Investigating the Influence of High-Speed Gantry Rotation in Cardiac CT on Motion Artifacts in Aortic Stenosis Patients Not Premedicated with β-Blockers: The FAST-CCT Randomized Trial Protocol
Abstract
:1. Introduction
1.1. Role and Challenges of CCTA
1.2. Technical Advancements in Cardiac CTA
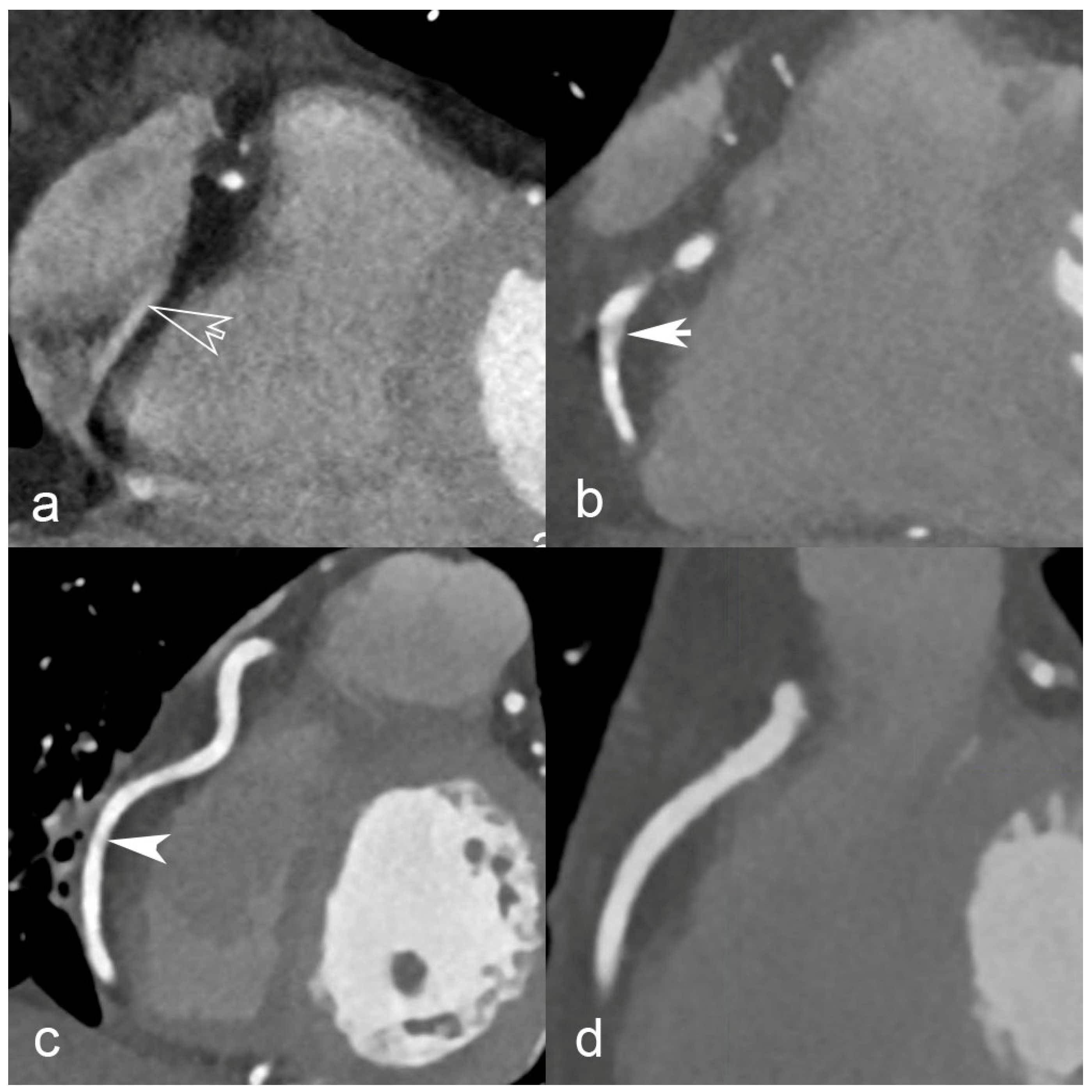
- (a)
- Mechanical stress. The gantry, including the X-ray tube, detectors, and other components, must be designed and built to withstand the increased rotational forces and vibrations associated with higher speeds. This requires robust engineering and precision manufacturing to ensure the gantry can handle the increased load without compromising performance or longevity.
