Abstract
Background: The incidence of acute myocardial infarction (AMI) in the younger population has been increasing gradually in recent years. The objective of the present study is to investigate the safety and effectiveness of drug-eluting balloons (DEBs) in young patients with AMI. Methods: All consecutive patients with AMI aged ≤ 45 years were retrospectively enrolled. The primary endpoint was a device-oriented composite endpoint (DOCE) of cardiac death, target vessel myocardial infarction (MI), or target lesion revascularization (TLR). The secondary study endpoints included heart failure and major bleeding events. Results: A total of 276 young patients presenting with AMI were finally included. The median follow-up period was 1155 days. Patients treated with DEBs had a trend toward a lower incidence of DOCEs (3.0% vs. 11.0%, p = 0.12) mainly driven by the need for TLR (3.0% vs. 9.1%, p = 0.19) than those treated with DESs. No significant differences between the two groups were detected in the occurrence of cardiac death (0.0% vs. 0.5%, p = 0.69), MI (0.0% vs. 1.4%, p = 0.40), heart failure (0.0% vs. 1.9%, p = 0.39), or major bleeding events (1.5% vs 4.8%, p = 0.30). Multivariate regression analysis showed that DEBs were associated with a trend toward a lower risk of DOCEs (HR 0.13, 95% CI [0.02, 1.05], p = 0.06). Conclusions: The findings of the present study suggested that DEBs might be a potential treatment option in young patients with AMI. A larger scale, randomized, multicenter study is required to investigate the safety and effectiveness of DEBs in this setting.
1. Introduction
Although the incidence of acute myocardial infarction (AMI) in the general population has significantly declined during the last decades, this decreasing tendency has not been uniformly observed in the young population [1]. In fact, the proportion of AMI hospitalizations attributable to younger individuals has been increasing gradually in recent years and accounts for up to 4–10% of all AMIs [2,3]. Moreover, the long-term prognosis in the young population is unfavorable, as it indicates that the death rate is up to 12–20%, and the rate of major adverse cardiac events (MACE) reaches 23–30% during the 15-year follow-up period [4,5,6]. Additionally, AMI occurring at a young age carries significant morbidity, psychological consequences, and economic burdens for patients and families because it affects those who are in the stage of full productivity [7,8]. Therefore, there is a great need to advance more effective awareness and treatment strategies specific to this important clinical entity.
According to current guidelines, percutaneous coronary intervention (PCI) with a drug-eluting stent (DES) is the preferred revascularization strategy for patients of all ages who present with AMI for the benefit of timely recovering coronary artery blood flow as well as decreasing the risk of repeat revascularization [9]. However, permanent vascular implants after implanting DES could lead to an increased risk of late and very late stent thrombosis, and recent studies suggested that younger age was an independent risk factor for late stent thrombosis (LST) and very late stent thrombosis (VLST) [10,11,12]. Additionally, intervention with a stent has been demonstrated to be associated with increased stress disorder in younger individuals [13]. Therefore, the concept of avoiding permanent implants may be especially attractive for younger patients with AMI.
A drug-eluting balloon (DEB) is a novel treatment strategy that has emerged in recent years and has been proven to be safe and effective in treating patients with in-stent restenosis and shown promising results in other indications such as small vessel disease, diffuse disease, bifurcations, chronic total occlusions, and calcified complex lesions [14,15]. The advantage of DEB is that it could rapidly provide a homogeneous distribution and high-concentration of antirestenotic drugs into the target lesion of the culprit coronary artery without using durable polymers and stent structures, thus fulfilling the requirements of “leaving nothing behind” to avoid long-term, stent-related complications as well as reduce the risk of stress disorders after PCI. However, to date, there are no data investigating the safety and effectiveness of DEBs among young patients with AMI. The objective of the present study is to investigate the safety and effectiveness of DEBs compared with DESs for young patients presenting with AMI.
