Competing Risks of Coronary Heart Disease Mortality versus Other Causes of Death in 10 Cohorts of Middle-Aged Men of the Seven Countries Study Followed for 60 Years to Extinction
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| USA | FINLAND | THE NETHERLANDS | ITALY | GREECE | JAPAN | |
|---|---|---|---|---|---|---|
| Age | 49 | 49 | 50 | 49 | 50 | 50 |
| years | (44–55) | (45–54) | (45–54) | (45–53) | (45–54) | (45–55) |
| Body mass index | 25.5 | 23.3 | 24.1 | 24.7 | 22.7 | 21.8 |
| kg/m squared | (23.3–27.5) | (21.5–25.6) | (22.2–25.7) | (22.6–27.4) | (20.8–24.8) | (20.4–23.3) |
| Systolic blood pressure | 135 | 140 | 140.5 | 140 | 132 | 130 |
| mm Hg | (125–150) | (130–154) | (130–155) | (130–155) | (122–147) | (118–146) |
| Heart rate | 71 | 65 | 71 | 70 | 63 | 62 |
| beats/min | (63–80) | (58–75) | (64–80) | (62–78) | (56–70) | (55–69) |
| Serum cholesterol | 236.3 | 258 | 232 | 198 | 200 | 164 |
| mg/dL | (210–268) | (225.5–291) | (206–227.3) | (175–224) | (177–229) | (143–184) |
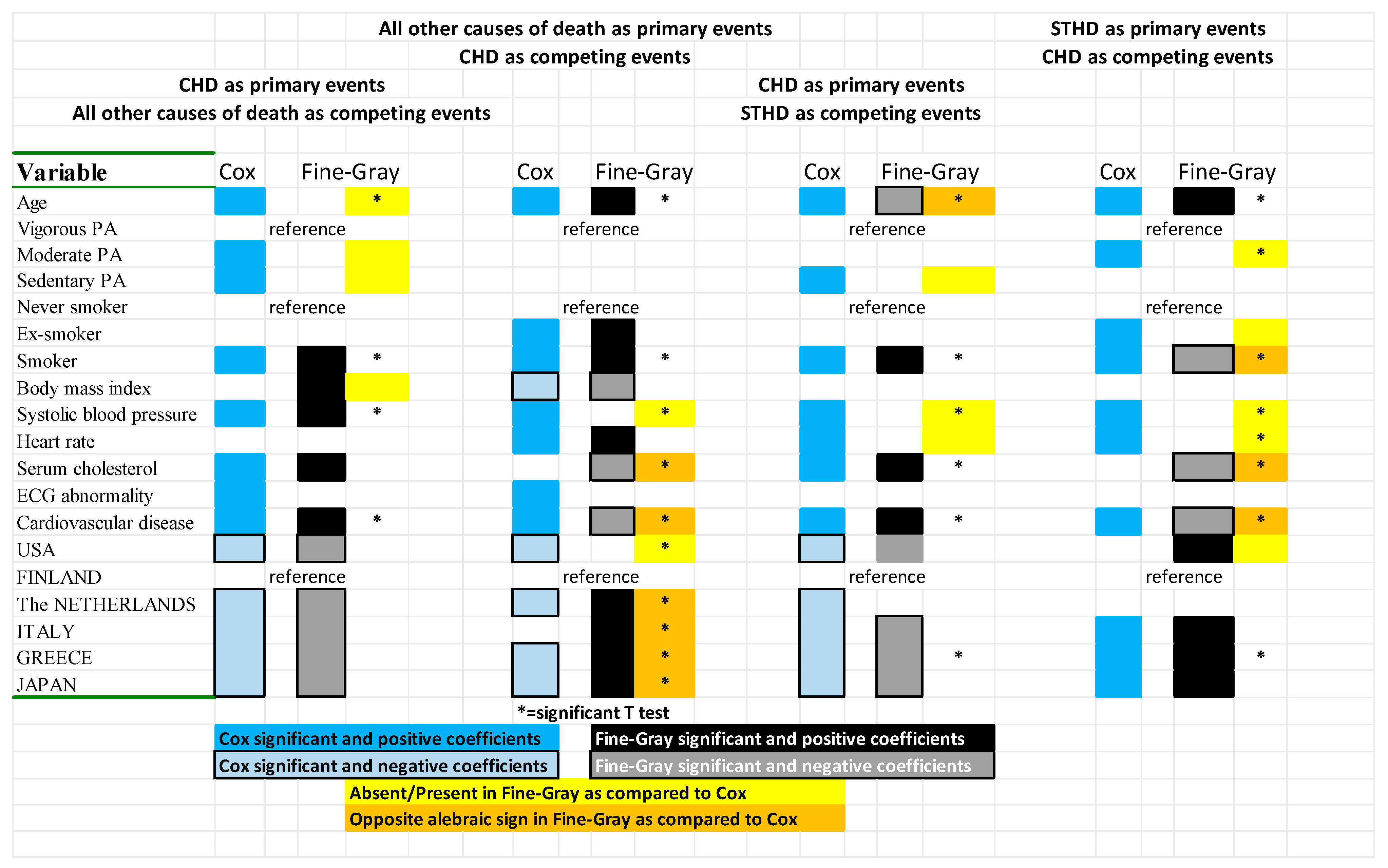
References
- Keys, A. Coronary heart disease in seven countries. Circulation 1970, 41 (Suppl. S1), 1–211. [Google Scholar] [CrossRef] [PubMed]
- Keys, A. (Ed.) Seven Countries Study. A Multivariate Analysis of Death and Coronary Heart Disease; Harvard University Press: Cambridge, MA, USA, 1980; pp. 1–381. [Google Scholar]
- Kromhout, D.; Menotti, A.; Blackburn, H. Prevention of Coronary Heart Disease. Diet, Lifestyle and Risk Factors in the Seven Countries Study; Kluwer Publishers: Norwell, MA, USA; Dordrecht, The Netherlands, 2002; pp. 1–267. [Google Scholar]
- Menotti, A.; Puddu, P.E.; Kafatos, A.G.; Tolonen, H.; Adachi, H.; Jacobs, D.R., Jr. Cardiovascular mortality in 10 cohorts of middle-aged men followed-up 60 years until extinction: The Seven Countries Study. J. Cardiovasc. Dev. Dis. 2023, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the sub-distribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Gray, B. mprsk: Subdistribution Analysis of Competing Risks. Cmprsk R Package, Version 2.2-11, 2022. Available online: https://CRAN.R-project.org/package=cmprsk (accessed on 20 September 2023).
