Nomogram and Risk Calculator for Postoperative Tracheostomy after Heart Valve Surgery
Abstract
:1. Introduction
2. Methods
2.1. Ethical Statement
2.2. Study Population
2.3. Data Collection
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Development of the Nomogram and Risk Calculator
3.3. Validation and Assessment of the Model
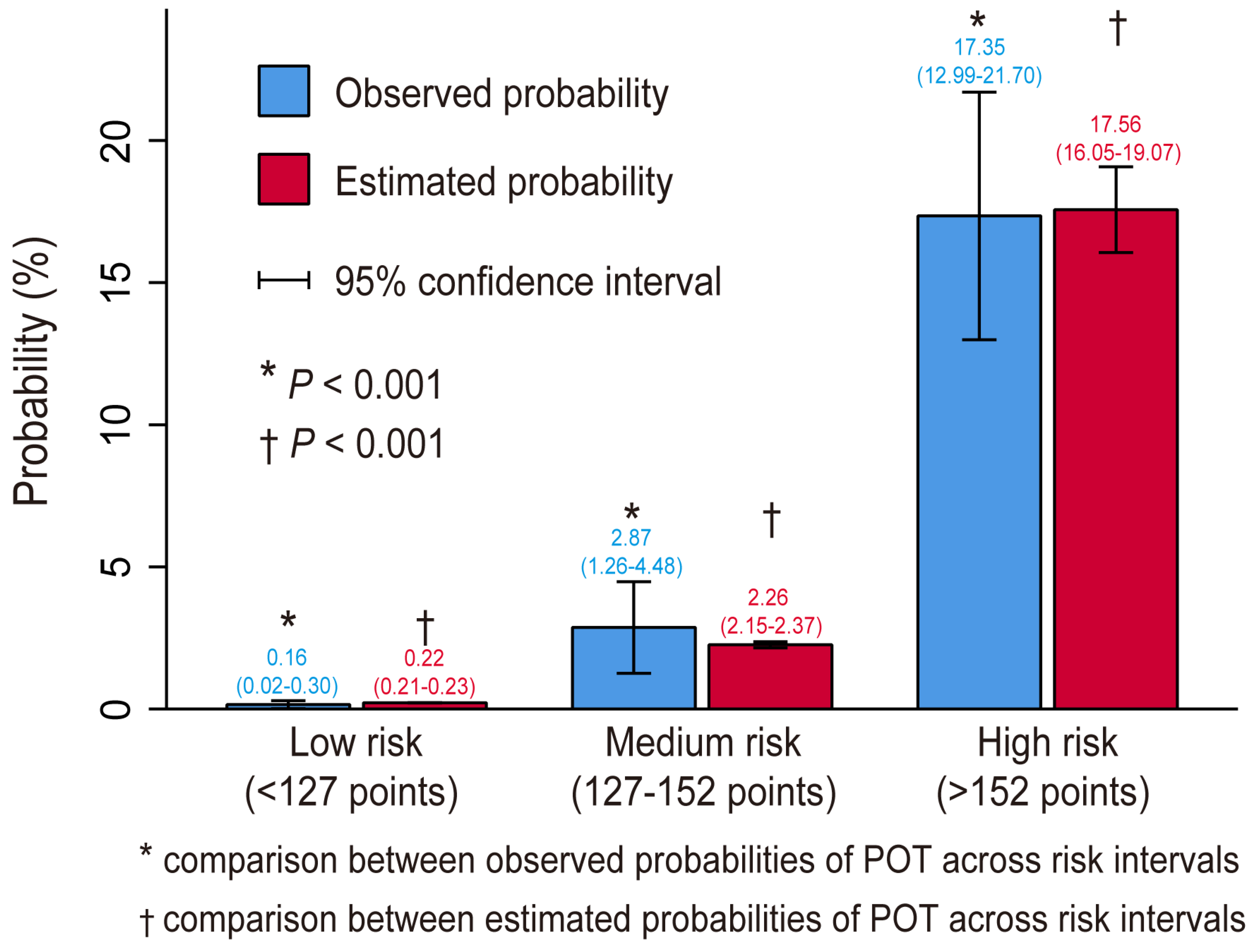
3.4. Risk Stratification
3.5. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the receiver operating characteristic curve |
| CABG | coronary artery bypass grafting |
| CI | confidence interval |
| HVS | heart valve surgery |
| ICU | intensive care unit |
| NYHA | New York Heart Association |
| OR | odds ratio |
| RBC | red blood cell |
| ROC | receiver operating characteristic curve |
| POT | postoperative tracheostomy |
References
- Kohne, J.G.; MacLaren, G.; Rider, E.; Carr, B.D.; Mallory, P.; Gebremariam, A.; Friedman, M.L.; Barbaro, R.P. Tracheostomy Practices and Outcomes in Children during Respiratory Extracorporeal Membrane Oxygenation. Pediatr. Crit. Care Med. 2022. Publish Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Cotts, T.; Hirsch, J.; Thorne, M.; Gajarski, R. Tracheostomy after pediatric cardiac surgery: Frequency, indications, and outcomes. J. Thorac. Cardiovasc. Surg. 2011, 141, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Sareh, S.; Toppen, W.; Ugarte, R.; Sanaiha, Y.; Hadaya, J.; Seo, Y.J.; Aguayo, E.; Shemin, R.; Benharash, P. Impact of Early Tracheostomy on Outcomes after Cardiac Surgery: A National Analysis. Ann. Thorac. Surg. 2021, 111, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.C.; Bush, W.J.; Mella, J.S.; Fridell, J.A.; Ekser, B.; Mihaylov, P.; Kubal, C.A.; Mangus, R.S. Tracheostomy Post Liver Transplant: Predictors, Complications, and Outcomes. Ann. Transpl. 2020, 25, e920630-1. [Google Scholar] [CrossRef]
- Songdechakraiwut, T.; Aftab, M.; Chatterjee, S.; Green, S.Y.; Price, M.D.; Preventza, O.; de la Cruz, K.I.; LeMaire, S.A.; Coselli, J.S. Tracheostomy after Thoracoabdominal Aortic Aneurysm Repair: Risk Factors and Outcomes. Ann. Thorac. Surg. 2019, 108, 778–784. [Google Scholar] [CrossRef]
