Cardiovascular Toxicities of Radiation Therapy and Recommended Screening and Surveillance
Abstract
:1. Introduction
2. Radiation Therapy
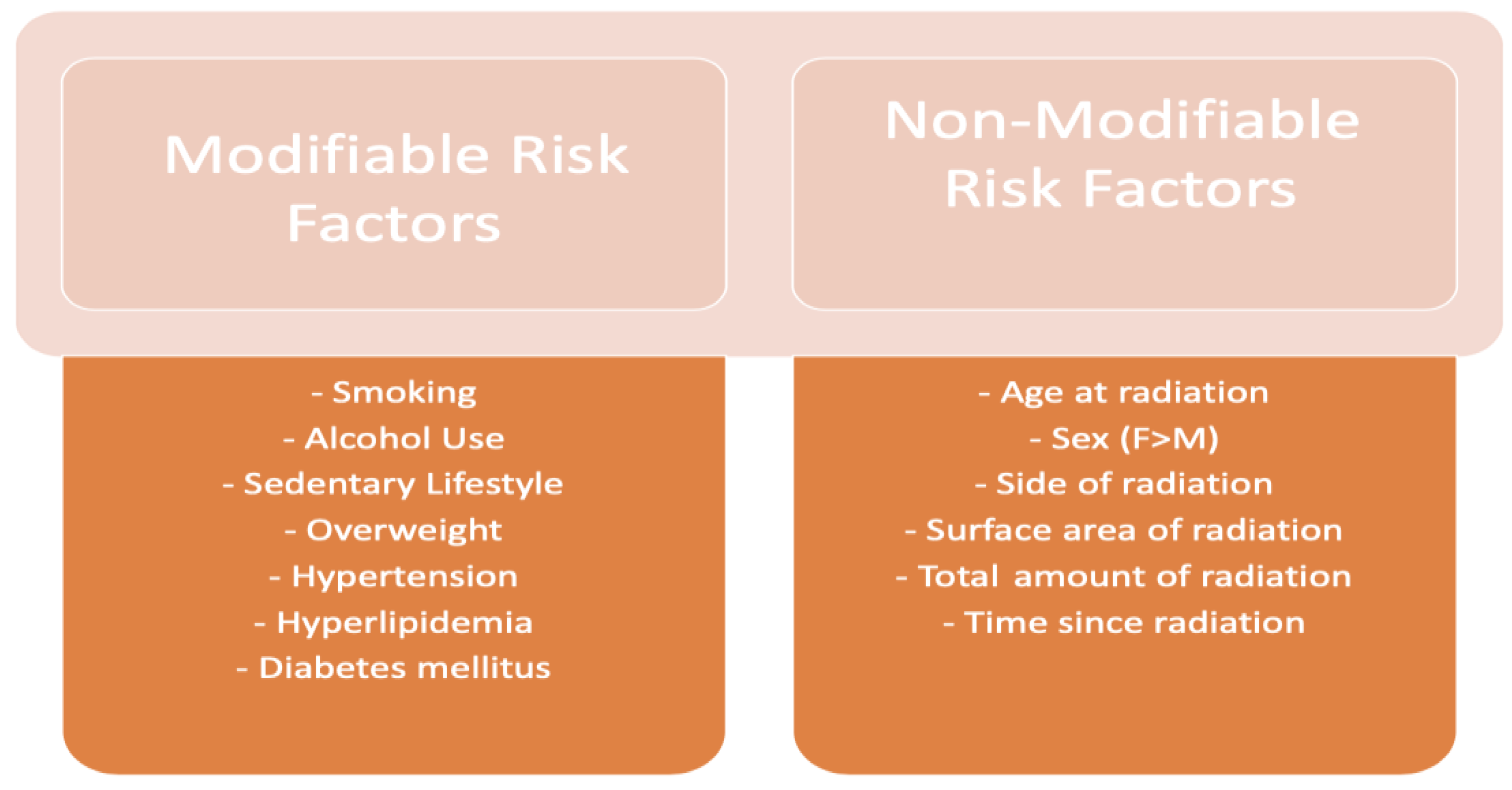
3. Factors Associated with Increased Risk of Cardiotoxicity
4. Cardiovascular Complications of Radiation Therapy
5. Adverse Cardiovascular Effects: Pathophysiology and Management
5.1. Pericardial Disease
5.1.1. Incidence and Pathophysiology
5.1.2. Management
5.2. Coronary Artery Disease
5.2.1. Incidence and Pathophysiology
5.2.2. Management
5.3. Cardiomyopathy
5.3.1. Incidence and Pathophysiology
5.3.2. Management
5.4. Valvular Disease
5.4.1. Incidence and Pathophysiology
5.4.2. Management
5.5. Conduction System Disease
5.5.1. Incidence and Pathophysiology
5.5.2. Management
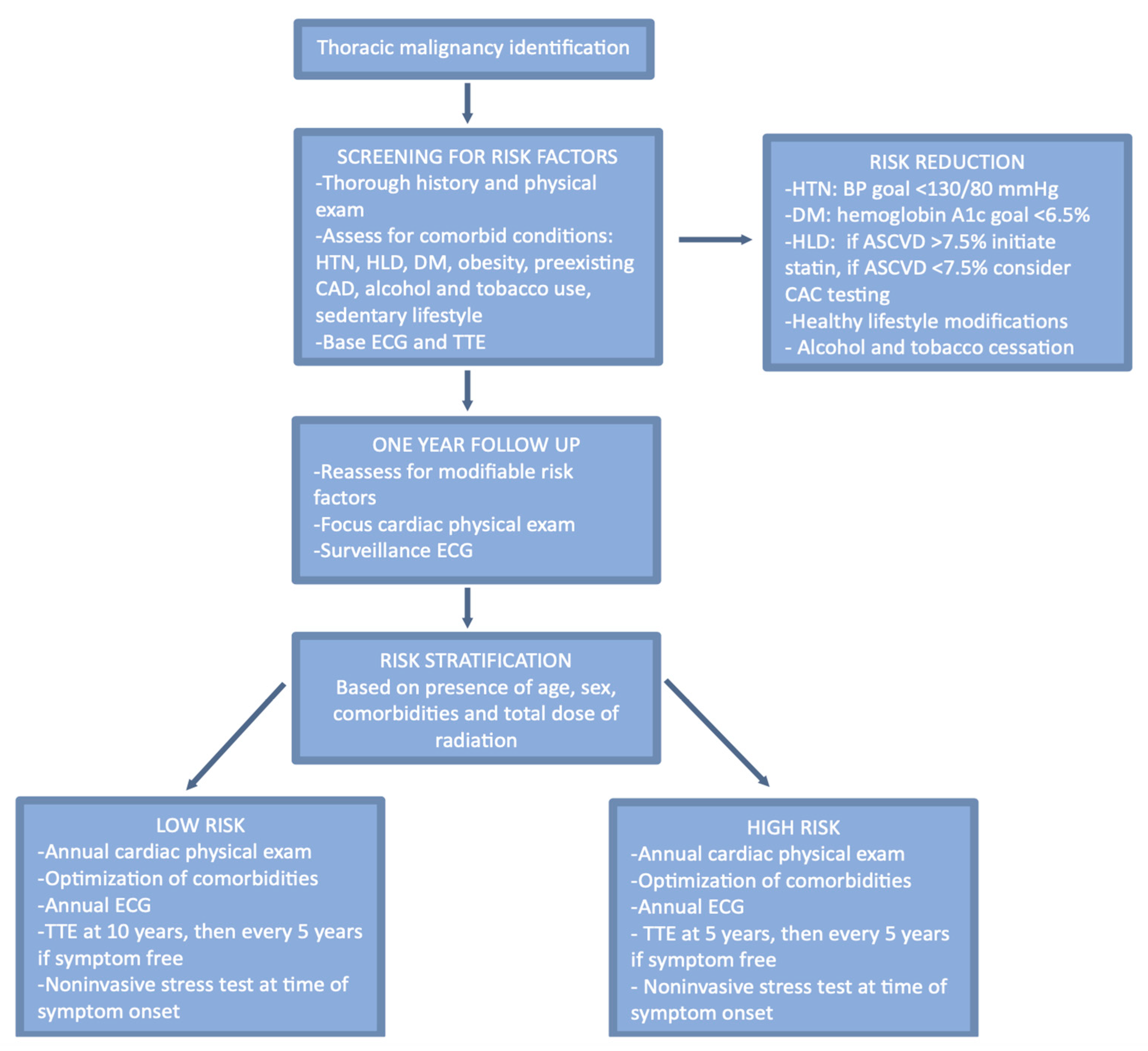
6. Risk Assessment and Pre-Radiation Optimization
6.1. Risk Assessment
6.2. Optimization of Pre-Existing Conditions
6.3. Optimization of Radiation Administration
7. Surveillance and Follow-Up
8. Pre-Surgical Risk Stratification
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramin, C.; Schaeffer, M.L.; Zheng, Z.; Connor, A.E.; Hoffman-Bolton, J.; Lau, B.; Visvanathan, K. All-Cause and Cardiovascular Disease Mortality among Breast Cancer Survivors in CLUE II, a Long-Standing Community-Based Cohort. JNCI J. Natl. Cancer Inst. 2021, 113, 137–145. [Google Scholar] [CrossRef]
- Groarke, J.D.; Nguyen, P.L.; Nohria, A.; Ferrari, R.; Cheng, S.; Moslehi, J. Cardiovascular complications of radiation therapy for thoracic malignancies: The role for non-invasive imaging for detection of cardiovascular disease. Eur. Hear. J. 2013, 35, 612–623. [Google Scholar] [CrossRef]
- Liu, X.-C.; Zhou, P.-K. Tissue Reactions and Mechanism in Cardiovascular Diseases Induced by Radiation. Int. J. Mol. Sci. 2022, 23, 14786. [Google Scholar] [CrossRef]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-induced cardiovascular disease: Review of an underrecognized pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Y.; Fradley, M.; Dasu, N.; Dasu, K.; Shah, A.; Levine, A. Gender disparity in cardiovascular mortality following radiation therapy for Hodgkin’s lymphoma: A systematic review. Cardio-Oncol. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-M.; Okwuosa, T.M.; Scarabelli, T.; Moudgil, R.; Yeh, E.T. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J. Am. Coll. Cardiol. 2017, 70, 2552–2565. [Google Scholar] [CrossRef]