- Puddu, P.E.; Piras, P.; Menotti, A. Competing risks and lifetime coronary heart disease incidence during 50 years of follow-up. Int. J. Cardiol. 2016, 219, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.E.; Piras, P.; Menotti, A. Lifetime competing risks between coronary heart disease mortality and other causes of death during 50 years of follow-up. Int. J. Cardiol. 2017, 228, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Haller, B.; Schmidt, G.; Ulm, K. Applying competing risks regression models: An overview. Lifetime Data Anal. 2013, 19, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Wolbers, M.; Koller, M.T.; Stel, V.S.; Schaer, B.; Jager, K.J.; Leffondré, K.; Heinze, G. Competing risks analyses: Objectives and approaches. Eur. Heart J. 2014, 35, 2936–2941. [Google Scholar] [CrossRef]
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef]
- Austin, P.C.; Fine, J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat. Med. 2017, 36, 4391–4400. [Google Scholar] [CrossRef]
- Feinstein, M.; Ning, H.; Kang, J.; Bertoni, A.; Carnethon, M.; Lloyd-Jones, D.M. Racial differences in risks for first cardiovascular events and non-cardiovascular death: The Atherosclerosis Risk in Communities Study (ARIC), the Cardiovascular Health Study (CHS), and the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2012, 126, 50–59. [Google Scholar] [CrossRef]
- van Kempen, B.J.H.; Ferket, B.S.; Kavousi, M.; Leening, M.J.G.; Steyerberg, E.W.; Ikrama, M.A.; Witteman, J.C.M.; Hofman, A.; Franco, O.H.; Hunink, M.G.M. Performance of Framingham cardiovascular disease (CVD) predictions in the Rotterdam Study taking into account competing risks and disentangling CVD into coronary heart disease (CHD) and stroke. Int. J. Cardiol. 2014, 171, 413–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, S.S.; Ning, H.; Sinha, A.; Wilkins, J.; Allen, N.B.; Vu, T.H.T.; Berry, J.D.; Lloyd-Jones, D.M.; Sweis, R. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J. Am. Heart Assoc. 2021, 10, e021751. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.; Wells, S.; Mehta, S. Are competing-risk models superior to standard Cox models for predicting cardiovascular risk in older adults? Analysis of a whole-of-country primary prevention cohort aged ≥65 years. Int. J. Epidemiol. 2022, 51, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, V. Ten-year mortality from coronary heart disease among 172,000 men classified by occupational physical activity. Scand. J. Work. Environ. Health 1979, 5, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Alberti Fidanza, A.; Seccareccia, F.; Torsello, S.; Fidanza, F. Diet of two rural population groups of middle-aged men in Italy. Internat J. Vit. Nutr. Res. 1988, 58, 442–451. [Google Scholar]
