Mapping of Neuro-Cardiac Electrophysiology: Interlinking Epilepsy and Arrhythmia
Abstract
:1. Introduction
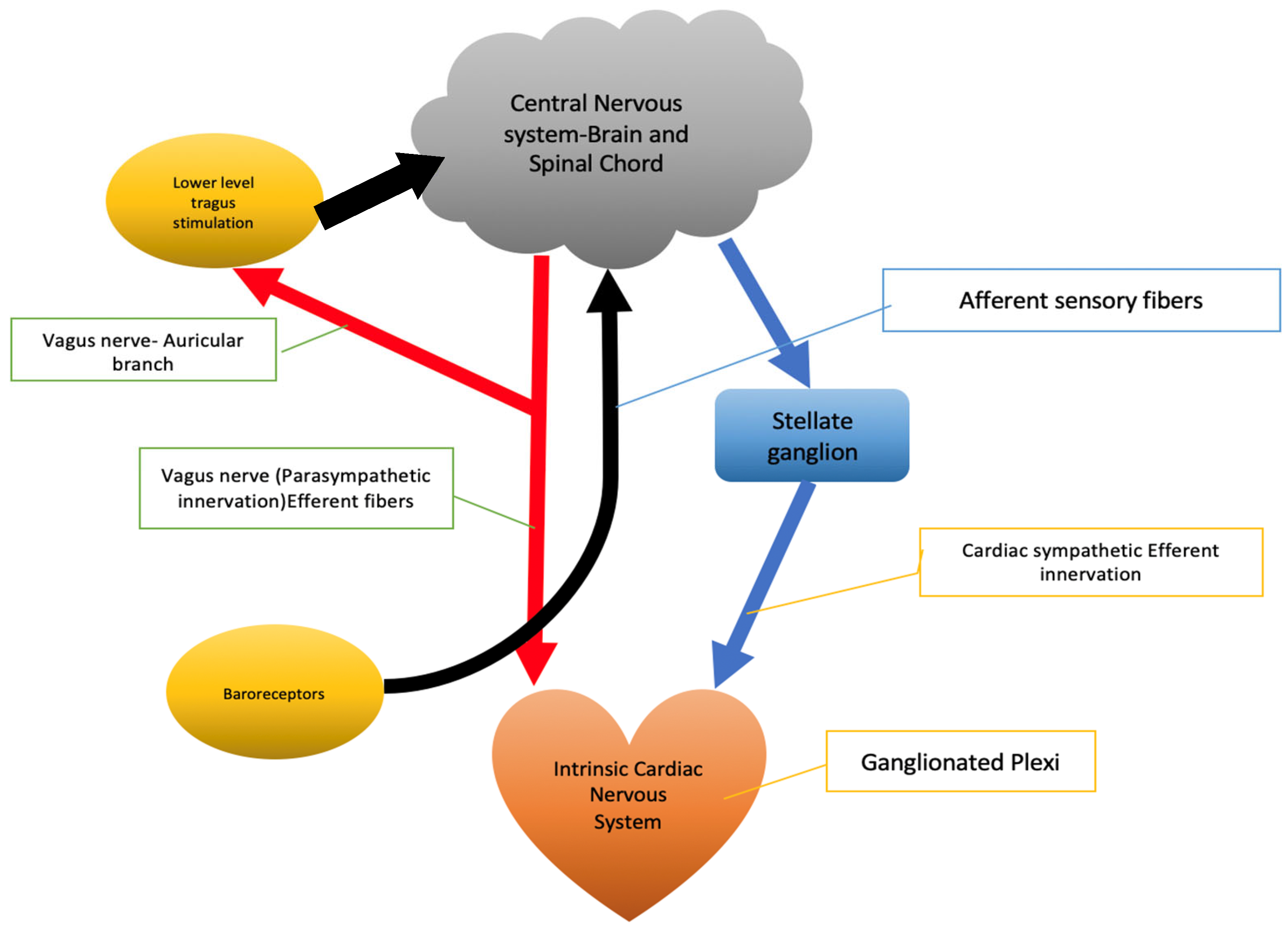
2. Brain–Heart Interplay
3. Ictal Arrhythmias
3.1. Pathophysiology of Heart Rate Changes in Epilepsy
3.2. Ictal Tachycardia
3.3. Ictal Bradycardia
4. Postictal Arrhythmia
5. Long QT Syndrome and Epilepsy
6. Sudden Unexplained Death in Epilepsy
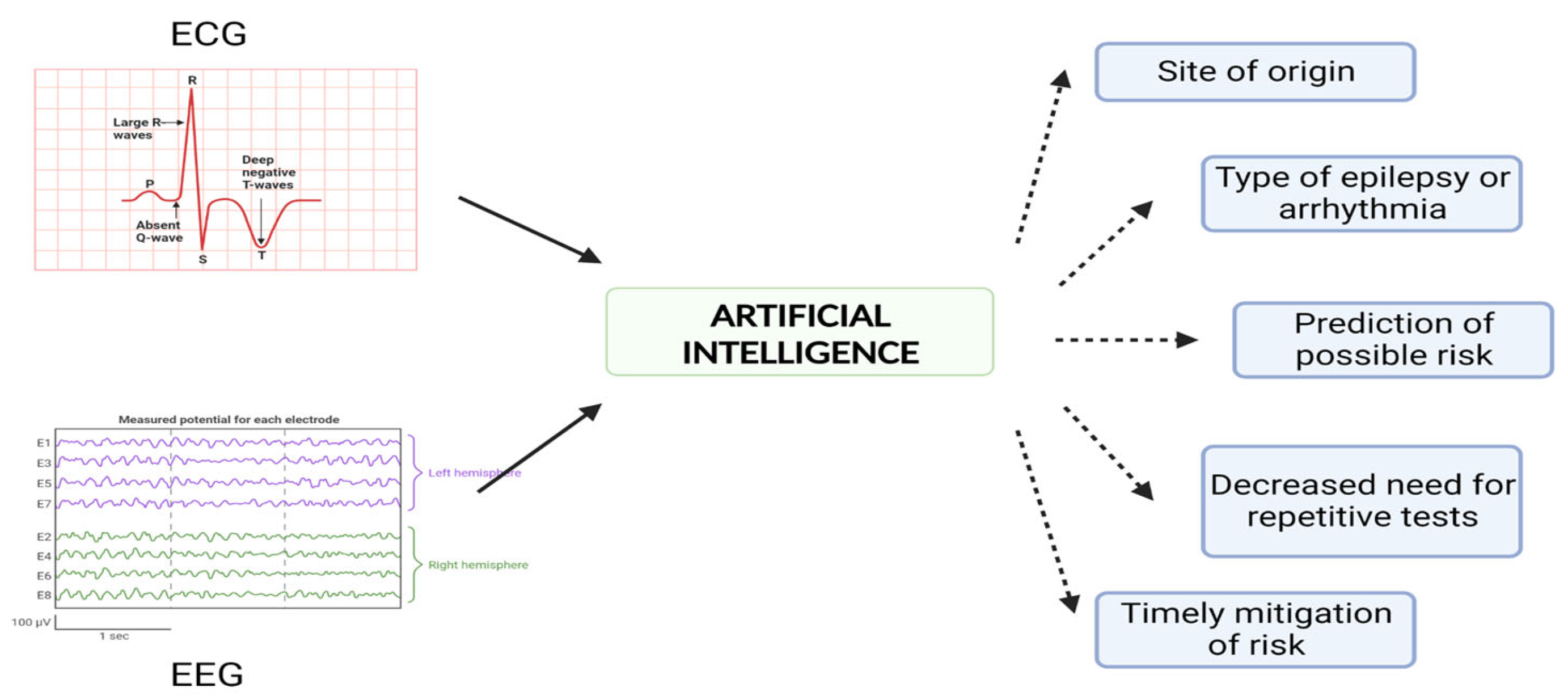
7. Discussion
- A
- LQTS: Simultaneous EEG and ECG recording becomes important in detecting the QT interval and T wave abnormalities, and in the evaluation of convulsive syncope [83].
- B
- Brugada syndrome: An arrhythmic disorder that may coexist with epileptic disorder. Simultaneous ECG and EEG recording can help in distinguishing arrhythmic from epileptic forms, as epileptic forms carry a higher risk of sudden death [84].
- C
- Myocardial-ischemia-related ST segment changes: Ventricular arrhythmia may precede the development of ST segment changes during myocardial ischemia. It is sometimes associated with syncope and can be monitored using simultaneous EEG and ECG recording.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epilepsy WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 23 April 2023).