- Fukada, J.; Shigematsu, N.; Takeuchi, H.; Ohashi, T.; Saikawa, Y.; Takaishi, H.; Hanada, T.; Shiraishi, Y.; Kitagawa, Y.; Fukuda, K. Symptomatic pericardial effusion after chemoradiation therapy in esophageal cancer patients. Int. J. Radiat. Oncol. 2013, 87, 487–493. [Google Scholar] [CrossRef]
- Veinot, J.P.; Edwards, W.D. Pathology of radiation-induced heart disease: A surgical and autopsy study of 27 cases. Hum. Pathol. 1996, 27, 766–773. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Crake, T.; Manisty, C.; Westwood, M. Pericardial Disease in Cancer Patients. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 60. [Google Scholar] [CrossRef]
- Lee, P.J.; Mallik, R. Cardiovascular Effects of Radiation Therapy: Practical Approach to Radiation Therapy-Induced Heart Disease. Cardiol. Rev. 2005, 13, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Marinko, T. Pericardial disease after breast cancer radiotherapy. Radiol. Oncol. 2018, 53, 1. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Andreis, A.; Agosti, A.; Piroli, F.; Avondo, S.; Casula, M.; Paneva, E.; Squarotti, G.B.; Giustetto, C.; De Ferrari, G.M. Usefulness of Beta-blockers to Control Symptoms in Patients with Pericarditis. Am. J. Cardiol. 2021, 146, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lioni, C.; et al. ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 36. [Google Scholar] [CrossRef] [PubMed]
- Seifert, F.C.; Miller, D.C.; Oesterle, S.N.; Oyer, P.E.; Stinson, E.B.; Shumway, N.E. Surgical treatment of constrictive pericarditis: Analysis of outcome and diagnostic error. Circulation 1985, 72, 3. [Google Scholar]
- Bertog, S.C.; Thambidorai, S.K.; Parakh, K.; Schoenhagen, P.; Ozduran, V.; Houghtaling, P.L.; Lytle, B.W.; Blackstone, E.H.; Lauer, M.S.; Klein, A.L. Constrictive pericarditis: Etiology and cause-specific survival after pericardiectomy. J. Am. Coll. Cardiol. 2004, 43, 1445–1452. [Google Scholar] [CrossRef]