- Rose, G.; Blackburn, H. Cardiovascular Survey Methods; World Health Organization: Geneva, Switzerland, 1968; pp. 1–188. [Google Scholar]
- Anderson, J.T.; Keys, A. Cholesterol in serum and lipoprotein fractions: Its measurement and stability. Clin. Chem. 1956, 2, 145–159. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Diseases and Causes of Death; 8th Revision; WHO: Geneva, Switzerland, 1965. [Google Scholar]
- Menotti, A.; Puddu, P.E.; Lanti, M.; Kromhout, D.; Tolonen, H.; Parapid, B.; Kircanski, B.; Kafatos, A.; Adachi, H. Epidemiology of typical coronary heart disease versus heart disease of uncertain etiology (atypical) fatalities and their relationships with classic coronary risk factors. Int. J. Cardiol. 2013, 168, 3963–3967. [Google Scholar] [CrossRef]
- Kromhout, D.; Menotti, A.; Alberti-Fidanza, A.; Puddu, P.E.; Hollman, P.; Kafatos, A.; Tolonen, H.; Adachi, H.; Jacobs, D.R., Jr. Comparative ecologic relationships of saturated fat, sucrose, food groups and a Mediterranean food pattern score to 50-year coronary heart disease mortality rates among 16 cohorts of the Seven Countries Study. Eur. J. Clin. Nutr. 2018, 72, 1102–1110. [Google Scholar] [CrossRef]
- Menotti, A.; Kromhout, D.; Puddu, P.E.; Alberti-Fidanza, A.; Hollman, P.; Kafatos, A.; Tolonen, H.; Adachi, H.; Jacobs, D.R., Jr. Baseline fatty acids, food groups, a diet score and 50-year all-cause mortality rates. An ecological analysis of the Seven Countries Study. Ann. Med. 2017, 49, 718–727. [Google Scholar] [CrossRef]
- De Longeril, M.; Salen, P. Mediterranean type of diet for the prevention of coronary heart disease. A global perspective from the Seven Countries Study to the most recent dietary trials. Int. J. Vitam. Nutr. Res. 2001, 71, 166–172. [Google Scholar] [CrossRef]
- Carballo-Casla, A.; Ortolá, R.; García-Esquinas, E.; Oliveira, A.; Sotos-Prieto, M.; Lopes, C.; Lopez-Garcia, E.; Rodríguez-Artalejo, F. The Southern European Atlantic Diet and all-cause mortality in older adults. BMC Med. 2021, 19, 36. [Google Scholar] [CrossRef]