- Jarosz, K.; Kubisa, B.; Andrzejewska, A.; Mrówczyńska, K.; Hamerlak, Z.; Bartkowska-Sniatkowska, A. Adverse outcomes after percutaneous dilatational tracheostomy versus surgical tracheostomy in intensive care patients: Case series and literature review. Ther. Clin. Risk Manag. 2017, 13, 975–981. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Du, Y.; Song, Y.; Le, S.; Wang, H.; Zhang, A.; Huang, X.; Wu, L.; Du, X. A Predictive Scoring Model for Postoperative Tracheostomy in Patients Who Underwent Cardiac Surgery. Front. Cardiovasc. Med. 2022, 8, 799605. [Google Scholar] [CrossRef]
- Robba, C.; Galimberti, S.; Graziano, F.; Wiegers, E.J.A.; Lingsma, H.F.; Iaquaniello, C.; Stocchetti, N.; Menon, D.; Citerio, G. Tracheostomy practice and timing in traumatic brain-injured patients: A CENTER-TBI study. Intens. Care Med. 2020, 46, 983–994. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Song, Y.; Wang, H.; Zhang, A.; Wu, L.; Huang, X.; Ye, P.; Du, X. Predictors and outcomes of postoperative tracheostomy in patients undergoing acute type A aortic dissection surgery. Bmc Cardiovasc. Disor. 2022, 22, 94. [Google Scholar] [CrossRef]
- Mastropietro, C.W.; Benneyworth, B.D.; Turrentine, M.; Wallace, A.S.; Hornik, C.P.; Jacobs, J.P.; Jacobs, M.L. Tracheostomy after Operations for Congenital Heart Disease: An Analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann. Thorac. Surg. 2016, 101, 2285–2292. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, R.; Chen-Yoshikawa, T.F.; Hamaji, M.; Kawaguchi, A.; Kayawake, H.; Hijiya, K.; Motoyama, H.; Aoyama, A.; Date, H. Effect of early tracheostomy on clinical outcomes in critically ill lung transplant recipients. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Ma, W.; Liu, S.; Zhu, J.; Sun, L.; Lu, J.; Cheng, W. Predictors of Prolonged Mechanical Ventilation in Adults after Acute Type-A Aortic Dissection Repair. J. Cardiothor. Vasc. An. 2017, 31, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Comas, G.M.; Leshnower, B.G.; Halkos, M.E.; Thourani, V.H.; Puskas, J.D.; Guyton, R.A.; Kilgo, P.D.; Chen, E.P. Acute Type A Dissection: Impact of Antegrade Cerebral Perfusion under Moderate Hypothermia. Ann. Thorac. Surg. 2013, 96, 2135–2141. [Google Scholar] [CrossRef]