- Hull, M.C.; Morris, C.G.; Pepine, C.J.; Mendenhall, N.P. Valvular Dysfunction and Carotid, Subclavian, and Coronary Artery Disease in Survivors of Hodgkin Lymphoma Treated With Radiation Therapy. JAMA 2003, 290, 2831–2837. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Hancock, S.L.; Vagelos, R.H.; Lee, B.K.; Schnittger, I. Diastolic dysfunction after mediastinal irradiation. Am. Heart J. 2005, 150, 977–982. [Google Scholar] [CrossRef]
- Wu, W.; Masri, A.; Popovic, Z.B.; Smedira, N.G.; Lytle, B.W.; Marwick, T.H.; Griffin, B.P.; Desai, M.Y. Long-term survival of patients with radiation heart disease undergoing cardiac surgery. Circulation 2013, 127, 1476–1484. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Oliveira, G.H. Heart transplantation outcomes in radiation-induced restrictive cardiomyopathy. J. Card. Fail. 2016, 22, 475–478. [Google Scholar] [CrossRef]
- Saxena, P.; Joyce, L.D.; Daly, R.C.; Kushwaha, S.S.; Schirger, J.A.; Rosedahl, J.; Dearani, J.A.; Kara, T.; Edwards, B.S. Cardiac transplantation for radiation-induced cardiomyopathy: The mayo clinic experience. Ann. Thorac. Surg. 2014, 98, 2115–2121. [Google Scholar] [CrossRef]
- Özyurt, H.; Çevik, O.; Özgen, Z.; Özden, A.S.; Çadırcı, S.; Elmas, M.A.; Ercan, F.; Gören, M.Z.; Şener, G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. 2014, 48, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Berretta, M.; Buccolo, S.; Iovine, M.; Paccone, A.; Cavalcanti, E.; Taibi, R.; Montopoli, M.; Botti, G.; Maurea, N. Polydatin Reduces Cardiotoxicity and Enhances the Anticancer Effects of Sunitinib by Decreasing Pro-Oxidative Stress, Pro-Inflammatory Cytokines, and NLRP3 Inflammasome Expression. Front. Oncol. 2021, 11, 680758. [Google Scholar] [CrossRef] [PubMed]
- Cutter, D.J.; Schaapveld, M.; Darby, S.C.; Hauptmann, M.; van Nimwegen, F.A.; Krol, A.D.G.; Janus, C.P.M.; van Leeuwen, F.E.; Aleman, B.M.P. Risk for valvular heart disease after treatment for Hodgkin lymphoma. JNCI J. Natl. Cancer Inst. 2015, 107, djv008. [Google Scholar] [CrossRef] [PubMed]
- Murbraech, K.; Wethal, T.; Smeland, K.B.; Holte, H.; Loge, J.H.; Holte, E.; Rösner, A.; Dalen, H.; Kiserud, C.E.; Aakhus, S. Valvular Dysfunction in Lymphoma Survivors Treated With Autologous Stem Cell Transplantation: A National Cross-Sectional Study. JACC Cardiovasc. Imaging 2016, 9, 230–239. [Google Scholar] [CrossRef]