- Liu, Y.; Bai, F.; Tang, Z.; Liu, N.; Liu, Q. Integrative transcriptomic, proteomic, and machine learning approach to identifying feature genes of atrial fibrillation using atrial samples from patients with valvular heart disease. BMC Cardiovasc. Disord. 2021, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Altman, N.R. Evidence-based medicine: Neuroimaging of seizures. Neuroimaging Clin. N. Am. 2003, 13, 211–224. [Google Scholar] [CrossRef] [PubMed]
- van der Lende, M.; Surges, R.; Sander, J.W.; Thijs, R.D. Cardiac arrhythmias during or after epileptic seizures. J. Neurol. Neurosurg. Psychiatry 2016, 87, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.; Rheims, S. Ictal and Interictal Cardiac Manifestations in Epilepsy. A Review of Their Relation with an Altered Central Control of Autonomic Functions and with the Risk of SUDEP. Front. Neurol. 2021, 12, 642645. [Google Scholar] [CrossRef]
- Desai, R.; Rupareliya, C.; Patel, U.; Naqvi, S.; Patel, S.; Lunagariya, A.; Mahuwala, Z. Burden of Arrhythmias in Epilepsy Patients: A Nationwide Inpatient Analysis of 1.4 Million Hospitalizations in the United States. Cureus 2017, 9, e1550. [Google Scholar] [CrossRef]
- Basili, L.M.; Morano, A.; Fattouch, J.; Fanella, M.; Albini, M.; Avorio, F.; Irelli, E.C.; Manfredi, M.; Urani, C.; Strano, S.; et al. Ictal atrial fibrillation during focal seizures: A case report and literature review. Epileptic Disord. 2019, 21, 295–301. [Google Scholar]
- Costagliola, G.; Orsini, A.; Coll, M.; Brugada, R.; Parisi, P.; Striano, P. The brain-heart interaction in epilepsy: Implications for diagnosis, therapy, and SUDEP prevention. Ann. Clin. Transl. Neurol. 2021, 8, 1557–1568. [Google Scholar] [CrossRef]
- Shen, M.J.; Zipes, D.P. Role of the Autonomic Nervous System in Modulating Cardiac Arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Fedorov, V.V.; Beloshapko, G.G.; Glukhov, A.V.; Yushmanova, A.V.; Rosenshtraukh, L.V. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J. Am. Coll. Cardiol. 2004, 43, 483–490. [Google Scholar] [CrossRef]
- Frigy, A.; Csiki, E.; Caraşca, C.; Szabó, I.A.; Moga, V.-D. Autonomic influences related to frequent ventricular premature beats in patients without structural heart disease. Medicine 2018, 97, e11489. [Google Scholar] [CrossRef]
- Manolis, T.A.; Apostolopoulos, E.J.; Apostolaki, N.E.; Melita, H.; Manolis, A.S. The role of the autonomic nervous system in cardiac arrhythmias: The neuro-cardiac axis, more foe than friend? Trends Cardiovasc. Med. 2021, 31, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Kalla, M.; Paterson, D.J. The autonomic nervous system and cardiac arrhythmias: Current concepts and emerging therapies. Nat. Rev. Cardiol. 2019, 16, 707–726. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp. Physiol. 2008, 93, 165–176. [Google Scholar] [CrossRef]
- Scridon, A.; Şerban, R.C.; Chevalier, P. Atrial fibrillation: Neurogenic or myogenic? Arch. Cardiovasc. Dis. 2018, 111, 59–69. [Google Scholar] [CrossRef]
- Pauza, D.H.; Rysevaite-Kyguoliene, K.; Vismantaite, J.; Brack, K.E.; Inokaitis, H.; Pauza, A.G.; Rimasauskaite-Petraitienė, V.; Pauzaite, J.I.; Pauziene, N. A combined acetylcholinesterase and immunohistochemical method for precise anatomical analysis of intrinsic cardiac neural structures. Ann. Anat. 2014, 196, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Schievink, S.H.; van Boxtel, M.P.; Deckers, K.; van Oostenbrugge, R.J.; Verhey, F.R.; Köhler, S. Cognitive changes in prevalent and incident cardiovascular disease: A 12-year follow-up in the Maastricht Aging Study (MAAS). Eur. Heart J. 2017, 43, e2–e9. [Google Scholar] [CrossRef] [PubMed]