- Vancheri, F.; Tate, A.R.; Henein, M.; Backlund, L.; Donfrancesco, C.; Palmieri, L.; Strender, L.E. Time trends in ischaemic heart disease incidence and mortality over three decades (1990–2019) in 20 Western European countries: Systematic analysis of the Global Burden of Disease Study 2019. Eur. J. Prev. Cardiol. 2022, 29, 396–403. [Google Scholar] [CrossRef]
- Laffond, A.; Rivera-Picón, C.; Rodríguez-Muñoz, P.M.; Juárez-Vela, R.; Ruiz de Viñaspre-Hernández, R.; Navas-Echazarreta, N.; Sánchez-González, J.L. Mediterranean Diet for primary and secondary prevention of cardiovascular disease and mortality: An updated systematic review. Nutrients 2023, 15, 3356. [Google Scholar] [CrossRef]
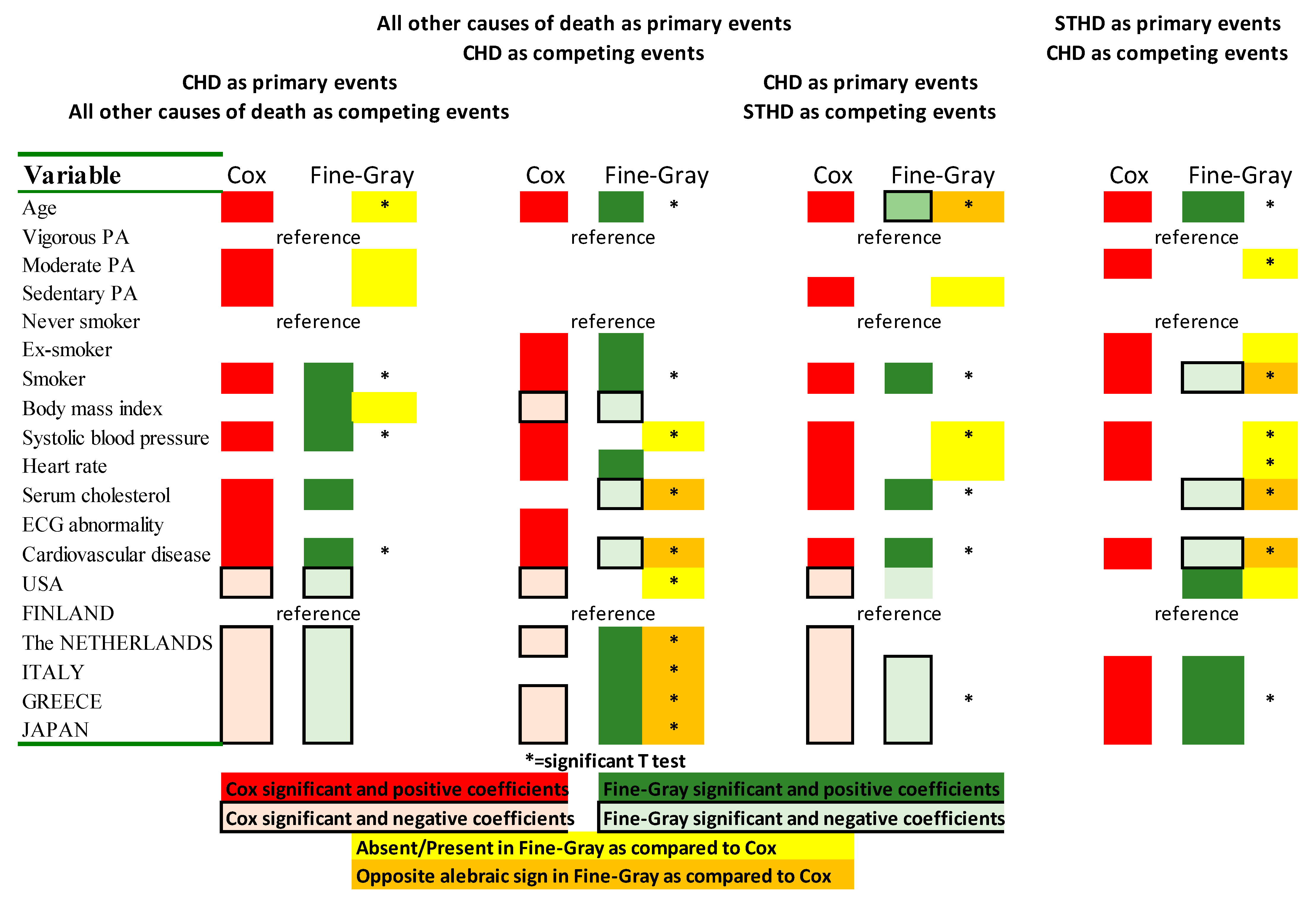
| USA | FINLAND | THE NETHERLANDS | ITALY | GREECE | JAPAN | |
|---|---|---|---|---|---|---|
| Age (years) | 49.4 (5.8) | 49.4 (5.5) | 49.9 (5.5) | 49.1 (5.1) | 49.3 (5.6) | 49.8 (5.7) |
| Sedentary physical activity (PA), % | 66.0 (0.9) | 10.2 (0.7) | 24.1 (1.4) | 9.7 (0.7) | 17.7 (1.1) | 5.7 (0.7) |
| Moderate PA, % | 34.0 (0.9) | 15.7 (0.9) | 64.6 (1.6) | 22.1 (1.0) | 33.7 (1.4) | 29.4 (1.4) |
| Vigorous PA, % | 0 | 74.1 (1.1) | 11.3 (1.1) | 68.2 (1.1) | 48.6 (1.4) | 64.9 (1.5) |
| Never smoker % | 19.9 (0.8) | 18.8 (1.0) | 7.3 (0.8) | 25.4 (1.1) | 24.1 (1.2) | 15.2 (1.1) |
| Ex-smoker % | 21.1 (0.8) | 18.3 (1.0) | 18.2 (1.3) | 13.6 (0.8) | 15.9 (1.1) | 9.9 (0.9) |
| Smoker (current), % | 59.0 (1.0) | 62.6 (1.2) | 74.5 (1.5) | 60.7 (1.2) | 60.0 (1.4) | 72.6 (0.9) |
| Body mass index (kg/m2) | 25.2 (3.2) | 23.7 (3.2) | 24.0 (2.7) | 25.2 (3.7) | 23.1 (3.2) | 22.0 (2.4) |
| Systolic Blood Pressure (mmHg) | 139.2 (20.8) | 143.9 (20.7) | 144.4 (19.8) | 143.6 (21.0) | 136.2 (20.5) | 135.0 (25.0) |
| Heart rate (beats/min) | 72.1 (12.4) | 67.7 (13.0) | 72.6 (12.6) | 71.3 (12.9) | 64.6 (12.6) | 63.0 (10.9) |
| Serum cholesterol mg/dL | 240.3 (45.3) | 261.0 (52.0) | 235.5 (44.4) | 201.7 (40.8) | 205.4 (42.8) | 165.2 (33.0) |
| CVD prevalence, % | 11.3 (0.6) | 19.4 (1.0) | 7.7 (0.9) | 5.4 (0.5) | 4.9 (0.6) | 0.6 (0.2) |
| ECG abnormalities, % | 0.2 (0.03) | 1.2 (0.3) | 3.3 (0.6) | 1.5 (0.3) | 1.3 (0.3) | 1.9 (0.4) |
| CHD | Stroke | HDUE | STHD | Ratio CHD/STHD | CVD | ALL CAUSES | ||
|---|---|---|---|---|---|---|---|---|
| N | Rates per 1000 | Rates per 1000 | ||||||
| USA | 2571 | 282 | 86 | 108 | 194 | 1.45 | 476 | 993 |
| FINLAND | 1677 | 352 | 101 | 61 | 162 | 2.18 | 514 | 998 |
| The NETHERLANDS | 878 | 267 | 84 | 65 | 149 | 1.79 | 416 | 998 |