- Lee, C.; Hahn, R.T. Valvular Heart Disease Associated With Radiation Therapy: A Contemporary Review. Struct. Heart 2022, 7, 100104. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, W.; Al-Hijji, M.A.; El Sabbagh, A.; Lewis, B.R.; Greason, K.; Sandhu, G.S.; Eleid, M.F.; Holmes, D.R.; Herrmann, J. Outcomes of Patients With Severe Symptomatic Aortic Valve Stenosis After Chest Radiation: Transcatheter Versus Surgical Aortic Valve Replacement. J. Am. Heart Assoc. 2019, 8, e012110. [Google Scholar] [CrossRef]
- Scarfò, I.; Denti, P.; Citro, R.; Buzzatti, N.; Alfieri, O.; La Canna, G. MitraClip for radiotherapy-related mitral valve regurgitation. Hell. J. Cardiol. 2019, 60, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Hardenbergh, P.H.; Constine, L.S.; Lipshultz, S.E. Radiation-associated cardiovascular disease. Crit. Rev. Oncol. 2003, 45, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lipsitz, S.R.; Colan, S.D.; Tarbell, N.J.; Treves, S.T.; Diller, L.; Greenbaum, N.; Mauch, P.; Lipshultz, S.E. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J. Clin. Oncol. 2004, 22, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, Y.; Zheng, R.; Lu, J.; Li, M.; Chen, L.; Huo, Y.; Xu, M.; Wang, T.; Zhao, Z.; et al. Hypertension Defined by 2017 ACC/AHA Guideline, Ideal Cardiovascular Health Metrics, and Risk of Cardiovascular Disease: A Nationwide Prospective Cohort Study. Lancet Reg. Healt West. Pac. 2022, 20, 100350. [Google Scholar] [CrossRef]
- Mancia(Chairperson), G.; Kreutz(Co-Chair), R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 85–89. [Google Scholar]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [Google Scholar] [CrossRef] [PubMed]
- Hooning, M.J.; Botma, A.; Aleman, B.M.P.; Baaijens, M.H.A.; Bartelink, H.; Klijn, J.G.M.; Taylor, C.W.; van Leeuwen, F.E. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. JNCI J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.K.; Sahijdak, W.M.; Haken, R.K.T.; Kessler, M.L.; Turrisi, A.T. Fraction size and dose parameters related to the incidence of pericardial effusions. Int. J. Radiat. Oncol. 1998, 40, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kepka, L.; Socha, J. Dose and fractionation schedules in radiotherapy for non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 1969–1982. [Google Scholar] [CrossRef]