2. Methods
2.1. Study Design and Population
All consecutive patients admitted to Beijing Chaoyang Hospital with AMI from 1 January 2017 to 1 January 2022 were retrospectively analyzed in this single-center study. Patients were included if they met the following criteria: (1) age 18 to 45 years; (2) presenting with ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI or presenting with non-ST-segment elevation myocardial infarction (NSTEMI) undergoing early invasive strategy (<24 h from symptom onset); and (3) receiving DES or DEB treatment. Patients were excluded if they met the following criteria: (1) presenting with stable or unstable angina pectoris; (2) cardiac arrest or cardiogenic shock; (3) mechanical complications; (4) ongoing malignant process; (5) receiving conservative treatment; (6) in-stent restenosis; (7) severe coronary artery tortuosity and calcification; (8) coronary artery ectasia; (9) undergoing plain old balloon angioplasty (POBA), a bare metal stent (BMS), or coronary bypass surgery; or (10) the combination use of DES and DEB in the target vessel segment. This study complied with the Declaration of Helsinki and has been approved by the ethical committee of Beijing Chaoyang Hospital.
2.2. Interventional Procedure
The PCI procedure was performed according to current international guidelines and local practice. All patients were administered 300 mg of aspirin and 300–600 mg of clopidogrel or 180 mg of ticagrelor as loading doses before the procedure. Unfractionated heparin was intravenously given as an initial bolus of 100 IU/kg body weight followed by additional boluses to maintain an activated clotting time of ≥250 s during the procedure.
In all cases, decisions regarding intervention strategy (DES or DEB), appropriate length and diameter of DES or DEB, inflation time and pressure of DES or DEB, administration of intra-aortic balloon pump, thrombus aspiration, and glycoprotein IIb/IIIa inhibitor during the procedure were left to the discretion of the operator. The procedure was considered successful if the visual postprocedural residual stenosis was ≤30% after PCI. A bailout stenting was performed if there was an apparent flow-limiting dissection (grade C–F) or residual stenosis >30% after DEB implantation.
After PCI, a dual antiplatelet therapy (DAPT) was prescribed using aspirin (100 mg/d) and either clopidogrel (75 mg/d) or ticagrelor (90 mg twice per day). DAPT was suggested for 3–6 months after DCB and 12 months in patients treated with DES.
2.3. Endpoints and Definitions
The primary endpoint was a device-oriented composite endpoint (DOCE) of cardiac death, target vessel myocardial infarction (MI), or target lesion revascularization (TLR) [16]. All deaths were considered cardiac unless a definite noncardiac cause could be documented. MI was defined as recurrent ischemic symptoms lasting ≥30 min together with either new electrocardiographic changes or elevation of troponin or creatine kinase MB isoenzyme level. Target vessel MI was defined as an MI not clearly attributable to a nontarget vessel. TLR was defined as any repeat percutaneous intervention or bypass surgery of the target lesion (including the whole DEB- or DES-treated segment plus 5 mm proximal and distal of the treated segment) due to restenosis, stent thrombosis, and coronary dissection. The secondary study endpoints included heart failure and major bleeding events. Heart failure was defined as any congestive heart failure (rales, dyspnoea, and New York Heart Association (NYHA) class III–IV) after the index procedure [17]. Major bleeding events were defined according to the International Society on Thrombosis and Hemostasis (ISTH) criteria [18].
2.4. Clinical Follow-Up
Clinical follow-up by reviewing hospital records, clinic visits, and telephone interviews was conducted during hospitalization and at 30 days, 3 months, 6 months, and 12 months after the index procedure and every three months thereafter. All adverse events were adjudicated by independent physicians who were not involved in the procedures.