- Salna, M.; Tipograf, Y.; Liou, P.; Chicotka, S.; Biscotti, M.; Agerstrand, C.; Abrams, D.; Brodie, D.; Bacchetta, M. Tracheostomy Is Safe During Extracorporeal Membrane Oxygenation Support. Asaio J. 2020, 66, 652–656. [Google Scholar] [CrossRef]
- Angel, L.F.; Amoroso, N.E.; Rafeq, S.; Mitzman, B.; Goldenberg, R.; Shekar, S.P.; Troxel, A.B.; Zhang, Y.; Chang, S.H.; Kwak, P.; et al. Percutaneous Dilational Tracheostomy for Coronavirus Disease 2019 Patients Requiring Mechanical Ventilation. Crit. Care Med. 2021. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Tripathi, S.; Swayampakula, A.K.; Deshpande, G.G.; Astle, M.; Wang, Y.; Welke, K.F. Illustration of the current practice and outcome comparison of early versus late tracheostomy after pediatric ECMO. Int. J. Artif. Organs 2020, 43, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Evans, P.T.; Prendergast, K.M.; Schneeberger, S.J.; Henson, C.P.; McGrane, S.; Kopp, E.B.; Collins, N.E.; Guillamondegui, O.D.; Dennis, B.M. Surgical outcomes and complications of bedside tracheostomy in the ICU for patients on ECMO. Perfusion 2022, 37, 26–30. [Google Scholar] [CrossRef]
- Schneider, H.; Hertel, F.; Kuhn, M.; Ragaller, M.; Gottschlich, B.; Trabitzsch, A.; Dengl, M.; Neudert, M.; Reichmann, H.; Wöpking, S. Decannulation and Functional Outcome after Tracheostomy in Patients with Severe Stroke (DECAST): A Prospective Observational Study. Neurocrit. Care 2017, 27, 26–34. [Google Scholar] [CrossRef]
- Xin, M.; Wang, L.; Li, C.; Hou, D.; Wang, H.; Wang, J.; Jia, M.; Hou, X. Percutaneous dilatation tracheotomy in patients on extracorporeal membrane oxygenation after cardiac surgery. Perfusion 2022, 1961970979. [Google Scholar] [CrossRef]
- Okada, M.; Watanuki, H.; Masato, T.; Sugiyama, K.; Futamura, Y.; Matsuyama, K. Impact of Tracheostomy Timing on Outcomes after Cardiovascular Surgery. J. Cardiothor. Vasc. An. 2022, 36, 2335–2338. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Fan, D.; Lu, H.; Xie, D.; Zhang, D.; Jiang, Y.; Li, P.; Teng, H. A Meta-Analysis of the Influencing Factors for Tracheostomy after Cervical Spinal Cord Injury. Biomed Res. Int. 2018, 2018, 5895830. [Google Scholar] [CrossRef] [PubMed]
- Humble, S.S.; Wilson, L.D.; McKenna, J.W.; Leath, T.C.; Song, Y.; Davidson, M.A.; Ehrenfeld, J.M.; Guillamondegui, O.D.; Pandharipande, P.P.; Patel, M.B. Tracheostomy risk factors and outcomes after severe traumatic brain injury. Brain Inj. 2016, 30, 1642–1647. [Google Scholar] [CrossRef]
