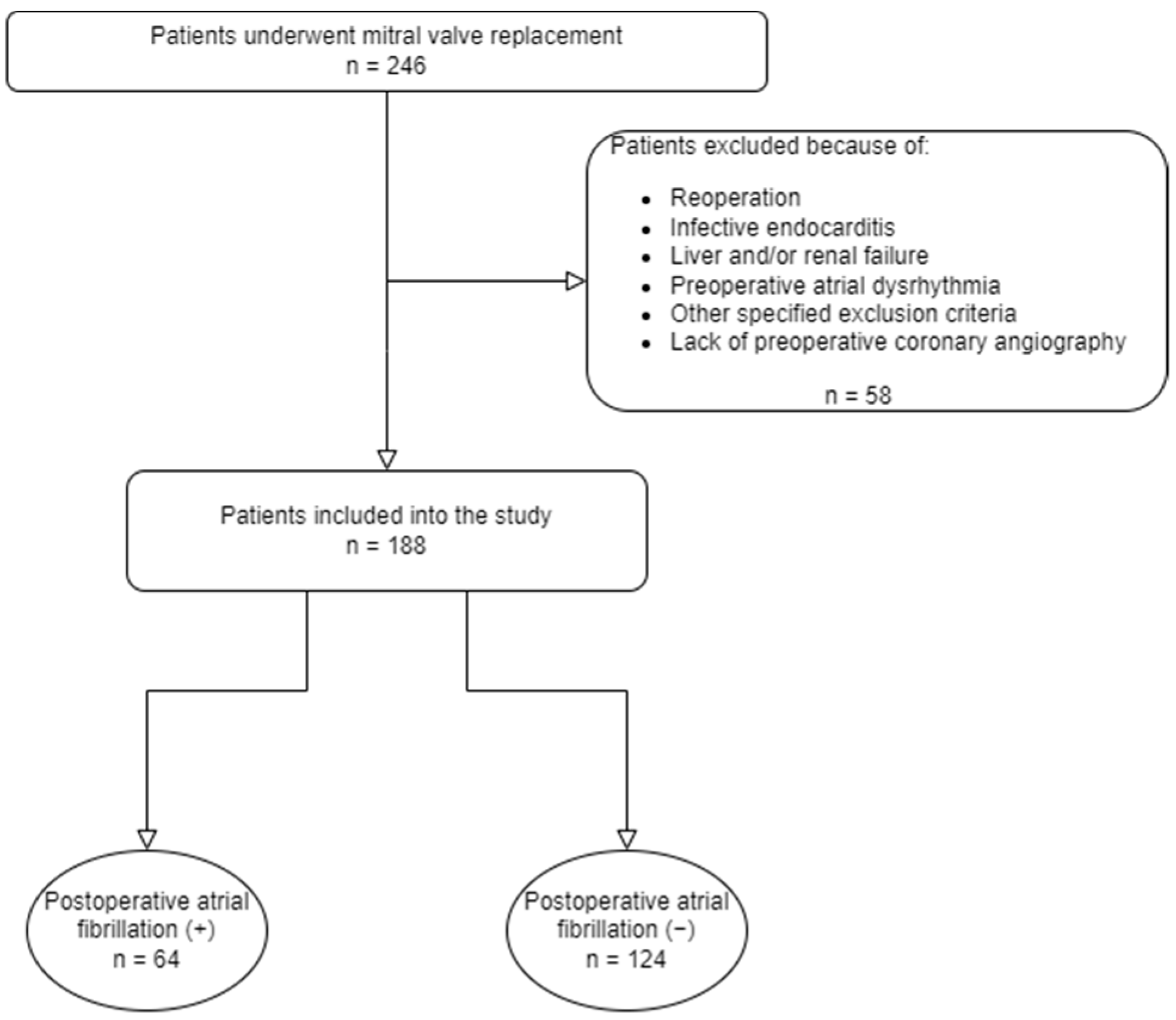
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Evaluation of Myocardial Blush Grading
2.3. Postoperative Atrial Fibrillation
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Echahidi, N.; Pibarot, P.; O’Hara, G.; Mathieu, P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 2008, 51, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Maisel, W.H.; Rawn, J.D.; Stevenson, W.G. Atrial fibrillation after cardiac surgery. Ann. Intern. Med. 2001, 135, 1061–1073. [Google Scholar] [CrossRef]
- Bramer, S.; van Straten, A.H.; Soliman Hamad, M.A.; Berreklouw, E.; Martens, E.J.; Maessen, J.G. The impact of new-onset postoperative atrial fibrillation on mortality after coronary artery bypass grafting. Ann. Thorac. Surg. 2010, 90, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Chami, M.F.; Kilgo, P.; Thourani, V.; Lattouf, O.M.; Delurgio, D.B.; Guyton, R.A.; Leon, A.R.; Puskas, J.D. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J. Am. Coll. Cardiol. 2010, 55, 1370–1376. [Google Scholar] [CrossRef] [Green Version]
- Mathew, J.P.; Parks, R.; Savino, J.S.; Friedman, A.S.; Koch, C.; Mangano, D.T.; Browner, W.S. Atrial fibrillation following coronary artery bypass graft surgery: Predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA 1996, 276, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.S.; Eagle, K.A.; Buckley, M.J.; DeSanctis, R.W. Atrial fibrillation following coronary artery bypass surgery. Prog. Cardiovasc. Dis. 1989, 31, 367–378. [Google Scholar] [CrossRef]
- Mostafa, A.; El-Haddad, M.A.; Shenoy, M.; Tuliani, T. Atrial fibrillation post cardiac bypass surgery. Avicenna J. Med. 2012, 2, 65–70. [Google Scholar] [CrossRef]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Ducceschi, V.; D’Andrea, A.; Liccardo, B.; Alfieri, A.; Sarubbi, B.; De Feo, M.; Santangelo, L.; Cotrufo, M. Perioperative clinical predictors of atrial fibrillation occurrence following coronary artery surgery. Eur. J. Cardio-Thorac. Surg. 1999, 16, 435–439. [Google Scholar] [CrossRef] [Green Version]
- van ‘t Hof, A.W.; Liem, A.; Suryapranata, H.; Hoorntje, J.C.; de Boer, M.J.; Zijlstra, F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef] [Green Version]