2.5. Statistical Analysis
Continuous data were expressed as mean ± SD or median and interquartile range and were compared using Student’s t-test or Mann–Whitney rank-sum test. Categorical data were expressed as numbers and percentages and compared using Pearson’s chi-square test or Fisher’s exact test. Event-free survival was estimated with the Kaplan–Meier method and compared using the log-rank test. Multivariate Cox proportional hazard regression models were performed to identify significant independent predictors of the endpoints. Prespecified subgroup analyses by age (≤40 vs. >40 years), infarct-related artery (left anterior descending artery vs. nonleft anterior descending artery), Killip class (Killip class I vs. II–III), thrombolysis in myocardial infarction (TIMI) flow pre-PCI (0–1 vs. 2–3), device diameter (<3 vs. ≥3 mm), device length (<30 vs. ≥30 mm), non-infarct-related artery stenosis (<70% vs. ≥70%), the use of thrombus aspiration, and additional administration of glycoprotein IIb/IIIa inhibitors were performed to evaluate the consistency of treatment effects. Interactions between treatment groups were analyzed using Cox proportional hazards models. A value of p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 26.0 software (SPPS Inc., Chicago, IL, USA).
3. Results
From January 2017 to January 2022, a total of 4309 patients with AMI were admitted to Beijing Chaoyang Hospital, and 347 of them were identified as young AMI patients who were at or under 45 years old. Among these, 71 patients were not eligible for this study due to the following reasons: cardiac arrest or cardiogenic shock (n = 2); not undergoing coronary angiography or PCI (n = 18); receiving medical treatment (n = 20); undergoing plain balloon angioplasty (n = 23); undergoing coronary bypass surgery (n = 2); coronary artery ectasia (n = 2); or the combination use of DES and DEB in the target vessel segment (n = 4). Thus, a total of 276 younger patients presenting with AMI were enrolled in the final analysis, including 67 patients receiving DEB treatment and 209 patients receiving DES treatment (Figure 1).
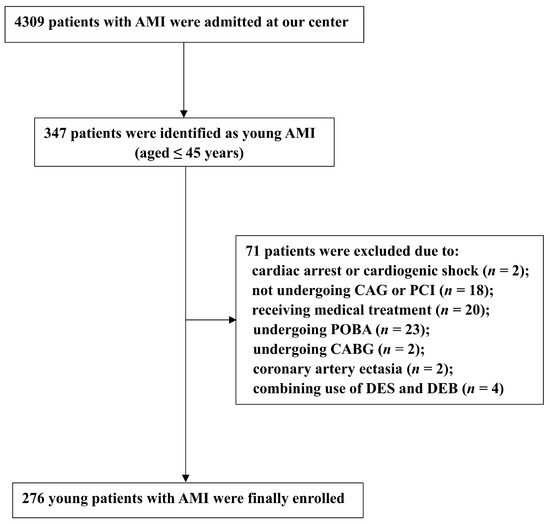
Figure 1.
Patient Flow Chart. AMI: acute myocardial infarction; CAG: coronary angiography; PCI: percutaneous coronary intervention; POBA: plain old balloon angioplasty; CABG: coronary bypass surgery; DEB: drug-eluting balloon; DES: drug-eluting stent.
Baseline demographic, clinical, laboratory, and angiographic characteristics are shown in Table 1 and Table 2. The mean age of the overall population was 39.3 ± 4.1 years, and 94.9% of them were men. Smoking (81.9%) was found to be the major risk factor for young AMI patients followed by overweightness (76.8%), hypertension (45.7%), family history of coronary artery disease (44.2%), hyperlipidemia (22.5%), and diabetes mellitus (18.5%). The majority (62.3%) of the young patients presented with STEMI. Multiple vessel disease (non-infarct-related artery with stenosis >70%) was observed in 63.0% of patients, and the left anterior descending coronary artery (LAD) was the most frequent infarct-related artery (41.7%).

Table 1.
Baseline and clinical characteristics of patients.

Table 2.
Procedural and angiographic characteristics of patients.