- Mu, Z.; Zhang, Z. Risk factors for tracheostomy after traumatic cervical spinal cord injury. J. Orthop. Surg. 2019, 27, 920545668. [Google Scholar] [CrossRef] [PubMed]
- Szylinska, A.; Listewnik, M.; Ciosek, Z.; Ptak, M.; Mikolajczyk, A.; Pawlukowska, W.; Rotter, I. The Relationship between Diabetes Mellitus and Respiratory Function in Patients Eligible for Coronary Artery Bypass Grafting. Int. J. Environ. Res. Public Health 2018, 15, 907. [Google Scholar] [CrossRef]
- Lauruschkat, A.H.; Arnrich, B.; Albert, A.A.; Walter, J.A.; Amann, B.; Rosendahl, U.P.; Alexander, T.; Ennker, J. Diabetes mellitus as a risk factor for pulmonary complications after coronary bypass surgery. J. Thorac. Cardiovasc. Surg. 2008, 135, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.C.; Arroliga, A.C.; Walts, P.A.; Feng, J.; Yared, J.; Lytle, B.W.; Blackstone, E.H. Ventilatory dependency after cardiovascular surgery. J. Thorac. Cardiovasc. Surg. 2007, 134, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Filsoufi, F.; Rahmanian, P.B.; Castillo, J.G.; Chikwe, J.; Adams, D.H. Predictors and early and late outcomes of respiratory failure in contemporary cardiac surgery. Chest 2008, 133, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.C.; Magruder, J.T.; Fraser, C.; Suarez-Pierre, A.; Alejo, D.; Bobbitt, J.; Fonner, C.E.; Canner, J.K.; Horvath, K.; Wehberg, K.; et al. Less Is More: Results of a Statewide Analysis of the Impact of Blood Transfusion on Coronary Artery Bypass Grafting Outcomes. Ann. Thorac. Surg. 2018, 105, 129–136. [Google Scholar] [CrossRef]
- Likosky, D.S.; Paone, G.; Zhang, M.; Rogers, M.A.M.; Harrington, S.D.; Theurer, P.F.; DeLucia, A.; Fishstrom, A.; Camaj, A.; Prager, R.L. Red Blood Cell Transfusions Impact Pneumonia Rates after Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2015, 100, 794–801. [Google Scholar] [CrossRef]
- LaPar, D.J.; Crosby, I.K.; Ailawadi, G.; Ad, N.; Choi, E.; Spiess, B.D.; Rich, J.B.; Kasirajan, V.; Fonner, E.; Kron, I.L.; et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2013, 145, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Naeem, S.S.; Sodha, N.R.; Sellke, F.W.; Ehsan, A. Impact of Packed Red Blood Cell and Platelet Transfusions in Patients Undergoing Dissection Repair. J. Surg. Res. 2018, 232, 338–345. [Google Scholar] [CrossRef]
