OX40 (CD134) Expression on T Regulatory Cells Is Related to Serious Hypertensive Disorders in Pregnancy
Abstract
:1. Introduction
2. Material and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.2.1. Isolation of Mononuclear Cells (PBMC) from Peripheral Blood
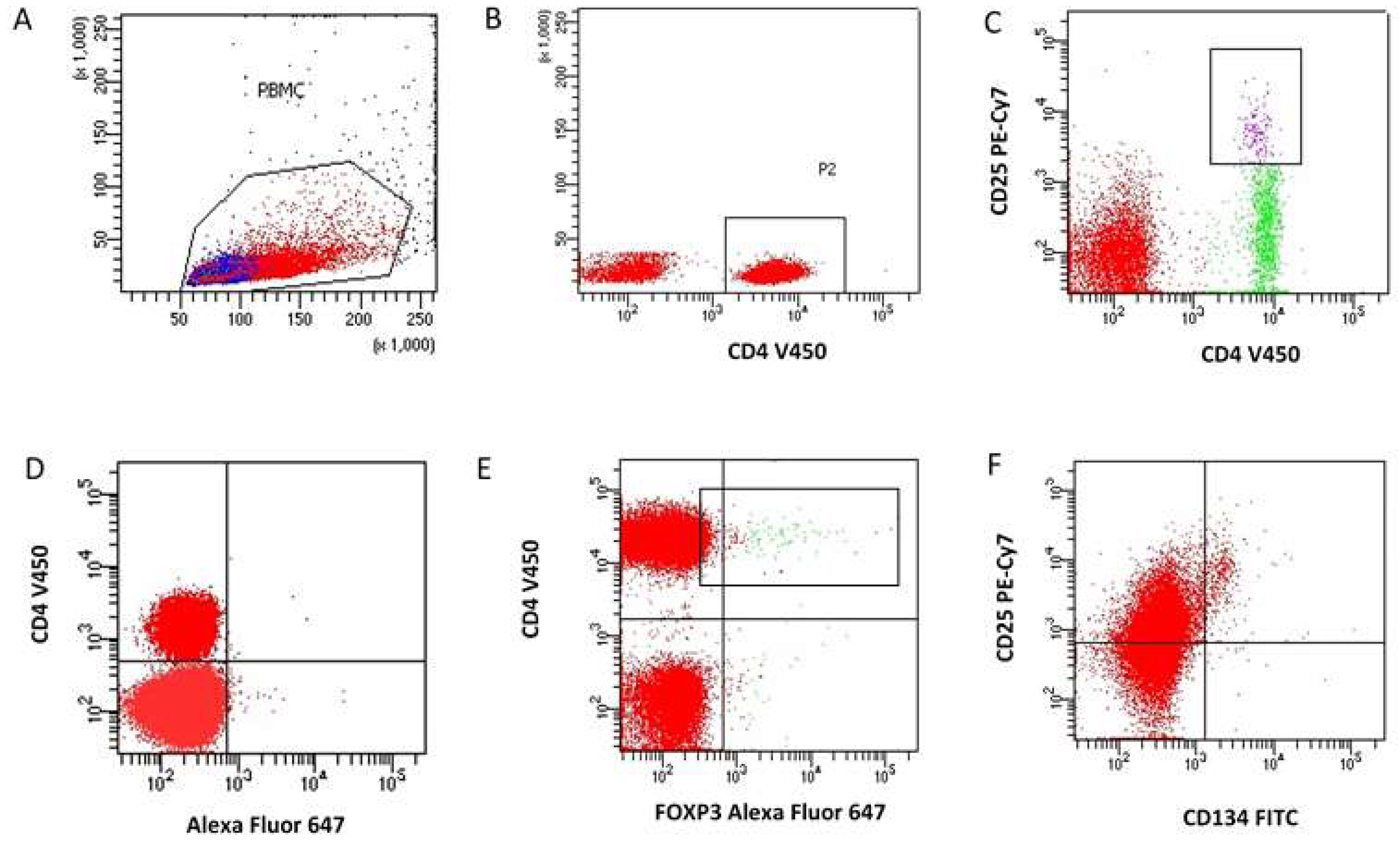
2.2.2. Determination of The Immunophenotype of Regulator T Cells
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sava, R.I.; March, K.L.; Pepine, C.J. Hypertension in Pregnancy: Taking Cues from Pathophysiology for Clinical Practice. Clin. Cardiol. 2018, 41, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.L.M.; Harper, L.M.; Tita, A.T.N. Hypertensive Disorders in Pregnancy. Obstet. Gynecol. 2018, 45, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Benny, P.; Yamasato, K.; Yunits, B.; Zhu, X.; Ching, T.; Garmire, L.X.; Berry, M.J.; Towner, D. Maternal Cardiovascular-Related Single Nucleotide Polymorphisms, Genes, and Pathways Associated with Early-Onset Preeclampsia. PLoS ONE 2019, 14, e0222672. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.G.; Seely, E.W. Hypertension in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2011, 40, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Nguyen-Hoang, L.; Smith, G.N.; Bergman, L.; O’Brien, P.; Hod, M.; Okong, P.; Kapur, A.; Maxwell, C.V.; McIntyre, H.D.; et al. Hypertensive Disorders of Pregnancy and Long-term Cardiovascular Health: FIGO Best Practice Advice. Int. J. Gynecol. Obstet. 2023, 160, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.; Yeh, C.; Bennett-Kunzier, N.; Kinzler, W.L. Long-Term Maternal Morbidity and Mortality Associated with Ischemic Placental Disease. Sem. Perinat. 2014, 38, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Prejbisz, A.; Dobrowolski, P.; Kosiński, P.; Bomba-Opoń, D.; Adamczak, M.; Bekiesińska-Figatowska, M.; Kądziela, J.; Konopka, A.; Kostka-Jeziorny, K.; Kurnatowska, I.; et al. Management of hypertension in pregnancy—Prevention, diagnosis, treatment and long-term prognosis. A position statement of the Polish Society of Hypertension, Polish Cardiac Society and Polish Society of Gynaecologists and Obstetricians. Arter. Hypertens. 2019, 23, 117–182. [Google Scholar] [CrossRef]
- Pennington, K.A.; Schlitt, J.M.; Jackson, D.L.; Schulz, L.C.; Schust, D.J. Preeclampsia: Multiple Approaches for a Multifactorial Disease. Dis. Model Mech. 2012, 5, 9–18. [Google Scholar] [CrossRef]
- Pasiński JSwierczewski, A.; Estemberg, D.; Kowalska-Koprek, U.; Karowicz-Bilińska, A. The influence of vitamin C and E use on concentration of endothelin-1 and lipid peroxides in the serum of pregnant women with arterial hypertension. Ginekol. Pol. 2013, 84, 32–37. [Google Scholar]