| ITALY | 1712 | 164 | 132 | 120 | 252 | 0.65 | 416 | 998 |
| GREECE | 1215 | 136 | 164 | 137 | 300 | 0.45 | 436 | 998 |
| JAPAN | 1010 | 70 | 177 | 51 | 229 | 0.31 | 299 | 957 |
| POOL | 9063 | 228 | 118 | 95 | 213 | 1.07 | 441 | 992 |
| A: Complete Direct Model | B: Complete Inverse Model | Test of Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Delta | Coeff | SE | HR | 95% C.L | Coeff | SE | HR | 95% C.L | |
| Age | 5 | 0.0036 | 0.0042 | 1.02 | 0.98 | 0.0359 | 0.0023 | 1.20 | 1.17 | 8.25 |
| 1.06 | 1.22 | |||||||||
| Vigorous PA | reference | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Moderate PA | 1 | 0.0700 | 0.0677 | 1.07 | 0.94 | 0.0216 | 0.0323 | 1.02 | 0.96 | 1.22 |
| 1.22 | 1.09 | |||||||||
| Sedentary PA | 1 | 0.1238 | 0.0757 | 1.13 | 0.98 | 0.0028 | 0.0033 | 1.00 | 0.92 | 1.45 |
| 1.31 | 1.09 | |||||||||
| Never smoker | reference | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Ex-smoker | 1 | −0.0156 | 0.0734 | 0.98 | 0.85 | 0.0798 | 0.0382 | 1.08 | 1.00 | 0.78 |
| 1.14 | 1.17 | |||||||||
| Smoker | 1 | 0.1223 | 0.0589 | 1.13 | 1.01 | 0.1182 | 0.0301 | 1.13 | 1.06 | 3.64 |
| 1.27 | 1.19 | |||||||||
| Body mass index | 3.5 | 0.0170 | 0.0070 | 1.06 | 1.01 | −0.0158 | 0.0045 | 0.95 | 0.92 | 0.15 |
| 1.11 | 0.98 | |||||||||
| Systolic blood pressure | 20 | 0.0060 | 0.0012 | 1.13 | 1.08 | 0.0004 | 0.0007 | 1.01 | 0.98 | 4.68 |
| 1.18 | 1.04 | |||||||||
| Heart rate | 13 | −0.0018 | 0.0019 | 0.98 | 0.93 | 0.0035 | 0.0011 | 1.05 | 1.02 | 0.78 |
| 1.02 | 1.08 | |||||||||
| Serum cholesterol | 50 | 0.0041 | 0.0005 | 1.23 | 1.17 | −0.0022 | 0.0003 | 0.90 | 0.87 | 3.34 |
| 1.29 | 0.92 | |||||||||
| ECG abnormality | 1 | 0.2219 | 0.1535 | 1.25 | 0.92 | −0.0195 | 0.1072 | 0.98 | 0.79 | 1.08 |
| 1.69 | 1.21 | |||||||||
| Cardiovascular disease | 1 | 0.3577 | 0.0700 | 1.43 | 1.25 | −0.1629 | 0.0557 | 0.85 | 0.76 | 2.18 |
| 1.64 | 0.95 | |||||||||
| USA | 1 | −0.2301 | 0.0773 | 0.79 | 0.68 | 0.0538 | 0.0522 | 1.06 | 0.95 | −1.89 |
| 0.92 | 1.17 | |||||||||
| FINLAND | reference | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| The NETHERLANDS | 1 | −0.2620 | 0.0904 | 0.77 | 0.64 | 0.0513 | 0.0585 | 1.05 | 0.94 | −1.96 |
| 0.92 | 1.18 | |||||||||
| ITALY | 1 | −0.6341 | 0.0811 | 0.53 | 0.45 | 0.3251 | 0.0484 | 1.38 | 1.26 | −3.27 |
| 0.62 | 1.52 | |||||||||
| GREECE | 1 | −0.8326 | 0.0928 | 0.43 | 0.36 | 0.2929 | 0.0477 | 1.34 | 1.22 | −5.17 |