- Mazer, C.D.; Whitlock, R.P.; Fergusson, D.A.; Hall, J.; Belley-Cote, E.; Connolly, K.; Khanykin, B.; Gregory, A.J.; de Médicis, É.; McGuinness, S.; et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N. Engl. J. Med. 2017, 377, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical Practice Guidelines from the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef]
- Ferraris, V.A.; Brown, J.R.; Despotis, G.J.; Hammon, J.W.; Reece, T.B.; Saha, S.P.; Song, H.K.; Clough, E.R.; Shore-Lesserson, L.J.; Goodnough, L.T.; et al. 2011 Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. Ann. Thorac. Surg. 2011, 91, 944–982. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, J.; Lu, Y.; Huang, X.; Du, X.; Sun, F.; Zhao, Y.; Xie, F.; Wang, D.; Liu, C. Prediction and prognosis of reintubation after surgery for Stanford type A aortic dissection. Front. Cardiovasc. Med. 2022, 9, 1004005. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, X.; Wang, H.; Le, S.; Yang, H.; Wang, F.; Du, X. Risk factors for postoperative pneumonia after cardiac surgery: A prediction model. J. Thorac. Dis. 2021, 13, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, Y.; Sun, M.; Huang, X.; Du, X.; Jiao, Z.; Sun, F.; Xie, F. Pneumonia after Cardiovascular Surgery: Incidence, Risk Factors and Interventions. Front. Cardiovasc. Med. 2022, 9, 1727. [Google Scholar] [CrossRef]
- Wang, D.; Ding, X.; Su, Y.; Yang, P.; Du, X.; Sun, M.; Huang, X.; Yue, Z.; Sun, F.; Xie, F.; et al. Incidence, Risk Factors, and Outcomes of Severe Hypoxemia after Cardiac Surgery. Front. Cardiovasc. Med. 2022, 9, 934533. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Wu, J.; Le, S.; Xie, F.; Li, X.; Wang, H.; Huang, X.; Zhang, A.; Du, X. Development and Validation of Nomogram Models for Postoperative Pneumonia in Adult Patients Undergoing Elective Cardiac Surgery. Front. Cardiovasc. Med. 2021, 8, 750828. [Google Scholar] [CrossRef]