- Gulec, S.; Atmaca, Y.; Kilickap, M.; Akyurek, O.; Aras, O.; Oral, D. Angiographic assessment of myocardial perfusion in patients with isolated coronary artery ectasia. Am. J. Cardiol. 2003, 91, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, Y.; Ozdemir, A.O.; Ozdol, C.; Oguz, D.; Gulec, S.; Kumbasar, D.; Erol, C. Angiographic evaluation of myocardial perfusion in patients with syndrome, X. Am. J. Cardiol. 2005, 96, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Esenboga, K.; Baskovski, E.; Sahin, E.; Ozyuncu, N.; Tan, T.S.; Candemir, B.; Turhan, S.; Tutar, E. Assessment of Myocardial Perfusion by Angiographic Methods in Tortuous Coronary Arteries. Angiology 2020, 71, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Wijesurendra, R.S.; Liu, A.; Notaristefano, F.; Ntusi, N.A.B.; Karamitsos, T.D.; Bashir, Y.; Ginks, M.; Rajappan, K.; Betts, T.R.; Jerosch-Herold, M.; et al. Myocardial Perfusion is Impaired and Relates to Cardiac Dysfunction in Patients with Atrial Fibrillation Both before and after Successful Catheter Ablation. J. Am. Heart Assoc. 2018, 7, e009218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariscalco, G.; Engström, K.G. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann. Thorac. Surg. 2009, 88, 1871–1876. [Google Scholar] [CrossRef]
- Bramer, S.; van Straten, A.H.; Soliman Hamad, M.A.; Berreklouw, E.; van den Broek, K.C.; Maessen, J.G. Body mass index predicts new-onset atrial fibrillation after cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2011, 40, 1185–1190. [Google Scholar] [CrossRef]
- Shingu, Y.; Kubota, S.; Wakasa, S.; Ooka, T.; Tachibana, T.; Matsui, Y. Postoperative atrial fibrillation: Mechanism, prevention, and future perspective. Surg. Today 2012, 42, 819–824. [Google Scholar] [CrossRef]
- Omae, T.; Kanmura, Y. Management of postoperative atrial fibrillation. J. Anesth. 2012, 26, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Magne, J.; Salerno, B.; Mohty, D.; Serena, C.; Rolle, F.; Piccardo, A.; Echahidi, N.; Le Guyader, A.; Aboyans, V. Echocardiography is useful to predict postoperative atrial fibrillation in patients undergoing isolated coronary bypass surgery: A prospective study. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 104–113. [Google Scholar] [CrossRef]
- Osranek, M.; Fatema, K.; Qaddoura, F.; Al-Saileek, A.; Barnes, M.E.; Bailey, K.R.; Gersh, B.J.; Tsang, T.S.; Zehr, K.J.; Seward, J.B. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: A prospective study. J. Am. Coll. Cardiol. 2006, 48, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Romeo, F.; Glauber, M.; de Siena, P.M.; Caputo, M.; Angelini, G.D. Preoperative anemia increases mortality and postoperative morbidity after cardiac surgery. J. Cardiothorac. Surg. 2014, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Alameddine, A.K.; Visintainer, P.; Alimov, V.K.; Rousou, J.A. Blood transfusion and the risk of atrial fibrillation after cardiac surgery. J. Card. Surg. 2014, 29, 593–599. [Google Scholar] [CrossRef]