- Janes, R.D.; Brandys, J.C.; Hopkins, D.A.; Johnstone, D.E.; Murphy, D.A.; Armour, J. Anatomy of human extrinsic cardiac nerves and ganglia. Am. J. Cardiol. 1986, 57, 299–309. [Google Scholar] [CrossRef]
- Hopkins, D.A.; Bieger, D.; de Vente, J.; Steinbusch, H.W. Vagal efferent projections: Viscerotopy, neurochemistry and effects of vagotomy. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1996; Volume 107, pp. 79–96. [Google Scholar] [CrossRef]
- Hanna, P.; Rajendran, P.S.; Ajijola, O.A.; Vaseghi, M.; Armour, J.A.; Ardell, J.L.; Shivkumar, K. Cardiac neuroanatomy—Imaging nerves to define functional control. Auton. Neurosci. 2017, 207, 48–58. [Google Scholar] [CrossRef]
- Ardell, J.L.; Andresen, M.C.; Armour, J.A.; Billman, G.E.; Chen, P.-S.; Foreman, R.D.; Herring, N.; O’Leary, D.S.; Sabbah, H.N.; Schultz, H.; et al. Translational neurocardiology: Preclinical models and cardioneural integrative aspects. J. Physiol. 2016, 594, 3877–3909. [Google Scholar] [CrossRef]
- Olshansky, B.; Sabbah, H.N.; Hauptman, P.J.; Colucci, W.S. Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 2008, 118, 863–871. [Google Scholar] [CrossRef]
- Soubhi Alhayek CVP. Beta 1 Receptors; StatsPearls: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Cardiovascular Physiology Concepts. Available online: https://www.cvphysiology.com/Blood%20Pressure/BP010 (accessed on 15 April 2023).
- Aksu, T.; Gopinathannair, R.; Gupta, D.; Pauza, D.H. Intrinsic cardiac autonomic nervous system: What do clinical electrophysiologists need to know about the “heart brain”? J. Cardiovasc. Electrophysiol. 2021, 32, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O. Effects of Seizures on Autonomic and Cardiovascular Function. Epilepsy Curr. 2004, 4, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Rocamora, R.; Kurthen, M.; Lickfett, L.; Von Oertzen, J.; Elger, C.E. Cardiac asystole in epilepsy: Clinical and neurophysiologic features. Epilepsia 2003, 44, 179–185. [Google Scholar] [CrossRef]
- Sevcencu, C.; Struijk, J.J. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010, 51, 725–737. [Google Scholar] [CrossRef]
- Sowden, N.; Booth, C.; Kaye, G. Syncope, Epilepsy and Ictal Asystole: A Case Series and Narrative Review. Heart Lung Circ. 2022, 31, 25–31. [Google Scholar] [CrossRef]
- Sánchez-Borque, P.; González-Giráldez, B.; Benezet-Mazuecos, J.; Miracle, A.; Crosa, J.; Rubio, J.M. Ictal asystole: A condition between neurology and cardiology. Int. J. Cardiol. 2019, 278, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, S.M.; Gelb, A.; Girvin, J.P.; Hachinski, V.C. Cardiovascular effects of human insular cortex stimulation. Neurology 1992, 42, 1727. [Google Scholar] [CrossRef]
- Thornton, J.M.; Aziz, T.; Schlugman, D.; Paterson, D.J. Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J. Physiol. 2002, 539, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Schindler, K.; Kwan, P.; Elger, C. Asystole induced by electrical stimulation of the left cingulate gyrus. Epileptic Disord. 2007, 9, 77–81. [Google Scholar]
- Strano, S.; Toni, D.; Ammirati, F.; Sanna, T.; Tomaino, M.; Brignole, M.; Mazza, A.; Nguyen, B.L.; Di Bonaventura, C.; Ricci, R.P.; et al. Neuro-arrhythmology: A challenging field of action and research: A review from the Task Force of Neuro-arrhythmology of Italian Association of Arrhythmias and Cardiac Pacing. J. Cardiovasc. Med. 2019, 20, 731–744. [Google Scholar] [CrossRef]
- Eggleston, K.S.; Olin, B.D.; Fisher, R.S. Ictal tachycardia: The head-heart connection. Seizure 2014, 23, 496–505. [Google Scholar] [CrossRef]