- Wang, D.; Abuduaini, X.; Huang, X.; Wang, H.; Chen, X.; Le, S.; Chen, M.; Du, X. Development and validation of a risk prediction model for postoperative pneumonia in adult patients undergoing Stanford type A acute aortic dissection surgery: A case control study. J. Cardiothorac. Surg. 2022, 17, 22. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Sheng, W.; Wang, H.; Le, S.; Huang, X.; Du, X. Development and validation of a nomogram model for pneumonia after redo cardiac surgery. J. Cardiovasc. Med. 2022, 23, 325–334. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.; Wang, H.; Le, S.; Du, X. Clinical risk score for postoperative pneumonia following heart valve surgery. Chin. Med. J.-Peking 2021, 134, 2447–2456. [Google Scholar] [CrossRef]
- Luc, N.F.; Rohner, N.; Girish, A.; Didar, S.S.U.; Neal, M.D.; Sen, G.A. Bioinspired artificial platelets: Past, present and future. Platelets 2022, 33, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Hadaya, J.; Tran, Z.; Dobaria, V.; Madrigal, J.; Xia, Y.; Sanaiha, Y.; Mendelsohn, A.H.; Benharash, P. Incidence and Outcomes of Laryngeal Complications Following Adult Cardiac Surgery: A National Analysis. Dysphagia 2022, 37, 1142–1150. [Google Scholar] [CrossRef]
- Jones, A.; Olverson, G.; Hwang, J.; Bhagat, R.; McGann, K.; Bradburn, K.; Miller, M.; Louis, C. The effect of tracheostomy on extracorporeal membrane oxygenation outcomes. J. Card Surg. 2022, 37, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P. The Role of the N-Terminal of the Prohormone Brain Natriuretic Peptide in Predicting Postoperative Multiple Organ Dysfunction Syndrome. J. Clin. Med. 2022, 11, 7217. [Google Scholar] [CrossRef] [PubMed]
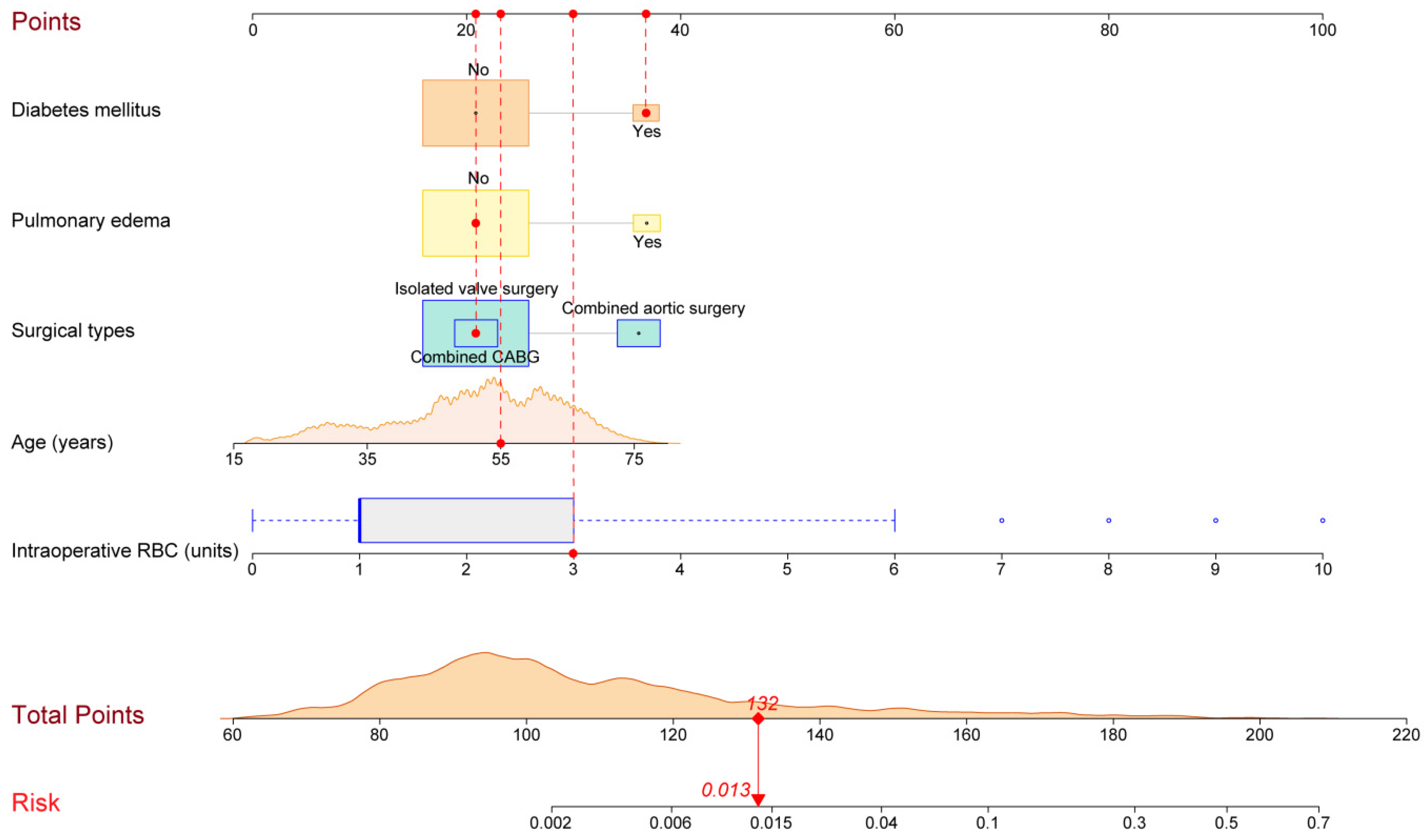
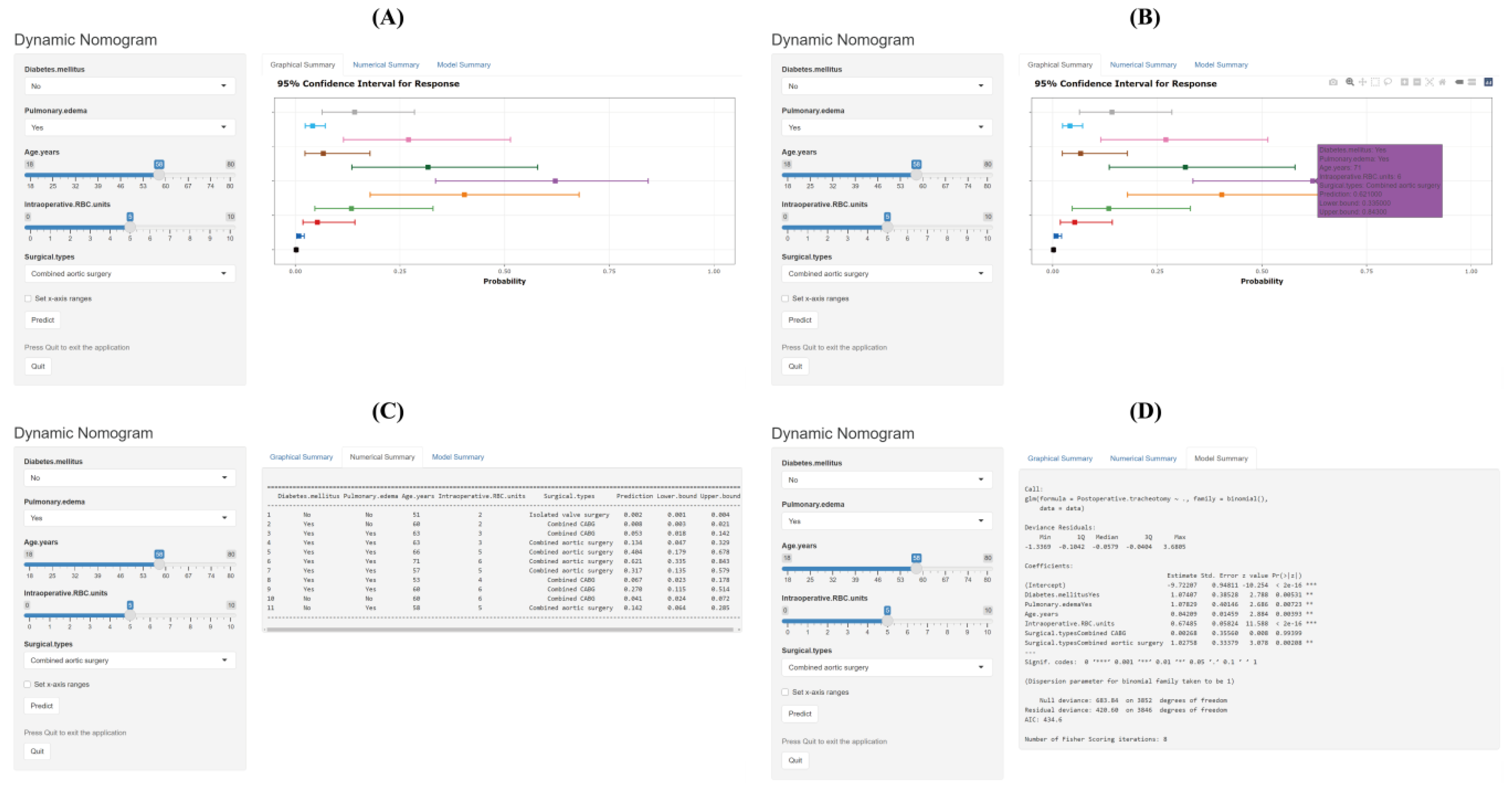
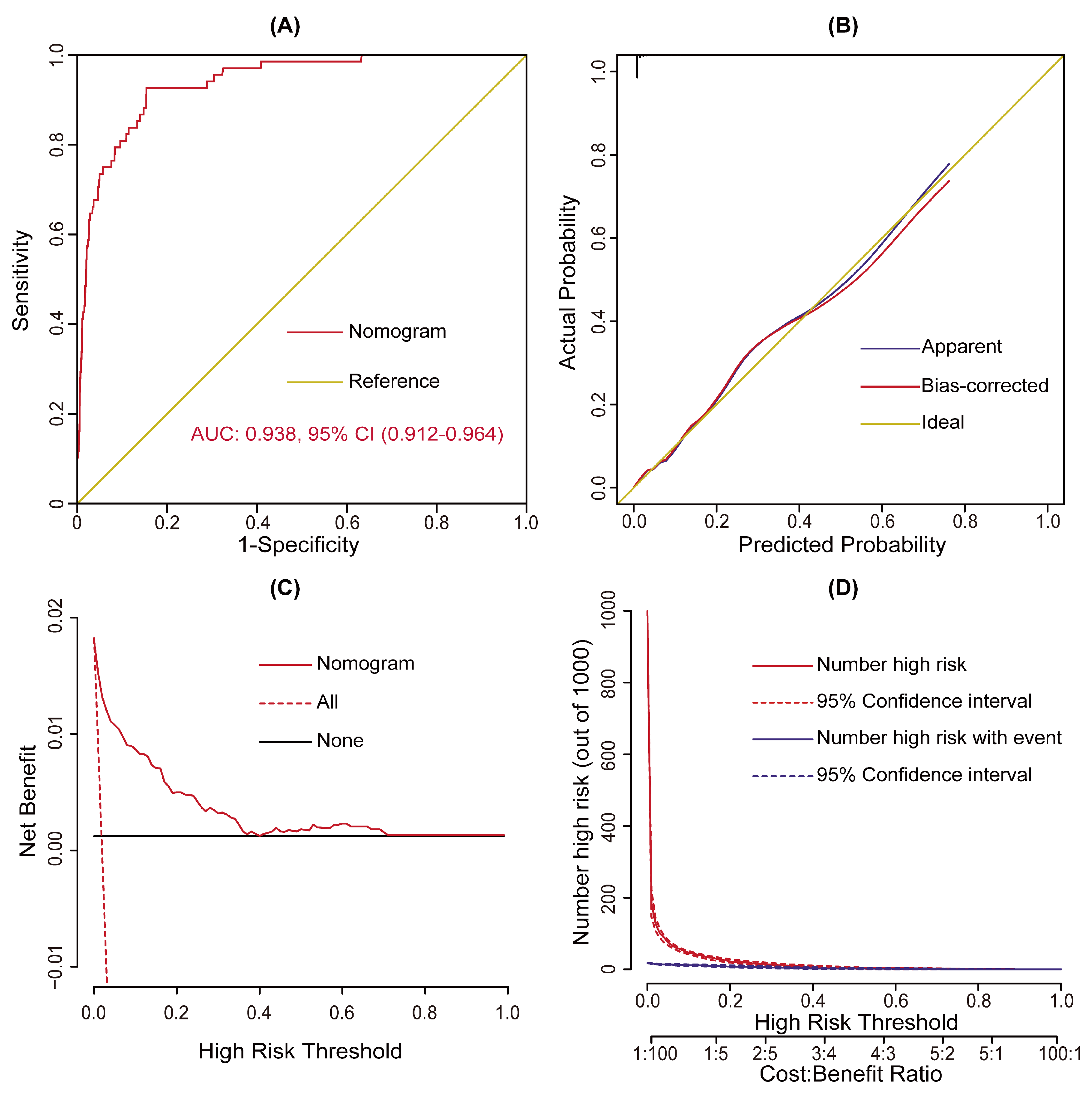
| Characteristic | Without POT n = 3785 (%) | With POT n = 68 (%) | χ2/Z/t | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Male | 2028 (53.6) | 45 (66.2) | 4.264 | 0.039 |
| Age (years) | 51.12 ± 12.53 | 58.82 ± 11.10 | 5.035 | <0.001 |
| Body mass index (kg/m2) | 23.02 ± 3.26 | 23.19 ± 4.16 | 0.323 | 0.748 |
| Smoking history | 1003 (26.5) | 26 (38.2) | 4.700 | 0.030 |
| Drinking history | 759 (20.1) | 14 (20.6) | 0.012 | 0.913 |
| Underlying conditions | ||||
| Hypertension | 899 (23.8) | 34 (50.0) | 25.079 | <0.001 |
| Diabetes mellitus | 209 (5.5) | 11 (16.2) | 14.085 | <0.001 |