- Porto, I.; Hamilton-Craig, C.; Brancati, M.; Burzotta, F.; Galiuto, L.; Crea, F. Angiographic assessment of microvascular perfusion--myocardial blush in clinical practice. Am. Heart J. 2010, 160, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, Y.; Duzen, V.; Ozdol, C.; Altin, T.; Tulunay, C.; Ertas, F.; Erol, C. Total blush score: A new index for the assessment of microvascular perfusion in idiopathic dilated cardiomyopathy. Coron. Artery Dis. 2008, 19, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Parwani, A.S.; Boldt, L.H.; Huemer, M.; Wutzler, A.; Blaschke, D.; Rolf, S.; Möckel, M.; Haverkamp, W. Atrial fibrillation-induced cardiac troponin I release. Int. J. Cardiol. 2013, 168, 2734–2737. [Google Scholar] [CrossRef] [PubMed]
- Range, F.T.; Schäfers, M.; Acil, T.; Schäfers, K.P.; Kies, P.; Paul, M.; Hermann, S.; Brisse, B.; Breithardt, G.; Schober, O.; et al. Impaired myocardial perfusion and perfusion reserve associated with increased coronary resistance in persistent idiopathic atrial fibrillation. Eur. Heart J. 2007, 28, 2223–2230. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C.; Bellocci, F.; Morgante, E.; Russo, M.A.; Maseri, A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997, 96, 1180–1184. [Google Scholar] [CrossRef]
- Boldt, A.; Wetzel, U.; Lauschke, J.; Weigl, J.; Gummert, J.; Hindricks, G.; Kottkamp, H.; Dhein, S. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004, 90, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Skalidis, E.I.; Hamilos, M.I.; Karalis, I.K.; Chlouverakis, G.; Kochiadakis, G.E.; Vardas, P.E. Isolated atrial microvascular dysfunction in patients with lone recurrent atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 2053–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesurendra, R.S.; Casadei, B. Atrial fibrillation: Effects beyond the atrium? Cardiovasc. Res. 2015, 105, 238–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Study Population (n = 188) | PoAF (+) (n = 64) | PoAF (−) (n = 124) | p Value |
|---|---|---|---|---|
| Age (years) | 56.69 ± 8.9 | 58.8 ± 6.07 | 55.6 ± 9.9 | 0.007 |
| Male gender | 74 (39.4) | 26 (40.6) | 48 (38.7) | 0.799 |
| BMI (kg/m2) | 26.36 ± 3.54 | 26.29 ± 3.53 | 26.4 ± 3.56 | 0.831 |
| Hypertension | 43 (22.9) | 19 (29.7) | 24 (19.4) | 0.157 |
| Hyperlipidemia | 23 (12.2) | 9 (14.1) | 14 (11.3) | 0.753 |