| 0.52 | 1.47 | |||||||||
| JAPAN | 1 | −1.2465 | 0.1363 | 0.29 | 0.22 | 0.4927 | 0.0551 | 1.64 | 1.47 | −5.13 |
| 0.38 | 1.92 | |||||||||
| A: Partial Direct Model | B: Partial Inverse Model | Test of Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Delta | Coeff | SE | HR | 95% C.L | Coeff | SE | HR | 95% C.L | |
| Age | 5 | −0.0072 | 0.0027 | 0.96 | 0.94 | 0.0080 | 0.0030 | 1.04 | 1.01 | −3.78 |
| 0.99 | 1.07 | |||||||||
| Vigorous PA | reference | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Moderate PA | 1 | −0.0020 | 0.0454 | 1.00 | 0.91 | 0.0056 | 0.0385 | 1.01 | 0.93 | −0.13 |
| 1.09 | 1.08 | |||||||||
| Sedentary PA | 1 | 0.0391 | 0.0508 | 1.04 | 0.93 | −0.0359 | 0.0510 | 0.96 | 0.87 | 1.04 |
| 1.15 | 1.07 | |||||||||
| Never smoker | reference | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Ex-smoker | 1 | −0.0099 | 0.0506 | 0.99 | 0.90 | 0.0258 | 0.0476 | 1.03 | 0.93 | −0.51 |
| 1.09 | 1.13 | |||||||||
| Smoker | 1 | 0.1085 | 0.0410 | 1.11 | 1.03 | −0.1092 | 0.0379 | 0.90 | 0.83 | 3.39 |
| 1.21 | 0.97 | |||||||||
| Body mass index | 3.5 | 0.0015 | 0.0048 | 1.01 | 0.97 | −0.0001 | 0.0051 | 1.00 | 0.97 | 0.23 |
| 1.04 | 1.04 | |||||||||
| Systolic blood pressure | 20 | −0.0001 | 0.0007 | 1.00 | 0.97 | 0.0001 | 0.0007 | 1.00 | 0.97 | −0.23 |
| 1.03 | 1.03 | |||||||||
| Heart rate | 13 | −0.0001 | 0.0012 | 1.00 | 0.97 | 0.0002 | 0.0013 | 1.00 | 0.97 | −0.20 |
| 1.03 | 1.04 | |||||||||
| Serum cholesterol | 50 | 0.0014 | 0.0003 | 1.07 | 1.04 | −0.0019 | 0.0004 | 0.91 | 0.87 | 6.80 |
| 1.11 | 0.94 | |||||||||
| ECG abnormality | 1 | 0.0289 | 0.0952 | 1.03 | 0.85 | −0.0391 | 0.1030 | 0.96 | 0.87 | 0.48 |
| 1.24 | 1.18 | |||||||||
| Cardiovascular disease | 1 | 0.0863 | 0.0396 | 1.09 | 1.01 | −0.1326 | 0.0638 | 0.88 | 0.77 | 2.92 |
| 1.18 | 0.99 | |||||||||
| USA | 1 | −0.1247 | 0.0489 | 0.88 | 0.80 | 0.2196 | 0.0701 | 1.25 | 1.09 | −4.03 |
| 0.97 | 1.43 | |||||||||
| FINLAND | reference | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| The NETHERLANDS | 1 | −0.0311 | 0.0544 | 0.97 | 0.87 | 0.0783 | 0.0896 | 1.08 | 0.91 | −1.04 |
| 1.08 | 1.29 | |||||||||
| ITALY | 1 | −0.4491 | 0.0566 | 0.64 | 0.57 | 0.5178 | 0.0645 | 1.68 | 1.48 | −11.27 |
| 0.71 | 1.90 | |||||||||
| GREECE | 1 | −0.6844 | 0.0729 | 0.50 | 0.44 | 0.6467 | 0.0640 | 1.91 | 1.68 | −13.72 |
| 0.58 | 2.16 | |||||||||