| Chronic obstructive pulmonary disease | 481 (12.7) | 17 (25.0) | 8.968 | 0.003 |
| Cerebrovascular disease | 1325 (35.0) | 36 (52.9) | 9.405 | 0.002 |
| Peripheral vascular disease | 1582 (41.8) | 34 (50.0) | 1.846 | 0.174 |
| Renal insufficiency | 296 (7.8) | 19 (27.9) | 36.024 | <0.001 |
| Gastrointestinal tract disease | 307 (8.1) | 9 (13.2) | 2.330 | 0.127 |
| Atrial fibrillation | 882 (23.3) | 14 (20.6) | 0.276 | 0.599 |
| Pulmonary edema | 221 (5.8) | 11 (16.2) | 12.615 | <0.001 |
| Cardiac surgery history | 299 (7.9) | 11 (16.2) | 6.185 | 0.013 |
| General surgery history | 1124 (29.7) | 19 (27.9) | 0.099 | 0.754 |
| NYHA class III–IV | 679 (17.9) | 18 (26.5) | 3.281 | 0.070 |
| Pulmonary artery hypertension | 1218 (32.2) | 19 (27.9) | 0.551 | 0.458 |
| Pericardial effusion | 587 (15.5) | 15 (22.1) | 2.174 | 0.140 |
| Diameter of the left atrium (cm) | 4.5 (3.9, 5.3) | 4.6 (3.9, 5.1) | 0.337 | 0.736 |
| Diameter of the left ventricle (cm) | 5.3 (4.6, 6.0) | 5.0 (4.6, 5.8) | 0.563 | 0.574 |
| Diameter of the right atrium (cm) | 3.9 (3.5, 4.5) | 4.1 (3.6, 4.9) | 1.635 | 0.102 |
| Diameter of the right ventricle (cm) | 3.6 (3.3, 4.0) | 3.7 (3.4, 4.4) | 1.557 | 0.120 |
| Left ventricular ejection fraction (%) | 62 (58, 66) | 62 (57, 66) | 0.144 | 0.866 |
| Laboratory values | ||||
| White blood cell count (×109/L) | 5.6 (4.7, 6.8) | 6.0 (4.9, 8.7) | 2.307 | 0.021 |
| Red blood cell count (×1012/L) | 4.3 (3.9, 4.6) | 4.1 (3.7, 4.7) | 1.787 | 0.074 |
| Hemoglobin (g/L) | 129 (118, 141) | 124 (114, 141) | 1.216 | 0.224 |
| Platelet count (×109/L) | 176 (142, 215) | 152 (107, 209) | 2.677 | 0.007 |
| Serum creatinine (μmol/L) | 71.7 (61.0, 84.4) | 80.6 (66.6, 101.6) | 3.701 | <0.001 |
| Serum albumin (g/L) | 40.5 (38.0, 42.7) | 39.3 (36.7, 41.7) | 2.824 | 0.005 |
| Serum globulin (g/L) | 24.3 (21.6, 27.1) | 24.9 (21.2, 29.5) | 0.803 | 0.422 |
| Operative variables | ||||
| Surgical types | 53.035 | <0.001 | ||
| Isolated valve surgery | 2871 (75.9) | 26 (38.2) | ||
| Combined CABG | 463 (12.2) | 18 (26.5) | ||
| Combined aortic surgery | 451 (11.9) | 24 (35.3) | ||
| Cardiopulmonary bypass time (minutes) | 108 (85, 138) | 153 (105, 236) | 5.128 | <0.001 |