- Mackenzie, F.; Greer, I.A. Preventing Eclampsia. Curr. Obstet. Gynaecol. 1996, 6, 159–164. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T Cells: Fates, Functions, and Faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- Mjösberg, J.; Berg, G.; Jenmalm, M.C.; Ernerudh, J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol. Reprod. 2010, 82, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Żabińska, M.; Krajewska, M.; Kościelska-Kasprzak, K.; Jakuszko, K.; Bartoszek, D.; Myszka, M.; Klinger, M. CD4(+)CD25(+)CD127(-) and CD4(+)CD25(+)Foxp3(+) Regulatory T Cell Subsets in Mediating Autoimmune Reactivity in Systemic Lupus Erythematosus Patients. Arch. Immunol. Ther. Exp. 2016, 64, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Scheinecker, C.; Göschl, L.; Bonelli, M. Treg Cells in Health and Autoimmune Diseases: New Insights from Single Cell Analysis. J. Autoimmun. 2020, 110, 102376. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Paroni, M.; Maglie, S.; Alfen, J.S.; Kastirr, I.; Gruarin, P.; De Simone, M.; Pagani, M.; Abrignani, S. Plasticity of Human CD4 T Cell Subsets. Front. Immunol. 2014, 5, 630. [Google Scholar] [CrossRef] [PubMed]
- Plitas, G.; Rudensky, A.Y. Regulatory T Cells: Differentiation and Function. Cancer Immunol. Res. 2016, 4, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Malhotra, R.; Mayer, G.; Gorochov, G.; Miyara, M. Human FOXP3 + T Regulatory Cell Heterogeneity. Clin. Transl. Immunol. 2018, 7, e1005. [Google Scholar] [CrossRef] [PubMed]
- Kwak-Kim, J.; Bao, S.; Lee, S.K.; Kim, J.W.; Gilman-Sachs, A. Immunological modes of pregnancy loss: Inflammation, immune effectors, and stress. Am. J. Reprod. Immunol. 2014, 72, 129–140. [Google Scholar] [CrossRef]
- Dimova, T.; Nagaeva, O.; Stenqvist, A.-C.; Hedlund, M.; Kjellberg, L.; Strand, M.; Dehlin, E.; Mincheva-Nilsson, L. Maternal Foxp3 expressing CD4+ CD25+ and CD4+ CD25– regulatory T-cell populations are enriched in human early normal pregnancy decidua: A phenotypic study of paired decidual and peripheral blood samples. Am. J. Reprod. Immunol. 2011, 66 (Suppl. 1), 44–56. [Google Scholar] [CrossRef]
- Quinn, K.H.; Lacoursiere, D.Y.; Cui, L.; Bui, J.; Parast, M.M. The unique pathophysiology of early-onset severe preeclampsia: Role of decidual T regulatory cells. J. Reprod. Immunol. 2011, 91, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, A.; Haensch, G.M.; Mahnke, K.; Schmitt, E.; Toermer, A.; Meuer, S.; Sohn, C. Distinct subsets of regulatory T cells during pregnancy: Is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin. Immunol. 2008, 129, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.; Svensson, J.; Johansson, E.; Hellström, L.; Casas, R.; Jenmalm, M.C.; Boij, R.; Matthiesen, L.; Jönsson, J.-I.; Berg, G.; et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17β-estradiol. J. Immunol. 2009, 183, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, J.Y.; Hur, S.E.; Kim, C.J.; Na, B.J.