| JAPAN | 1 | −0.9020 | 0.1118 | 0.41 | 0.33 | 0.6721 | 0.0720 | 1.96 | 1.70 | −11.84 |
| 0.51 | 2.25 | |||||||||
| Cox Direct Models | Cox Inverse Models | Test of Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHD vs. OTHERS (Complete vs. Table 3A) | CHD vs. STHD (Partial vs. Table 4A) | OTHERS vs. CHD (Complete vs. Table 3B) | STHD vs. CHD (Partial vs. Table 4B) | Versus Those of Table 3 | Versus Those of Table 4 | |||||
| Variable | Coeff ± SE | t-Test | Coeff ± SE | t-Test | Coeff ± SE | t-Test | Coeff ± SE | t-Test | A B | A B |
| Age | 0.0678 ± 0.0044 | 15.29 | 0.0597 ± 0.0044 | 13.48 | 0.0986 ± 0.0025 | 39.80 | 0.1188 ± 0.0048 | 24.54 | 10.51 18.54 | 12.86 19.48 |
| Vigorous PA | reference | ----- | reference | ----- | reference | ----- | reference | ----- | ----- ----- | ----- ----- |
| Moderate PA | 0.1430 ± 0.0660 | 2.17 | 0.1212 ± 0.0673 | 1.80 | 0.4142 ± 0.0329 | 1.26 | 0.1579 ± 0.0619 | 2.55 | 0.77 0.43 | 1.52 2.09 |
| Sedentary PA | 0.1928 ± 0.0768 | 2.51 | 0.1689 ± 0.0778 | 2.17 | 0.0638 ± 0.0417 | 1.53 | 0.0890 ± 0.0778 | 1.14 | 0.64 1.46 | 1.40 1.34 |
| Never smoker | reference | ----- | reference | ----- | reference | ----- | reference | ----- | ----- ----- | ----- ----- |
| Ex-smoker | 0.0678 ± 0.0736 | 0.922 | 0.1069 ± 0.0738 | 1.45 | 0.1257 ± 0.0400 | 3.14 | 0.1410 ± 0.0718 | 1.96 | 0.80 0.83 | 1.30 1.34 |
| Smoker | 0.4259 ± 0.0595 | 7.16 | 0.5027 ± 0.0593 | 8.48 | 0.4198 ± 0.0317 | 13.25 | 0.3745 ± 0.0573 | 6.54 | 3.63 6.90 | 5.47 7.04 |
| Body mass index | 0.0094 ± 0.0073 | 1.28 | −0.0050 ± 0.0073 | −0.69 | −0.0136 ± 0.004 | −3.21 | −0.0108 ± 0.0077 | −1.41 | −0.76 0.36 | −0.75 −1.16 |
| Systolic blood pressure | 0.0121 ± 0.0011 | 10.77 | 0.0087 ± 0.0011 | 7.76 | 0.0071 ± 0.0007 | 10.84 | 0.0112 ± 0.0011 | 9.77 | 3.77 7.00 | 6.60 8.18 |
| Heart rate | 0.0018 ± 0.0018 | 0.97 | 0.0040 ± 0.0018 | 2.21 | 0.0052 ± 0.0010 | 5.11 | 0.0062 ± 0.0019 | 3.23 | 1.36 1.15 | 1.91 2.56 |
| Serum cholesterol | 0.0046 ± 0.0005 | 9.78 | 0.0035 ± 0.0005 | 7.48 | −0.0001 ± 0.000 | −0.44 | 0.0004 ± 0.0006 | 0.74 | 0.70 4.87 | 3.75 3.44 |
| ECG abnormality | 0.3587 ± 0.1518 | 2.36 | 0.1105 ± 0.1522 | 0.73 | 0.2073 ± 0.0939 | 2.21 | 0.2142 ± 0.1589 | 1.35 | 0.63 1.59 | 0.45 1.34 |
| Cardiovascular disease | 0.5533 ± 0.0660 | 8.39 | 0.4983 ± 0.0658 | 7.57 | 0.2409 ± 0.0025 | 5.16 | 0.3206 ± 0.0842 | 3.81 | 2.03 5.55 | 5.37 4.29 |