- Ravindran, K.; Powell, K.L.; Todaro, M.; O’brien, T.J. The pathophysiology of cardiac dysfunction in epilepsy. Epilepsy Res. 2016, 127, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Glasscock, E. Genomic biomarkers of SUDEP in brain and heart. Epilepsy Behav. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Ackerman, M.J. The long QT syndrome: Ion channel diseases of the heart. Mayo Clin. Proc. 1998, 73, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Richichi, C.; Brewster, A.L.; Bender, R.A.; Simeone, T.A.; Zha, Q.; Yin, H.Z.; Weiss, J.H.; Baram, T.Z. Mechanisms of seizure-induced ‘transcriptional channelopathy’ of hyperpolarization-activated cyclic nucleotide gated (HCN) channels. Neurobiol. Dis. 2008, 29, 297–305. [Google Scholar] [CrossRef]
- Wannamaker, B.B. Autonomic nervous system and epilepsy. Epilepsia 1985, 26 (Suppl. S1), S31–S39. [Google Scholar] [CrossRef] [PubMed]
- Provini, F.; Plazzi, G.; Tinuper, P.; Vandi, S.; Lugaresi, E.; Montagna, P. Nocturnal frontal lobe epilepsy. A clinical and polygraphic overview of 100 consecutive cases. Brain 1999, 122 Pt 6, 1017–1031. [Google Scholar] [CrossRef]
- Zijlmans, M.; Flanagan, D.; Gotman, J. Heart rate changes and ECG abnormalities during epileptic seizures: Prevalence and definition of an objective clinical sign. Epilepsia 2002, 43, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Rugg-Gunn, F.J.; Simister, R.J.; Squirrell, M.; Holdright, D.R.; Duncan, J.S. Cardiac arrhythmias in focal epilepsy: A prospective long-term study. Lancet 2004, 364, 2212–2219. [Google Scholar] [CrossRef]
- Garcia, M.; D’giano, C.; Estelles, S.; Leiguarda, R.; Rabinowicz, A. Ictal tachycardia: Its discriminating potential between temporal and extratemporal seizure foci. Seizure 2001, 10, 415–419. [Google Scholar] [CrossRef]
- Leutmezer, F.; Schernthaner, C.; Lurger, S.; Pötzelberger, K.; Baumgartner, C. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia 2003, 44, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Reeves, A.L.; Novak, P.; Low, P.A.; Sharbrough, F.W. Time-frequency mapping of R-R interval during complex partial seizures of temporal lobe origin. J. Auton. Nerv. Syst. 1999, 77, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.A.; Sperling, M.R.; O’Connor, M.J. Cardiac rhythm during temporal lobe seizures. Neurology 1992, 42, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Işık, U.; Ayabakan, C.; Tokel, K.; Özek, M.M. Ictal electrocardiographic changes in children presenting with seizures. Pediatr. Int. 2012, 54, 27–31. [Google Scholar] [CrossRef]
- Moseley, B.D.; Nickels, K.; Britton, J.; Wirrell, E. How common is ictal hypoxemia and bradycardia in children with partial complex and generalized convulsive seizures? Epilepsia 2010, 51, 1219–1224. [Google Scholar] [CrossRef]
- Opherk, C.; Coromilas, J.; Hirsch, L.J. Heart rate and EKG changes in 102 seizures: Analysis of influencing factors. Epilepsy Res. 2002, 52, 117–127. [Google Scholar] [CrossRef]
- Weil, S.; Arnold, S.; Eisensehr, I.; Noachtar, S. Heart rate increase in otherwise subclinical seizures is different in temporal versus extratemporal seizure onset: Support for temporal lobe autonomic influence. Epileptic Disord. 2005, 7, 199–204. [Google Scholar]
- Galimberti, C.A.; Marchioni, E.; Barzizza, F.; Manni, R.; Sartori, I.; Tartara, A. Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996, 37, 742–747. [Google Scholar] [CrossRef]
- Monté, C.J.; Boon, P.; Arends, J. Epileptic seizures associated with syncope: Ictal bradycardia and ictal asystole. Epilepsy Behav. 2019, 90, 168–171. [Google Scholar] [CrossRef]