| Diabetes mellitus | 25 (13.3) | 11 (17.2) | 14 (11.3) | 0.367 |
| COPD | 40 (21.3) | 16 (25) | 24 (19.4) | 0.479 |
| Current smoking | 22 (11.7) | 9 (14.1) | 13 (10.5) | 0.628 |
| Preoperative NYHA status | 1.97 ± 0.58 | 2.02 ± 0.49 | 1.94 ± 0.63 | 0.387 |
| Preoperative ACEi/ARB use | 40 (21.3) | 17 (26.6) | 23 (18.5) | 0.278 |
| Preoperative statin use | 22 (11.7) | 9 (14.1) | 13 (10.5) | 0.628 |
| Preoperative EF | 56.5 (49–63.75) | 55 (50–60) | 60 (48–65) | 0.34 |
| Preoperative LVEDD | 5.65 (5.3–6.1) | 5.8 (5.4–6.2) | 5.6 (5.2–6.1) | 0.04 |
| Preoperative LVESD | 4.1 (3.8–4.4) | 4.1 (4–4.5) | 4 (3.73–4.3) | 0.045 |
| Preoperative SPAP (mmHg) | 45 (38–53.5) | 44.5 (35.25–50) | 45 (38–54.75) | 0.669 |
| Preoperative LA diameter (cm) | 5.15 (4.63–6.1) | 5.9 (5.2–6.47) | 4.9 (4.5–5.8) | <0.001 |
| Preoperative WBC (×109/L) | 8.34 ± 1.65 | 8.49 ± 1.78 | 8.27 ± 1.59 | 0.393 |
| Preoperative hemoglobin (g/dL) | 13.84 ± 1.25 | 12.64 ± 0.89 | 14.46 ± 0.91 | <0.001 |
| Preoperative ALT (U/L) | 41.5 (35–48) | 43 (36–51) | 41 (35–46.75) | 0.15 |
| Preoperative AST (U/L) | 36 (31–44.75) | 35 (31–43.7) | 37 (31.25–45) | 0.503 |
| Preoperative urea (mg/dL) | 35 (29–43) | 35 (30.25–42.75) | 35 (28.25–43) | 0.705 |
| Preoperative creatinine (mg/dL) | 0.83 (0.76–0.9) | 0.82 (0.76–0.86) | 0.83 (0.77–0.98) | 0.074 |
| Preoperative TSH (mIU/L) | 2.3 (1.66–2.8) | 2.3 (1.53–2.6) | 2.35 (1.7–3.1) | 0.101 |
| Operation type | ||||
| MVR | 134 (71.3) | 47 (73.4) | 87 (70.2) | 0.764 |
| MVR + tricuspid annuloplasty | 54 (28.7) | 17 (26.6) | 37 (29.8) | |
| Prosthetic valve size (mm) | 29 (27–31) | 29 (27–31) | 29 (27–31) | 0.088 |
| CPB time (minutes) | 102.89 ± 20.22 | 108.19 ± 14.17 | 100.15 ± 22.29 | 0.003 |
| X-clamp time (minutes) | 74.66 ± 16.13 | 78.81 ± 10.96 | 72.52 ± 17.9 | 0.003 |
| Postoperative inotrope | 35 (18.6) | 14 (21.9) | 21 (16.9) | 0.531 |
| Postop hospital length (days) | 6.44 ± 1.02 | 7.2 ± 0.93 | 6.05 ± 0.83 | <0.001 |
| PoAF (+) (n = 64) | PoAF (−) (n = 124) | p Value | |
|---|---|---|---|
| Left anterior descending artery | |||
| 1 | 1 (1.6) | - | |
| 2 | 14 (21.9) | 3 (2.4) | <0.001 |
| 3 | 49 (76.6) | 121 (97.6) | |
| Circumflex artery | |||
| 1 | 4 (6.3) | - | |
| 2 | 8 (12.5) | 4 (3.2) | <0.001 |
| 3 | 52 (81.3) | 120 (96.8) | |
| Right coronary artery | |||
| 2 | 11 (17.2) | 6 (4.8) | 0.005 |
| 3 | 53 (82.8) | 118 (95.2) |
| Study Population (n = 188) | PoAF (+) (n = 64) | PoAF (−) (n = 124) | p Value | |
|---|---|---|---|---|
| Left anterior descending artery | 2.9 ± 0.32 | 2.75 ± 0.47 | 2.98 ± 0.15 | <0.001 |