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Lédée, N.; Petitbarat, M.; Chevrier, L.; Vitoux, D.; Vezmar, K.; Rahmati, M.; Dubanchet, S.; Gahéry, H.; Bensussan, A.; Chaouat, G. The Uterine Immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am. J. Reprod. Immunol. 2016, 75, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Josefowicz, S.Z.; Arvey, A.; Treuting, P.M.; Rudensky, A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012, 150, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Care, A.S.; Bourque, S.L.; Morton, J.S.; Hjartarson, E.P.; Robertson, S.A.; Davidge, S.T. Reduction in regulatory T cells in early pregnancy causes uterine artery dysfunction in mice. Hypertension 2018, 72, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Motomura, K.; Galaz, J.; Gershater, M.; Lee, E.D.; Romero, R.; Gomez-Lopez, N. Cellular immune responses in the pathophysiology of preeclampsia. J. Leukocyte Biol. 2022, 111, 237–260. [Google Scholar] [CrossRef]
- Hsu, P.; Santner-Nanan, B.; Dahlstrom, J.E.; Fadia, M.; Chandra, A.; Peek, M.; Nanan, R. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am. J. Pathol. 2012, 181, 2149–2160. [Google Scholar] [CrossRef]
- Wagner, M.I.; Jöst, M.; Spratte, J.; Schaier, M.; Mahnke, K.; Meuer, S.; Zeier, M.; Steinborn, A. Differentiation of ICOS+ and ICOS– recent thymic emigrant regulatory T cells (RTE T regs) during normal pregnancy, pre-eclampsia and HELLP syndrome. Clin. Exp. Immunol. 2016, 183, 129. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Li, J.; Kulkarni, A.B.; Perruche, S.; Chen, W.J. A Critical Function for TGF-β Signaling in the Development of Natural CD4+CD25+Foxp3+ Regulatory T Cells. Nat. Immunol. 2008, 9, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.; So, T.; Duan, W.; Soroosh, P. The Significance of OX40 and OX40L to T-Cell Biology and Immune Disease. Immunol. Rev. 2009, 229, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.N.; Budhu, S.; Gasmi, B.; Zappasodi, R.; Hirschhorn-Cymerman, D.; Plitt, T.; De Henau, O.; Zamarin, D.; Holmgaard, R.B.; Murphy, J.T.; et al. The New Era of Cancer Immunotherapy. Adv. Cancer Res. 2015, 128, 1–68. [Google Scholar]
- Imura, A.; Hori, T.; Imada, K.; Ishikawa, T.; Tanaka, Y.; Maeda, M.; Imamura, S.; Uchiyama, T. The Human OX40/Gp34 System Directly Mediates Adhesion of Activated T Cells to Vascular Endothelial Cells. J. Exp. Med. 1996, 183, 2185–2195. [Google Scholar] [CrossRef]
- Piconese, S.; Valzasina, B.; Colombo, M.P. OX40 Triggering Blocks Suppression by Regulatory T Cells and Facilitates Tumor Rejection. J. Exp. Med. 2008, 205, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Darmochwal-Kolarz, D.; Suzuki, D.; Sakai, M.; Ito, M.; Shima, T.; Shiozaki, A.; Rolinski, J.; Saito, S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 2007, 149, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-Eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018, 27, 314–340. [Google Scholar] [CrossRef]