- Tinuper, P.; Bisulli, F.; Cerullo, A.; Carcangiu, R.; Marini, C.; Pierangeli, G.; Cortelli, P. Ictal bradycardia in partial epileptic seizures: Autonomic investigation in three cases and literature review. Brain 2001, 124 Pt 12, 2361–2371. [Google Scholar] [CrossRef]
- Howell, S.J.; Blumhardt, L.D. Cardiac asystole associated with epileptic seizures: A case report with simultaneous EEG and ECG. J. Neurol. Neurosurg. Psychiatry 1989, 52, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Van Rijckevorsel, K.; Saussu, F.; De Barsy, T. Bradycardia, an epileptic ictal manifestation. Seizure 1995, 4, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Shmuely, S.; van der Lende, M.; Lamberts, R.; Sander, J.; Thijs, R. The heart of epilepsy: Current views and future concepts. Seizure 2017, 44, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Elnazeir, M.; Badugu, P.; Narayanan, S.; Hussain, A.; Bhagat, R.N.; Jones, C.M.; Holiday, V.N.; Evans, M.S.; Palade, A.E. Generalized tonic-clonic seizures with post-ictal atrial fibrillation. Epilepsy Behav. Rep. 2020, 13, 100343. [Google Scholar] [CrossRef]
- Poh, M.Z.; Loddenkemper, T.; Reinsberger, C.; Swenson, N.C.; Goyal, S.; Madsen, J.R.; Picard, R.W. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 2012, 78, 1868–1876. [Google Scholar] [CrossRef]
- Devinsky, O.; Hesdorffer, D.C.; Thurman, D.J.; Lhatoo, S.; Richerson, G. Sudden unexpected death in epilepsy: Epidemiology, mechanisms, and prevention. Lancet Neurol. 2016, 15, 1075–1088. [Google Scholar] [CrossRef]
- Nei, M.; Ho, R.T.; Abou-Khalil, B.W.; Drislane, F.W.; Liporace, J.; Romeo, A.; Sperling, M.R. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004, 45, 338–345. [Google Scholar] [CrossRef]
- Bardai, A.; Lamberts, R.J.; Blom, M.T.; Spanjaart, A.M.; Berdowski, J.; van der Staal, S.R.; Brouwer, H.J.; Koster, R.W.; Sander, J.W.; Thijs, R.D.; et al. Epilepsy Is a Risk Factor for Sudden Cardiac Arrest in the General Population. PLoS ONE 2012, 7, e42749. [Google Scholar] [CrossRef]
- Volders, P.G. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm 2010, 7, 1900–1906. [Google Scholar] [CrossRef]
- Paton, J.; Boscan, P.; Pickering, A.; Nalivaiko, E. The yin and yang of cardiac autonomic control: Vago-sympathetic interactions revisited. Brain. Res. Brain. Res. Rev. 2005, 49, 555–565. [Google Scholar] [CrossRef]
- Krishnaiengar, S.; Fitzgerald, J.; Nagaraju, D.; Zarroli, K.; Bautista, R. Prolonged post-ictal atrial fibrillation following seizures. Epilepsy Behav. Rep. 2021, 16, 100481. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Larsen, A.; Aznar-Lain, G.; Benito, B.; Principe, A.; Ley, M.; Campo, A.T.; Rocamora, R. Post-ictal atrial fibrillation detected during video-EEG monitoring: Case report, proposed physiopathologic mechanism and therapeutic considerations. Epilepsy Behav. Case. Rep. 2017, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Schwartz, P.J.; Crampton, R.S.; Tzivoni, D.; Locati, E.H.; MacCluer, J.; Hall, W.J.; Weitkamp, L.; Vincent, G.M.; Garson, A.; et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 1991, 84, 1136–1144. [Google Scholar] [CrossRef]
- Splawski, I.; Shen, J.; Timothy, K.W.; Lehmann, M.H.; Priori, S.; Robinson, J.L.; Moss, A.J.; Schwartz, P.J.; Towbin, J.A.; Vincent, G.M.; et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 2000, 102, 1178–1185. [Google Scholar] [CrossRef]
- Rashid, U.; Virk, A.O.; Nawaz, R.; Mahmood, T.; Fatima, Z. Overt long QT syndrome in children presenting with seizure disorders in Pakistan. Ann. Pediatr. Cardiol. 2021, 14, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Galtrey, C.M.; Levee, V.; Arevalo, J.; Wren, D. Long QT syndrome masquerading as epilepsy. Pract. Neurol. 2019, 19, 56–61. [Google Scholar] [CrossRef]