- Lin, S.H.; Hobbs, B.P.; Verma, V.; Tidwell, R.S.; Smith, G.L.; Lei, X.; Corsini, E.M.; Mok, I.; Wei, X.; Yao, L.; et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 14. [Google Scholar] [CrossRef]
- Simonetto, C.; Eidemüller, M.; Gaasch, A.; Pazos, M.; Schönecker, S.; Reitz, D.; Kääb, S.; Braun, M.; Harbeck, N.; Niyazi, M.; et al. Does deep inspiration breath-hold prolong life? Individual risk estimates of ischaemic heart disease after breast cancer radiotherapy. Radiother. Oncol. 2019, 131, 202–207. [Google Scholar] [CrossRef]
- Hancock, S.L.; Tucker, M.A.; Hoppe, R.T. Factors Affecting Late Mortality From Heart Disease After Treatment of Hodgkin’s Disease. JAMA 1993, 270, 1949–1955. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European He-matology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Nkomo, V.T.; Badano, L.P.; Bergler-Klein, J.; Bogaert, J.; Davin, L.; Cosyns, B.; Coucke, P.; Dulgheru, R.; Edvardsen, T.; et al. European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardio-vascular Magnetic Resonance; American Society of Nuclear Cardiology; Society for Cardiovascular Magnetic Resonance; Society of Cardiovascular Computed Tomography. Expert consensus for multi-modality imaging evaluation of cardiovascular com-plications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Murabito, A.; Hirsch, E.; Ghigo, A. Mechanisms of Anthracycline-Induced Cardiotoxicity: Is Mitochondrial Dysfunction the Answer? Front. Cardiovasc. Med. 2020, 7, 35. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narowska, G.; Gandhi, S.; Tzeng, A.; Hamad, E.A. Cardiovascular Toxicities of Radiation Therapy and Recommended Screening and Surveillance. J. Cardiovasc. Dev. Dis. 2023, 10, 447. https://doi.org/10.3390/jcdd10110447
Narowska G, Gandhi S, Tzeng A, Hamad EA. Cardiovascular Toxicities of Radiation Therapy and Recommended Screening and Surveillance. Journal of Cardiovascular Development and Disease. 2023; 10(11):447. https://doi.org/10.3390/jcdd10110447
Chicago/Turabian StyleNarowska, Gabriela, Sakshi Gandhi, Allison Tzeng, and Eman A. Hamad. 2023. "Cardiovascular Toxicities of Radiation Therapy and Recommended Screening and Surveillance" Journal of Cardiovascular Development and Disease 10, no. 11: 447. https://doi.org/10.3390/jcdd10110447