- Smith, D.D.; Merriam, A.A.; Jung, J.; Gyamfi-Bannerman, C. Effect of Maternal Age and Fetal Number on the Risk of Hypertensive Disorders of Pregnancy. Am. J. Perinatol. 2018, 35, 311–316. [Google Scholar]
- Masturzo, B.; Di Martino, D.; Prefumo, F.; Cavoretto, P.; Germano, C.; Gennarelli, G.; Roletti, E.; Bottazzoli, E.; Fusè, F.; Ferrazzi, E.; et al. Higher Rate of Early-Onset Preeclampsia in Pregnancies Following Oocyte Donation According to Increasing Maternal Age. Arch. Gynecol. Obstet. 2019, 300, 861–867. [Google Scholar] [CrossRef]
- Sibai, B.M.; Hauth, J.; Caritis, S.; Lindheimer, M.D.; MacPherson, C.; Klebanoff, M.; VanDorsten, J.P.; Landon, M.; Miodovnik, M.; Paul, R.; et al. Hypertensive Disorders in Twin versus Singleton Gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am. J. Obstet. Gynecol. 2000, 182, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Heimrath, J.; Czekański, A.; Krawczenko, A.; Duś, D. The role of endothelium in the pathogenesis of pregnancy-induced hypertension. Postep. Hig. Med. Dosw. 2007, 61, 48–57. [Google Scholar]
- Nwosu, Z.; Omabe, M. Maternal and Fetal Consequences of Preeclampsia. Internet J. Gynecol. Obstet. 2009, 13, 1–10. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wang, X.Y.; Lu, K.M.; Yu, C.H.; Su, M.T.; Kang, L.; Hsu, K.F.; Chen, P.F.; Lin, S.H. Maternal Th17/Treg Cytokines and Small Extracellular Vesicles in Plasma as Potential Biomarkers for Preeclampsia. Int. J. Med. Sci. 2022, 19, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, S.; Smith, J.; Sferruzzi-Perri, A.; Ledwozyw, A.; Kishore, M.; Haas, R.; Mauro, C.; Williams, D.; Farsky, S.; Marelli-Berg, F.; et al. Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc. Natl. Acad. Sci. USA 2016, 113, E8415–E8424. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.M.; Zimmerman, M.C.; Moore, T.A. Oxidative Stress in Early Pregnancy and the Risk of Preeclampsia. Pregnancy Hypertens. 2019, 18, 99–102. [Google Scholar] [CrossRef]
- Haram, K.; Mortensen, J.H.; Myking, O.; Magann, E.F.; Morrison, J.C. The Role of Oxidative Stress, Adhesion Molecules and Antioxidants in Preeclampsia. Curr. Hypertens Rev. 2019, 15, 105–112. [Google Scholar] [CrossRef]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, immunity, and hypertension. Am. Heart Assoc. Hyperten. 2011, 57, 132–140. [Google Scholar] [CrossRef]
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Höpner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L.; et al. Expression of Ectonucleotidase CD39 by Foxp3+ Treg Cells: Hydrolysis of Extracellular ATP and Immune Suppression. Blood 2007, 110, 1225–1232. [Google Scholar] [CrossRef]