- Medford, B.A.; Bos, J.M.; Ackerman, M.J. Epilepsy misdiagnosed as long QT syndrome: It can go both ways. Congenit. Heart Dis. 2014, 9, E135–E139. [Google Scholar] [CrossRef]
- Feldman, A.E.; Gidal, B.E. QTc prolongation by antiepileptic drugs and the risk of torsade de pointes in patients with epilepsy. Epilepsy Behav. 2013, 26, 421–426. [Google Scholar] [CrossRef]
- Nashef, L.; So, E.L.; Ryvlin, P.; Tomson, T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012, 53, 227–233. [Google Scholar] [CrossRef]
- Chahal, C.A.A.; Salloum, M.N.; Alahdab, F.; Gottwald, J.A.; Tester, D.J.; Anwer, L.A.; So, E.L.; Murad, M.H.; Louis, E.K.S.; Ackerman, M.J.; et al. Systematic Review of the Genetics of Sudden Unexpected Death in Epilepsy: Potential Overlap with Sudden Cardiac Death and Arrhythmia-Related Genes. J. Am. Heart Assoc. 2020, 9, e012264. [Google Scholar] [CrossRef]
- Leung, H.; Kwan, P.; Elger, C. Finding the missing link between ictal bradyarrhythmia, ictal asystole, and sudden unexpected death in epilepsy. Epilepsy Behav. 2006, 9, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Schuele, S.U.; Bermeo, A.C.; Locatelli, E.; Burgess, R.C.; Lüders, H.O. Ictal asystole: A benign condition? Epilepsia 2008, 49, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Ho, R.T.; Sperling, M.R. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000, 41, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Clough, P.; Cooper, P.; Scheepers, B.; Fitzpatrick, A.P. Misdiagnosis of epilepsy: Many seizure-like attacks have a cardiovascular cause. J. Am. Coll. Cardiol. 2000, 36, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Defalla, B.; Chadwick, D. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. QJM 1999, 92, 15–23. [Google Scholar] [CrossRef]
- Pitney, M.R.; Beran, R.G.; Jones, A. A simultaneous electrocardiogram is important when electroencephalography is used in the evaluation of loss of consciousness. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 246–248. [Google Scholar] [CrossRef]
- Kendirli, M.T.; Aparci, M.; Kendirli, N.; Tekeli, H.; Karaoglan, M.; Senol, M.G.; Togrol, E. Diagnostic Role of ECG Recording Simultaneously with EEG Testing. Clin. EEG Neurosci. 2015, 46, 214–217. [Google Scholar] [CrossRef]
- Anderson, J.H.; Bos, J.M.; Cascino, G.D.; Ackerman, M.J. Prevalence and spectrum of electroencephalogram-identified epileptiform activity among patients with long QT syndrome. Heart Rhythm 2014, 11, 53–57. [Google Scholar] [CrossRef]
- Sandorfi, G.; Clemens, B.; Csanadi, Z. Electrical storm in the brain and in the heart: Epilepsy and Brugada syndrome. Mayo Clin. Proc. 2013, 88, 1167–1173. [Google Scholar] [CrossRef]
- Britton, J.W.; Frey, L.C.; Hopp, J.L.; Korb, P.; Koubeissi, M.Z.; Lievens, W.E.; Pestana-Knight, E.M.; Louis, E.K.S. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; American Epilepsy Society: Chicago, IL, USA, 2016. [Google Scholar]
- Benbadis, S.R.; Beniczky, S.; Bertram, E.; MacIver, S.; Moshé, S.L. The role of EEG in patients with suspected epilepsy. Epileptic Disord. 2020, 22, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Oto, M.M. The misdiagnosis of epilepsy: Appraising risks and managing uncertainty. Seizure 2017, 44, 143–146. [Google Scholar] [CrossRef]
- Nizam, A.; Mylavarapu, K.; Thomas, D.; Briskin, K.; Wu, B.; Saluja, D.; Wong, S. Lacosamide-induced second-degree atrioventricular block in a patient with partial epilepsy. Epilepsia 2011, 52, e153–e155. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Alexander, S.; Brickel, N. Effect of lamotrigine on the PR interval in healthy subjects. Br. J. Clin. Pharmacol. 2011, 71, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Michel, V.-H.; Adam, C.; Dinkelacker, V.; Pichit, P.; Boudali, Y.; Dupont, S.; Baulac, M.; Navarro, V. Characterization of seizure-induced syncopes: EEG, ECG, and clinical features. Epilepsia 2014, 55, 146–155. [Google Scholar] [CrossRef]