| Aortic cross clamp time (minutes) | 72 (53, 95) | 92 (58, 137) | 4.003 | <0.001 |
| Transfusion of red blood cells (units) | 1 (1, 3) | 8 (5, 9) | 12.326 | <0.001 |
| Characteristic | Coefficient | Standard Error | OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | 0.042 | 0.015 | 1.043 (1.014–1.073) | 0.004 |
| Diabetes mellitus | 1.074 | 0.385 | 2.927 (1.376–6.229) | 0.005 |
| Pulmonary edema | 1.078 | 0.401 | 2.940 (1.338–6.457) | 0.007 |
| Transfusion of red blood cell (units) | 0.675 | 0.058 | 1.964 (1.752–2.201) | <0.001 |
| Surgical types | 0.004 | |||
| Isolated valve surgery | Reference | Reference | Reference | Reference |
| Combined CABG | 0.003 | 0.356 | 1.003 (0.499–2.013) | 0.994 |
| Combined aortic surgery | 1.028 | 0.334 | 2.794 (1.453–5.375) | 0.002 |
| Intercept | −9.722 | 0.948 | <0.001 | <0.001 |
| Risk Intervals | Low Risk (<127 Points) | Medium Risk (127–152 Points) | High Risk (>152 Points) |
|---|---|---|---|
| Estimated probability (%) | <1 | 1–5 | >5 |
| Observed probability, % (95% CI) | 0.16 (0.02–0.30) | 2.87 (1.26–4.48) | 17.35 (12.99–21.70) |
| No. of patients (%) | 3141 (81.5) | 418 (10.9) | 294 (7.6) |
| Variables | Without POT n = 3785 (%) | With POT n = 68 (%) | χ2/Z | p Value |
|---|---|---|---|---|
| Readmission to ICU | 119 (3.1) | 26 (38.2) | 227.125 | <0.001 |
| ICU stay (days) | 2.8 (1.9, 3.9) | 18.8 (11.4, 27.1) | 12.611 | <0.001 |
| Hospital stay (days) | 14 (11, 19) | 37 (25, 48) | 11.839 | <0.001 |
| Mortality | 72 (1.9) | 39 (57.4) | 734.110 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Sun, B.; Liu, L.; Lei, Y.; Su, Y. Nomogram and Risk Calculator for Postoperative Tracheostomy after Heart Valve Surgery. J. Cardiovasc. Dev. Dis. 2023, 10, 73. https://doi.org/10.3390/jcdd10020073
Ding X, Sun B, Liu L, Lei Y, Su Y. Nomogram and Risk Calculator for Postoperative Tracheostomy after Heart Valve Surgery. Journal of Cardiovascular Development and Disease. 2023; 10(2):73. https://doi.org/10.3390/jcdd10020073
Chicago/Turabian StyleDing, Xiangchao, Bing Sun, Liang Liu, Yuan Lei, and Yunshu Su. 2023. "Nomogram and Risk Calculator for Postoperative Tracheostomy after Heart Valve Surgery" Journal of Cardiovascular Development and Disease 10, no. 2: 73. https://doi.org/10.3390/jcdd10020073