- Matrougui, K.; Zakaria, A.E.; Kassan, M.; Choi, S.; Nair, D.; Gonzalez-Villalobos, R.A.; Chentoufi, A.A.; Kadowitz, P.; Belmadani, S.; Partyka, M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am. J. Pathol. 2011, 178, 434–441. [Google Scholar] [CrossRef]
- Maganto-García, E.; Bu, D.-X.; Tarrio, M.L.; Alcaide, P.; Newton, G.; Griffin, G.K.; Croce, K.J.; Luscinskas, F.W.; Lichtman, A.H.; Grabie, N. Foxp3+ -inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J. Immunol. 2011, 187, 3521–3529. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Amaral, L.M.; Wallace, K.; Thomas, A.J.; Campbell, N.; Scott, J.; Herse, F.; Haase, N.; Moseley, J.; Wallukat, G.; et al. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R884–R891. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, D.C.; Amaral, L.M.; Wallace, K.; Campbell, N.; Thomas, A.J.; Scott, J.; Herse, F.; Wallukat, G.; Dechend, R.; LaMarca, B. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1192–R1199. [Google Scholar] [CrossRef] [PubMed]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, A.; Schmitt, E.; Kisielewicz, A.; Rechenberg, S.; Seissler, N.; Mahnke, K.; Schaier, M.; Zeier, M.; Sohn, C. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin. Exp. Immunol. 2012, 167, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015, 6, 639. [Google Scholar] [CrossRef] [PubMed]
- Sadlon, T.; Brown, C.Y.; Bandara, V.; Hope, C.M.; Schjenken, J.; Pederson, S.M.; Breen, J.; Forrest, A.; Beyer, M.; Robertson, S.; et al. Unravelling the molecular basis for regulatory T-cell plasticity and loss of function in disease. Clin. Transl. Immunol. 2018, 7, e1011. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Zhang, B.; Chan, H.Y.; Sharkey, D.J.; Fullston, T.; Robertson, S.A. miRNA Regulation of immune tolerance in early pregnancy. Am. J. Reprod. Immunol. 2016, 75, 272–280. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Perlman, S. Differential effects of IL-12 on Tregs and non-Treg T cells: Roles of IFNγ, IL-2 and IL-2R. PLoS ONE 2012, 7, e46241. [Google Scholar] [CrossRef]
- Hori, S. Lineage stability and phenotypic plasticity of Foxp3+ regulatory T cells. Immunol. Rev. 2014, 259, 159–172. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Walunas, T.L.; Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996, 14, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. OX40 and OX40L Interaction in Cancer. Curr. Med. Chem. 2021, 28, 5659–5673. [Google Scholar] [CrossRef] [PubMed]