- (b)
- Heat dissipation. Faster rotation times mean shorter exposure times for each angular view, resulting in higher X-ray tube power requirements to maintain image quality. This increased power generation leads to higher heat generation, and effective heat dissipation becomes crucial. Designing tubes that can deliver higher currents and equipped with efficient cooling mechanisms to dissipate the excess heat and prevent overheating is essential for maintaining optimal system performance and preventing damage.
- (c)
- Data acquisition and processing. Faster rotation speeds generate a larger amount of data in a shorter period of time. This increases demand on data acquisition and processing systems, including detector speed, readout capabilities, data transfer rates, and computational power. The system must handle the increased data volume and process it efficiently to reconstruct high-quality images within the desired time frame.
2. Materials and Methods
2.1. Rationale, Aim, and Design of the Study
2.2. Sample Size, Inclusion, and Exclusion Criteria
2.3. Characteristics of Participants, Randomization, and Blinding
2.4. Processes and Interventions
2.5. Measured Outcomes
2.6. Data Management
2.7. Safety Considerations
2.8. Type of Data
2.9. Statistical Analysis
2.10. Ethical Considerations and Declarations
2.11. Study Status and Timeline
3. Discussion
3.1. Rationale of the FAST-CCTA Study
3.2. Limitations of the Study Design
3.3. Dissemination
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Underlying Cause of Death, 1999–2019 Request. Available online: https://wonder.cdc.gov/ucd-icd10.html (accessed on 29 July 2023).
- Jordan, L.C.; Khan, S.S.; Kissela, B.M.; Knutson, K.L.; Kwan, T.W.; Lackland, D.T.; Lewis, T.T.; Lichtman, J.H.; Longenecker, C.T.; Loop, M.S.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Bourassa, M.G. The history of cardiac catheterization. Can. J. Cardiol. 2005, 21, 1011–1014. [Google Scholar]
- Carpeggiani, C.; Picano, E.; Brambilla, M.; Michelassi, C.; Knuuti, J.; Kauffman, P.; Underwood, S.R.; Neglia, D.; EVINCI Study Investigators. Variability of radiation doses of cardiac diagnostic imaging tests: The RADIO-EVINCI study (RADIationdOse subproject of the EVINCI study). BMC Cardiovasc. Disord. 2017, 17, 63. [Google Scholar] [CrossRef]
- Schoepf, U.J.; Zwerner, P.L.; Savino, G.; Herzog, C.; Kerl, J.M.; Costello, P. Coronary CT angiography. Radiology 2007, 244, 48–63. [Google Scholar] [CrossRef]
- Collet, C.; Onuma, Y.; Andreini, D.; Sonck, J.; Pompilio, G.; Mushtaq, S.; La Meir, M.; Miyazaki, Y.; de Mey, J.; Gaemperli, O.; et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur. Heart J. 2018, 39, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Štěchovský, C.; Šakalyte, G.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N. Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [CrossRef]
- Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; MacLean, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Ghekiere, O.; Salgado, R.; Buls, N.; Leiner, T.; Mancini, I.; Vanhoenacker, P.; Dendale, P.; Nchimi, A. Image quality in coronary CT angiography: Challenges and technical solutions. Br. J. Radiol. 2017, 90, 20160567. [Google Scholar] [CrossRef] [PubMed]
- Pannu, H.K.; Alvarez, W.; Fishman, E.K. β-Blockers for Cardiac CT: A Primer for the Radiologist. Am. J. Roentgenol. 2006, 186, S341–S345. [Google Scholar] [CrossRef]