| USA | −0.3575 ± 0.0781 | −4.58 | −0.2720 ± 0.0795 | −3.42 | −0.1104 ± 0.049 | −5.16 | 0.0336 ± 0.0944 | 0.36 | −1.16 −2.29 | −1.58 −1.58 |
| FINLAND | reference | ----- | reference | ----- | reference | ----- | reference | ---- | ----- ----- | ----- ----- |
| The NETHERLANDS | −0.4223 ± 0.0898 | −4.70 | −0.1853 ± 0.0919 | −2.02 | −0.1124 ± 0.055 | −2.03 | −0.1109 ± 0.1171 | −0.95 | −1.26 −2.03 | −1.44 −1.28 |
| ITALY | −0.5893 ± 0.0814 | −7.24 | −0.5250 ± 0.0816 | −6.44 | 0.0809 ± 0.0457 | 1.77 | 0.4212 ± 0.0880 | 4.78 | 0.39 −3.67 | −0.76 −0.89 |
| GREECE | −1.0044 ± 0.0945 | −10.63 | −1.0339 ± 0.0962 | −10.74 | −0.1222 ± 0.047 | −2.58 | 0.1796 ± 0.0896 | 2.00 | −1.30 −6.18 | −2.89 −4.24 |
| JAPAN | −1.1885 ± 0.1357 | −8.76 | −0.7740 ± 0.1368 | −5.66 | 0.1587 ± 0.0539 | 2.95 | 0.8505 ± 0.1079 | 7.88 | 0.30 −4.33 | 0.72 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puddu, P.E.; Piras, P.; Kafatos, A.; Adachi, H.; Tolonen, H.; Menotti, A. Competing Risks of Coronary Heart Disease Mortality versus Other Causes of Death in 10 Cohorts of Middle-Aged Men of the Seven Countries Study Followed for 60 Years to Extinction. J. Cardiovasc. Dev. Dis. 2023, 10, 482. https://doi.org/10.3390/jcdd10120482
Puddu PE, Piras P, Kafatos A, Adachi H, Tolonen H, Menotti A. Competing Risks of Coronary Heart Disease Mortality versus Other Causes of Death in 10 Cohorts of Middle-Aged Men of the Seven Countries Study Followed for 60 Years to Extinction. Journal of Cardiovascular Development and Disease. 2023; 10(12):482. https://doi.org/10.3390/jcdd10120482
Chicago/Turabian StylePuddu, Paolo Emilio, Paolo Piras, Anthony Kafatos, Hisashi Adachi, Hanna Tolonen, and Alessandro Menotti. 2023. "Competing Risks of Coronary Heart Disease Mortality versus Other Causes of Death in 10 Cohorts of Middle-Aged Men of the Seven Countries Study Followed for 60 Years to Extinction" Journal of Cardiovascular Development and Disease 10, no. 12: 482. https://doi.org/10.3390/jcdd10120482
APA StylePuddu, P. E., Piras, P., Kafatos, A., Adachi, H., Tolonen, H., & Menotti, A. (2023). Competing Risks of Coronary Heart Disease Mortality versus Other Causes of Death in 10 Cohorts of Middle-Aged Men of the Seven Countries Study Followed for 60 Years to Extinction. Journal of Cardiovascular Development and Disease, 10(12), 482. https://doi.org/10.3390/jcdd10120482