- Gramaglia, I.; Weinberg, A.D.; Lemon, M.; Croft, M. Ox-40 ligand: A potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 1998, 161, 6510–6517. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Ishii, N.; Takano, H.; Miura, S.; Ndhlovu, L.C.; Nose, M.; Noda, T.; Sugamura, K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 2000, 191, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.R.; Gayle, R.B.; Ramsdell, F.; Srinivasan, S.; Sorensen, R.A.; Watson, M.L.; Seldin, M.F.; Clifford, K.N.; Grabstein, K.; Alderson, M.R. Identification of OX40 Ligand and Preliminary Characterization of Its Activities on OX40 Receptor. Circ. Shock 1994, 44, 30–34. [Google Scholar] [PubMed]
- Wu, J.; Cui, Y.; Zhu, W.; Bai, S.; Zhao, N.; Liu, B. Critical Role of OX40/OX40L in ILC2-Mediated Activation of CD4+T Cells during Respiratory Syncytial Virus Infection in Mice. Int. Immunopharmacol. 2019, 76, 105784. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J.L. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Bansal-Pakala, P.; Jember, A.G.; Croft, M. Signaling through OX40 (CD134) breaks peripheral T cell tolerance. Nat. Med. 2001, 7, 907–912. [Google Scholar] [CrossRef]
- Demirci, G.; Amanullah, F.; Kewalaramani, R.; Yagita, H.; Strom, T.B.; Sayegh, M.H.; Li, X.C. Critical role of OX40 in CD28 and CD154 independent rejection. J. Immunol. 2004, 172, 1691–1698. [Google Scholar] [CrossRef]
- Miller, M.L.; Alegre, M.L.; Chong, A.S. Transplantation Tolerance after Allograft Rejection. Curr. Opin. Organ Transplant. 2017, 22, 64–70. [Google Scholar] [CrossRef]
- Sugamura, K.; Ishii, N.; Weinberg, A.D. Therapeutic targeting of the effector T cell costimulatory molecule OX40. Nat. Rev. Immunol. 2004, 4, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Bornsen, L.; Christensen, J.R.; Ratzer, R.; Oturai, A.B.; Sorensen, P.S.; Sondergaard, H.B.; Sellebjerg, F. Effect of natalizumab oncirculating CD4+ T-cells in multiple sclerosis. PLoS ONE 2012, 7, e47578. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.C. OX40L-OX40 Interactions: A Possible Target for Gastrointestinal Autoimmune Diseases. N. Am. J. Med. Sci 2012, 4, 533–536. [Google Scholar] [PubMed]
- Tripathi, T.; Yin, W.; Xue, Y.; Zurawski, S.; Fujita, H.; Hanabuchi, S.; Liu, Y.J.; Oh, S.K.; Joo, H.M. Central Roles of OX40L-OX40 Interaction in the Induction and Progression of Human T Cell-Driven Acute Graft-versus-Host Disease. ImmunoHorizons 2019, 3, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Findlay, E.G.; Danks, L.; Madden, J.; Cavanagh, M.M.; McNamee, K.; McCann, F.; Snelgrove, R.J.; Shaw, S.; Feldmann, M.; Taylor, P.C.; et al. OX40L Blockade Is Therapeutic in Arthritis, despite Promoting Osteoclastogenesis. Proc. Nat. Acad. Sci. USA 2014, 111, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Lé, A.M.; Torres, T. OX40-OX40L Inhibition for the Treatment of Atopic Dermatitis—Focus on Rocatinlimab and Amlitelimab. Pharmaceutics 2022, 14, 2753. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, S.; Zhang, X.; Jia, K.; Wang, H.; Zhou, C.; He, Y. OX40 (CD134) and OX40 Ligand, Important Immune Checkpoints in Cancer. OncoTargets Ther. 2019, 12, 7347–7353. [Google Scholar] [CrossRef]