- Kalisz, K.; Buethe, J.; Saboo, S.S.; Abbara, S.; Halliburton, S.; Rajiah, P. Artifacts at Cardiac CT: Physics and Solutions. Radio Graph. 2016, 36, 2064–2083. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, J.; Xu, D.; Dong, Z.; Li, X.; Zhang, L. CTCA image quality improvement by using snapshot freeze technique under prospective and retrospective electrocardiographic gating. J. Comput. Assist. Tomogr. 2015, 39, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Depierre, A.; Topolsky, A.; Roguelov, C.; Dupre, M.; Rubimbura, V.; Eeckhout, E.; Qanadli, S.D.; Muller, O.; Mahendiran, T.; et al. Computed Tomography Angiography for the Diagnosis of Coronary Artery Disease Among Patients Undergoing Transcatheter Aortic Valve Implantation. J. Cardiovasc. Transl. Res. 2021, 14, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Schicchi, N.; Fogante, M.; Pirani, P.E.; Agliata, G.; Piva, T.; Tagliati, C.; Marcucci, M.; Francioso, A.; Giovagnoni, A. Third generation dual source CT with ultra-high pitch protocol for TAVI planning and coronary tree assessment: Feasibility, image quality and diagnostic performance. Eur. J. Radiol. 2020, 122, 108749. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Hughes, G.J.; Owens, P.E.; Marshall, A.J.; Roobottom, C.A. Thoracic aorta at multi-detector row CT: Motion artifact with various reconstruction windows. Radiology 2003, 228, 583–588. [Google Scholar] [CrossRef]
- Sondergaard, L.; Steinbruchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Ngo, A.T.; Olsen, N.T.; Chang, Y.; Franzen, O.W.; et al. Two-Year Outcomes in Patients with Severe Aortic Valve Stenosis Randomized to Transcatheter Versus Surgical Aortic Valve Replacement: The All-Comers Nordic Aortic Valve Intervention Randomized Clinical Trial. Circ. Cardiovasc. Interv. 2016, 9, e003665. [Google Scholar] [CrossRef]
- Rotzinger, D.C.; Si-Mohamed, S.A.; Yerly, J.; Boccalini, S.; Becce, F.; Boussel, L.; Meuli, R.A.; Qanadli, S.D.; Douek, P.C. Reduced-iodine-dose dual-energy coronary CT angiography: Qualitative and quantitative comparison between virtual monochromatic and polychromatic CT images. Eur. Radiol. 2021, 31, 7132–7142. [Google Scholar] [CrossRef]
- Harvey, L. REDCap: Web-based software for all types of data storage and collection. Spinal Cord 2018, 56, 625. [Google Scholar] [CrossRef]
- Wintersperger, B.J.; Nikolaou, K.; von Ziegler, F.; Johnson, T.; Rist, C.; Leber, A.; Flohr, T.; Knez, A.; Reiser, M.F.; Becker, C.R. Image Quality, Motion Artifacts, and Reconstruction Timing of 64-Slice Coronary Computed Tomography Angiography with 0.33-Second Rotation Speed. Investig. Radiol. 2006, 41, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.T.; Bax, J.J.; Davies, L.C. Cardiac CT and CT coronary angiography: Technology and application. Heart 2008, 94, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.M.; Rybicki, F.J.; Steigner, M. CT Coronary Angiography: 256-Slice and 320-Detector Row Scanners. Curr. Cardiol. Rep. 2010, 12, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ohnesorge, B.M.; Hofmann, L.K.; Flohr, T.G.; Schoepf, U.J. CT for imaging coronary artery disease: Defining the paradigm for its application. Int. J. Cardiovasc. Imaging 2005, 21, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Weustink Annick, C.; Meijboom Willem, B.; Mollet Nico, R.; Otsuka, M.; Pugliese, F.; van Mieghem, C.; Malago, R.; van Pelt, N.; Dijkshoorn Marcel, L.; Cademartiri, F.; et al. Reliable High-Speed Coronary Computed Tomography in Symptomatic Patients. J. Am. Coll. Cardiol. 2007, 50, 786–794. [Google Scholar] [CrossRef]