- Tomson, T.; Kennebäck, G. Arrhythmia, heart rate variability, and antiepileptic drugs. Epilepsia 1997, 38 (Suppl. S11), S48–S51. [Google Scholar] [CrossRef]
- Vaz-Da-Silva, M.; Nunes, T.; Almeida, L.; Gutierrez, M.J.; Litwin, J.S.; Soares-Da-Silva, P.; Litwin, F.J.S. Evaluation of Eslicarbazepine acetate on cardiac repolarization in a thorough QT/QTc study. J. Clin. Pharmacol. 2012, 52, 222–233. [Google Scholar] [CrossRef]
- Vandecasteele, K.; De Cooman, T.; Chatzichristos, C.; Cleeren, E.; Swinnen, L.; Ortiz, J.M.; Van Huffel, S.; Dümpelmann, M.; Schulze-Bonhage, A.; De Vos, M.; et al. The power of ECG in multimodal patient-specific seizure monitoring: Added value to an EEG-based detector using limited channels. Epilepsia 2021, 62, 2333–2343. [Google Scholar] [CrossRef]
- Shrivastava, S.; Cohen-Shelly, M.; Attia, Z.I.; Rosenbaum, A.N.; Wang, L.; Giudicessi, J.R.; Redfield, M.; Bailey, K.; Lopez-Jimenez, F.; Lin, G.; et al. Artificial Intelligence-Enabled Electrocardiography to Screen Patients with Dilated Cardiomyopathy. Am. J. Cardiol. 2021, 155, 121–127. [Google Scholar] [CrossRef]
- Siontis, K.C.; Liu, K.; Bos, J.M.; Attia, Z.I.; Cohen-Shelly, M.; Arruda-Olson, A.M.; Farahani, N.Z.; Friedman, P.A.; Noseworthy, P.A.; Ackerman, M.J. Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents. Int. J. Cardiol. 2021, 340, 42–47. [Google Scholar] [CrossRef]
- Potter, E.L.; Rodrigues, C.H.; Ascher, D.B.; Abhayaratna, W.P.; Sengupta, P.P.; Marwick, T.H. Machine Learning of ECG Waveforms to Improve Selection for Testing for Asymptomatic Left Ventricular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, B.; Meng, H.; Lv, Y.; Qiu, J.; Zhu, Z.; Xie, Y.; Li, Y.; Cheng, Y.; Zhao, W.; Liu, J.; et al. An Overview of EEG-based Machine Learning Methods in Seizure Prediction and Opportunities for Neurologists in this Field. Neuroscience 2022, 481, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.P.; Senadeera, M.; Jacobs, S.; Coghlan, S.; Le, V. Trust and medical AI: The challenges we face and the expertise needed to overcome them. J. Am. Med. Inform. Assoc. 2021, 28, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Babic, B.; Gerke, S.; Evgeniou, T.; Cohen, I.G. Direct-to-consumer medical machine learning and artificial intelligence applications. Nat. Mach. Intell. 2021, 3, 283–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senapati, S.G.; Bhanushali, A.K.; Lahori, S.; Naagendran, M.S.; Sriram, S.; Ganguly, A.; Pusa, M.; Damani, D.N.; Kulkarni, K.; Arunachalam, S.P. Mapping of Neuro-Cardiac Electrophysiology: Interlinking Epilepsy and Arrhythmia. J. Cardiovasc. Dev. Dis. 2023, 10, 433. https://doi.org/10.3390/jcdd10100433
Senapati SG, Bhanushali AK, Lahori S, Naagendran MS, Sriram S, Ganguly A, Pusa M, Damani DN, Kulkarni K, Arunachalam SP. Mapping of Neuro-Cardiac Electrophysiology: Interlinking Epilepsy and Arrhythmia. Journal of Cardiovascular Development and Disease. 2023; 10(10):433. https://doi.org/10.3390/jcdd10100433
Chicago/Turabian StyleSenapati, Sidhartha G., Aditi K. Bhanushali, Simmy Lahori, Mridula Sree Naagendran, Shreya Sriram, Arghyadeep Ganguly, Mounika Pusa, Devanshi N. Damani, Kanchan Kulkarni, and Shivaram P. Arunachalam. 2023. "Mapping of Neuro-Cardiac Electrophysiology: Interlinking Epilepsy and Arrhythmia" Journal of Cardiovascular Development and Disease 10, no. 10: 433. https://doi.org/10.3390/jcdd10100433