- Wang, X.; Ria, M.; Kelmenson, P.M.; Eriksson, P.; Higgins, D.C.; Samnegård, A.; Petros, C.; Rollins, J.; Bennet, A.M.; Wiman, B.; et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat. Genet. 2005, 37, 365–372. [Google Scholar] [CrossRef]
- Rabieyousefi, M.; Soroosh, P.; Satoh, K.; Date, F.; Ishii, N.; Yamashita, M.; Oka, M.; McMurtry, I.F.; Shimokawa, H.; Nose, M.; et al. Indispensable roles of OX40L-derived signal and epistatic genetic effect in immune-mediated pathogenesis of spontaneous pulmonary hypertension. BMC Immunol. 2011, 12, 67. [Google Scholar] [CrossRef]
- Yoshimatsu, J.; Matsumoto, H.; Narahara, H. Co-stimulatory molecule OX40 ligand in early human pregnancy. Int. J. Gynaecol. Obstet. 2006, 93, 240–241. [Google Scholar] [CrossRef]
- Rahmani, F.; Hadinedoushan, H.; Ghasemi, N. Relative Expression of OX40, OX40L mRNA, and OX40L Serum Levels in Women with Recurrent Spontaneous Abortion. Immunol. Investig. 2019, 48, 480–489. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, X.; Liu, C.; Lyu, J.; Liu, X.; Zhong, S.; Liang, Y.; Liu, P.; Huang, L.; Xiao, Z.; et al. Phenotypic and functional alteration of CD45+ immune cells in the decidua of preeclampsia patients analyzed by mass cytometry (CyTOF). Front. Immunol. 2023, 13, 1047986. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Study group n = 60 | Gestational hypertension | Chronic hypertension Superimposed pre-eclampsia Other chronic maternal diseases Threatening preterm delivery PROM Signes of infection |
| Pre-eclampsia | ||
| Control group n = 48 | Healthy pregnancy | Any maternal disease or pregnancy complication |
| Variable | Control Group (n = 48) n (%) or Median [Interquartile Range] (Min-Max) | Study Group (n = 60) n (%) or Median [Interquartile Range] (Min-Max) | p or Chi2, p | Gestational Hypertension (n = 30) n (%) lub Median [Interquartile Range] (Min-Max) | Pre-Eclampsia (n = 30) n (%) lub Median [Interquartile Range] (Min-Max) | p or Chi2, p |
|---|---|---|---|---|---|---|
| Maternal age (years) | 29 [27–32] (18–40) | 31 [27.5–35] (19–42) | 0.1425 | 31 [28–34] (19–41) | 30.5 [26–36] (21–42) | 0.9410 |
| Maternal age and parity Primiparas and multiparas 21–34 yo Primiparas ≤20 yo Multiparas ≥35 yo | 43 (89.6%) 5 (10.4%) | 50 (83.3%) 10 (16.7%) | 0.5136 | 25 (83.3%) 5 (16.7%) | 26 (86.7%) 4 (13.3%) | 1.0000 |
| Number of pregnancies | 2 [1–2] (1–4) | 1 [1–2] (1–3) | 0.1492 | 1 [1–2] (1–3) | 1 [1–2] (1–3) | 0.3942 |
| Number of pregnancies 1 2 3 4 | 23 (47.9%) 21 (43.7%) 3 (6.2%) 1 (2.1%) | 38 (63.3%) 17 (28.3%) 5 (8.3%) - | 0.2280 | 17 (56.7%) 11 (36.7%) 2 (6.7%) | 22 (70%) 6 (20%) 2 (10%) | 0.3514 |
| Parity | 1 [1–2] (1–3) | 1 [1–2] (1–3) | 0.3087 | 1 [1–2] (1–2) | 1 [1–2] (1–3) | 0.7182 |