- Flohr, T.; Bruder, H.; Stierstorfer, K.; Simon, J.; Schaller, S.; Ohnesorge, B. New Technical Developments in Multislice CT, Part 2: Sub-Millimeter 16-Slice Scanning and Increased Gantry Rotation Speed for Cardiac Imaging. Rofo 2002, 174, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Marwan, M.; Schepis, T.; Pflederer, T.; Bruder, H.; Allmendinger, T.; Petersilka, M.; Anders, K.; Lell, M.; Kuettner, A.; et al. High-pitch spiral acquisition: A new scan mode for coronary CT angiography. J. Cardiovasc. Comput. Tomogr. 2009, 3, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.; Küttner, A.; Bruder, H.; Stierstorfer, K.; Halliburton, S.S.; Schaller, S.; Ohnesorge, B.M. Performance Evaluation of a Multi-Slice CT System with 16-Slice Detector and Increased Gantry Rotation Speed for Isotropic Submillimeter Imaging of the Heart. Herz 2003, 28, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shirasaka, T.; Yamasaki, Y.; Kondo, M.; Hamasaki, H.; Mikayama, R.; Sakai, Y.; Kato, T.; Nishie, A.; Ishigami, K.; et al. Importance of the heart rate in ultra-high-resolution coronary CT angiography with 0.35 s gantry rotation time. Jpn. J. Radiol. 2022, 40, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Beeres, M.; Wichmann, J.L.; Paul, J.; Mbalisike, E.; Elsabaie, M.; Vogl, T.J.; Nour-Eldin, N.-E.A. CT chest and gantry rotation time: Does the rotation time influence image quality? Acta Radiol. 2015, 56, 950–954. [Google Scholar] [CrossRef]
- Belsack, D.; Tanaka, K.; Buls, N.; de Mey, J. 427 Diagnostic Performance of Coronary Ct Angiography By 0.23 ms Ct Gantry Rotation Time in Patients with High Heart Rates: A Preliminary Study. J. Cardiovasc. Comput. Tomogr. 2022, 16, S13–S14. [Google Scholar] [CrossRef]

| Standard Protocol | Fast Protocol | |
|---|---|---|
| Tube voltage (kVp) | 100 | 100 |
| Tube current (mA) | 500–1000 | 500–1000 |
| Noise index | 20 | 20 |
| Rotation time (s/rot) | 0.28 | 0.23 |
| Acquisition mode | Axial | Axial |
| Injection phase | Arterial | Arterial |
| Phases of cardiac cycle | 20–80% (prospective) | 20–80% (prospective) |
| Collimation (mm) | 256 × 0.625 | 256 × 0.625 |
| FOV (mm) | 220–250 | 220–250 |
| Matrix size (pixels) | 512 × 512 | 512 × 512 |
| Slice thickness (mm) | 0.625 | 0.625 |
| Slice incrementation (mm) | 0.6 | 0.6 |
| Reconstruction kernel | Standard | Standard |
| Reconstruction algorithm | DLIR H | DLIR H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahrni, G.; Gullo, G.; Touray, A.; Fournier, S.; Jouannic, A.-M.; Lu, H.; Racine, D.; Muller, O.; Pozzessere, C.; Qanadli, S.D.; et al. Investigating the Influence of High-Speed Gantry Rotation in Cardiac CT on Motion Artifacts in Aortic Stenosis Patients Not Premedicated with β-Blockers: The FAST-CCT Randomized Trial Protocol. J. Cardiovasc. Dev. Dis. 2023, 10, 424. https://doi.org/10.3390/jcdd10100424
Fahrni G, Gullo G, Touray A, Fournier S, Jouannic A-M, Lu H, Racine D, Muller O, Pozzessere C, Qanadli SD, et al. Investigating the Influence of High-Speed Gantry Rotation in Cardiac CT on Motion Artifacts in Aortic Stenosis Patients Not Premedicated with β-Blockers: The FAST-CCT Randomized Trial Protocol. Journal of Cardiovascular Development and Disease. 2023; 10(10):424. https://doi.org/10.3390/jcdd10100424
Chicago/Turabian StyleFahrni, Guillaume, Giuseppe Gullo, Aisha Touray, Stéphane Fournier, Anne-Marie Jouannic, Henri Lu, Damien Racine, Olivier Muller, Chiara Pozzessere, Salah D. Qanadli, and et al. 2023. "Investigating the Influence of High-Speed Gantry Rotation in Cardiac CT on Motion Artifacts in Aortic Stenosis Patients Not Premedicated with β-Blockers: The FAST-CCT Randomized Trial Protocol" Journal of Cardiovascular Development and Disease 10, no. 10: 424. https://doi.org/10.3390/jcdd10100424