| Circumflex artery | 2.89 ± 0.37 | 2.75 ± 0.56 | 2.97 ± 0.18 | 0.004 |
| Right coronary artery | 2.91 ± 0.29 | 2.83 ± 0.38 | 2.95 ± 0.21 | 0.018 |
| Total blush score | 8.7 ± 0.61 | 8.33 ± 0.84 | 8.9 ± 0.31 | <0.001 |
| Difference | |||||
|---|---|---|---|---|---|
| n | Agreement | MBG 1 | MBG 2 | MBG 3 | |
| Intraobserver variability | 40 | 38 (95) | 1 (2.5) | 1 (2.5) | - |
| Interobserver variability | 40 | 38 (95) | 2 (5) | - | - |
| Variable | Univariate Analysis | ||
|---|---|---|---|
| OR | 95% CI | p | |
| Age | 1.042 | (1.006–1.078) | 0.021 |
| Hypertension | 1.759 | (0.876–3.533) | 0.112 |
| Preoperative LVEDD | 1.648 | (0.968–2.806) | 0.066 |
| Preoperative LVESD | 1.768 | (0.992–3.15) | 0.053 |
| Preoperative LA diameter | 2.758 | (1.869–4.071) | <0.001 |
| Preoperative hemoglobin | 0.091 | (0.045–0.185) | <0.001 |
| CPB time | 1.021 | (1.005–1.037) | 0.011 |
| X-clamp time | 1.025 | (1.005–1.045) | 0.012 |
| Abnormal MBG score for LAD | 12.347 | (3.422–44.552) | <0.001 |
| Abnormal MBG score for Cx | 6.923 | (2.133–22.473) | 0.001 |
| Abnormal MBG score for RCA | 4.082 | (1.434–11.62) | 0.008 |
| Abnormal total blush score | 44.489 | (5.758–343.728) | <0.001 |
| Variable | Multivariate Analysis | ||
|---|---|---|---|
| OR | 95% CI | p | |
| Preoperative LA diameter | 2.057 | (1.166–3.63) | 0.013 |
| Preoperative hemoglobin | 0.12 | (0.058–0.245) | <0.001 |
| CPB time | 1.025 | (0.996–1.055) | 0.096 |
| Abnormal total blush score | 15.1 | (1.602–142.339) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çiçek, Ö.F.; Esenboğa, K.; Yalçın, M.U.; Durdu, M.S.; Altunkeser, B.B.; Büyükateş, M. Myocardial Blush Grade Predicts Postoperative Atrial Fibrillation following Mitral Valve Replacement: A Novel Perspective. J. Cardiovasc. Dev. Dis. 2023, 10, 275. https://doi.org/10.3390/jcdd10070275
Çiçek ÖF, Esenboğa K, Yalçın MU, Durdu MS, Altunkeser BB, Büyükateş M. Myocardial Blush Grade Predicts Postoperative Atrial Fibrillation following Mitral Valve Replacement: A Novel Perspective. Journal of Cardiovascular Development and Disease. 2023; 10(7):275. https://doi.org/10.3390/jcdd10070275
Chicago/Turabian StyleÇiçek, Ömer Faruk, Kerim Esenboğa, Muhammed Ulvi Yalçın, Mustafa Serkan Durdu, Bülent Behlül Altunkeser, and Mustafa Büyükateş. 2023. "Myocardial Blush Grade Predicts Postoperative Atrial Fibrillation following Mitral Valve Replacement: A Novel Perspective" Journal of Cardiovascular Development and Disease 10, no. 7: 275. https://doi.org/10.3390/jcdd10070275