| Parity 1 2 3 | 29 (60.4%) 17 (35.4%) 2 (4.2%) | 42 (70%) 16 (26.7%) 2 (3.3%) | 0.5796 | 20 (66.7%) 10 (33.3%) - | 22 (73.3%) 6 (20%) 2 (6.7%) | 0.2128 |
| Number of miscarriages | 0 [0–0] (0–1) | 0 [0–0] (0–2) | 0.2253 | 0 [0–0] (0–2) | 0 [0–0] (0–1) | 0.4096 |
| History of miscarriage 0 1 2 | 39 (81.2%) 9 (18.8%) - | 53 (89.9%) 5 (8.5%) 1 (1.7%) | 0.2043 | 26 (86.7%) 3 (10%) 1 (3.3%) | 26 (93.1%) 2 (6.9%) - | 0.5482 |
| Gestational age at sampling | 40 [39–40] (37–41) | 38 [36–39] (24–41) | <0.0001 | 39 [38–40] (33–40) | 36.5 [32–38] (24–41) | 0.0001 |
| Gestational age at delivery | 40 [39–41] (37–41) | 38 [37–39] (28–41) | <0.0001 | 39 [38–40] (33–41) | 38 [34–39] (28–41) | 0.0136 |
| Variable | Control Group (n = 48) Median [Interquartile Range] (Min-Max) | Study Group (n = 60) Median [Interquartile Range] (Min-Max) | p | Gestational Hypertension (n = 30) Median [Interquartile Range] (Min-Max) | Pre-Eclampsia (n = 30) Median [Interquartile Range] (Min-Max) | p |
|---|---|---|---|---|---|---|
| Peripheral lymphocytes (percentage) | 45.3 [37–60.9] (14.6–72.9) | 47.5 [39.4–54.7] (16.6–79.1) | 0.9501 | 47.6 [41.6–54.7] (23.4–61.7) | 47.3 [37.3–54] (16.6–79.1) | 0.7159 |
| Percentage of lymphocytes CD4+FoxP3+ | 37.4 [31.8–42.2] (17–56) | 37.9 [31.9–42.4] (18.2–60) | 0.7666 | 38.2 [36.1–42.3] (21.4–53.4) | 37.3 [27.8–42.8] (18.2–60) | 0.3912 |
| Percentage of CD4+CD25+FoxP3+ | 2.4 [0.9–3.9] (0.2–19) | 1.9 [0.8–3.4] (0.2–8.1) | 0.1677 | 1.4 [0.7–2] 90.2–7.5) | 2.1 [1–4] (0.3–8.1) | 0.0389 |
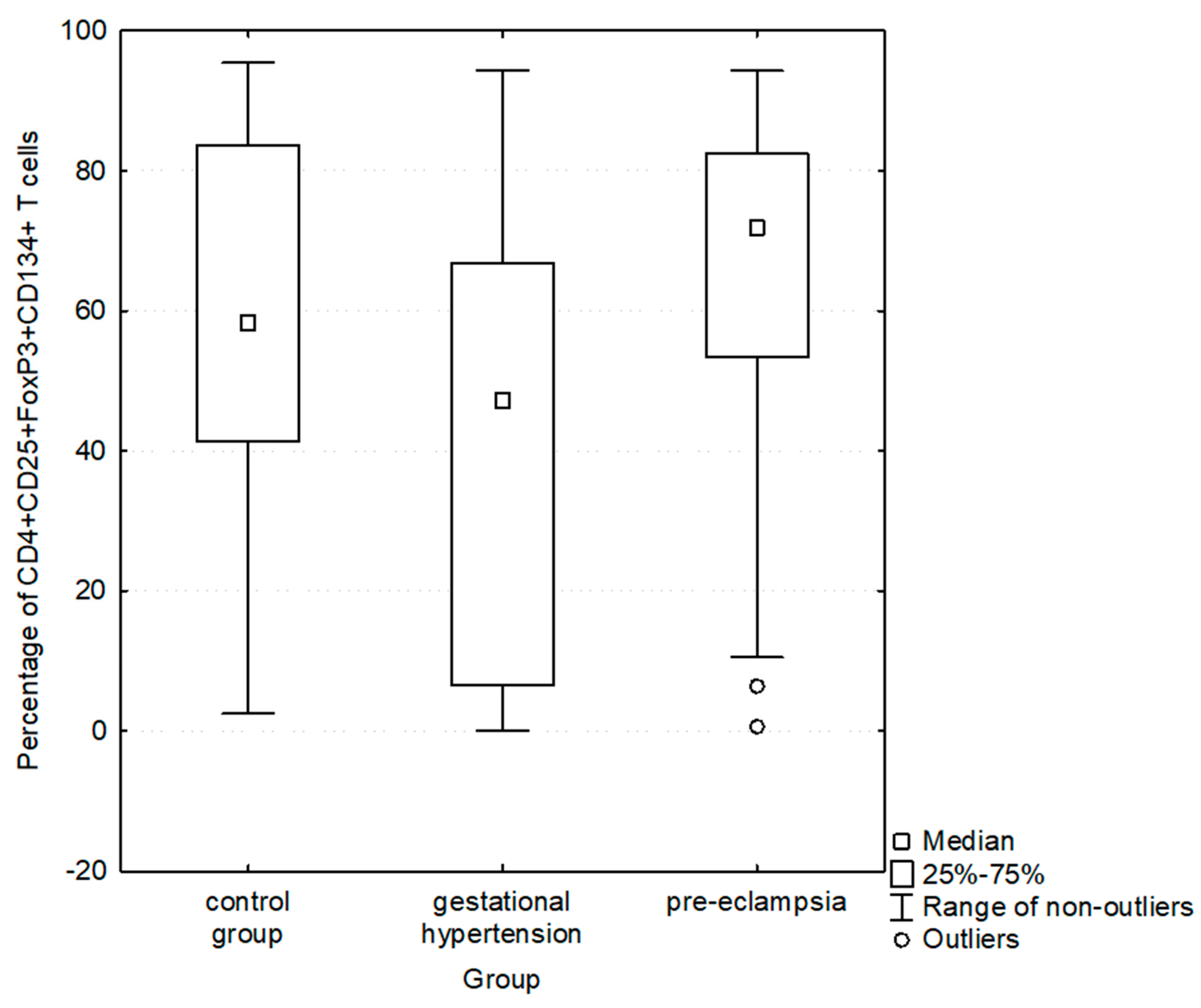
| Percentage of CD4+CD25+FoxP3+ expressing CD134+ | 58.3 [41.3–83.7] (2.5–95.4) | 59.5 [13.3–75.8] (0–94.3) | 0.3180 | 43.2 [6.5–65.6] (0–94.3) | 71.9 [53.4–82.4] (0.6–94.3) | 0.0014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatek, M.; Kojak, A.; Kwaśniewska, A. OX40 (CD134) Expression on T Regulatory Cells Is Related to Serious Hypertensive Disorders in Pregnancy. J. Cardiovasc. Dev. Dis. 2023, 10, 431. https://doi.org/10.3390/jcdd10100431
Kwiatek M, Kojak A, Kwaśniewska A. OX40 (CD134) Expression on T Regulatory Cells Is Related to Serious Hypertensive Disorders in Pregnancy. Journal of Cardiovascular Development and Disease. 2023; 10(10):431. https://doi.org/10.3390/jcdd10100431
Chicago/Turabian StyleKwiatek, Maciej, Agnieszka Kojak, and Anna Kwaśniewska. 2023. "OX40 (CD134) Expression on T Regulatory Cells Is Related to Serious Hypertensive Disorders in Pregnancy" Journal of Cardiovascular Development and Disease 10, no. 10: 431. https://doi.org/10.3390/jcdd10100431
APA StyleKwiatek, M., Kojak, A., & Kwaśniewska, A. (2023). OX40 (CD134) Expression on T Regulatory Cells Is Related to Serious Hypertensive Disorders in Pregnancy. Journal of Cardiovascular Development and Disease, 10(10), 431. https://doi.org/10.3390/jcdd10100431