Compared with patients treated with DESs, patients treated with DEBs had a lower prevalence of STEMI (47.8% vs. 67.0%, p = 0.01), fewer LAD lesions as an infarcted related artery (28.4% vs. 45.9, p = 0.01), smaller device number (1.0 [1.0, 1.0] vs. 2.0 [1.0, 2.0], p < 0.01), smaller device diameter (2.75 vs. 3.00, p < 0.01), shorter device length (26.00 [20.00, 30.00] vs. 30.00 [23.5, 46.00], p < 0.01), lower inflation pressure (9.31 ± 1.84 vs. 10.58 ± 1.76, p < 0.01), and lower frequency of using thrombus aspiration (9.0% vs. 23.0%, p = 0.01). Moreover, among those patients who had non-infarct-related arteries with stenosis > 70%, the rate of immediate complete revascularization was higher in the DEB group than in the DES group (53.2% vs. 22.8%, p < 0.01). No significant differences were observed with regard to other baseline characteristics.
Clinical follow-up data (Table 3) were obtained in all patients with a median follow-up period of 1155 days (IQR: 690, 1530 days). Despite no statistical difference, patients treated with DEBs had a trend toward a lower incidence of DOCEs (3.0% vs. 11.0%, p = 0.12) mainly driven by the need for TLR (3.0% vs. 9.1%, p = 0.19) than those treated with DESs. Specifically, in the DEB group, two patients had TLR due to coronary dissections and acute vessel closure; in the DES group, one patient had TLR due to early stent thrombosis which occurred on the third day after the index procedure, seven patients had TLR due to LST which occurred within 1 year, five patients had TLR due to VLST which occurred after 1 year, and six patients had TLR due to in-stent restenosis. Of the total of 12 patients who had LST and VLST, eight patients received DAPT at the time of the event. Additionally, no significant differences between the two groups were detected in the occurrence of cardiac death (0.0% vs. 0.5%, p = 0.69) and target vessel MI (0.0% vs. 1.4%, p = 0.40). There were no significant differences between the two groups with regard to the risk of heart failure (0.0% vs. 1.9%, p = 0.39) and major bleeding events (1.5% vs 4.8%, p = 0.30). Kaplan–Meier survival curves for the clinical outcomes are shown in Figure 2.

Table 3.
Clinical outcomes after the index procedure.
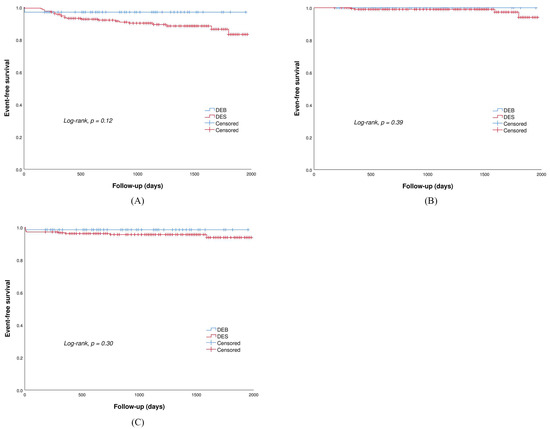
Figure 2.
Kaplan–Meier survival curve for (A) DOCE: device-oriented composite endpoint; (B) heart failure; (C) major bleeding events.
Multivariate regression analysis (Table 4) showed that NSTEMI (HR 3.43, 95% CI [1.36, 8.63], p = 0.01), Killip class ≥ 2 (HR 2.53, 95% CI [1.08, 5.95], p = 0.03), TIMI grade ≤ 1 (HR 4.25, 95% CI [1.25, 14.41], p = 0.02) and non-infarct-related artery with stenosis >70% (HR 4.02, 95% CI [1.33, 12.14], p = 0.01) were the independent predictors for DOCEs during the follow-up period. Additionally, DEBs were found to be associated with a trend toward a lower risk of DOCEs (HR 0.13, 95% CI [0.02, 1.05], p = 0.06) despite no statistical significance achieved. No independent factors for major bleeding events were observed by multivariate regression analysis (Supplementary Table S1).

Table 4.
Multivariate regression analysis of the predictors for DOCE.
In the subgroup analyses, the results of comparison between the two groups were consistent across the ten prespecified subgroups (Supplementary Figure S1). Furthermore, no significant differences between the two groups were observed with regard to clinical events occurring either within 1-year or beyond 1 year (Supplementary Tables S2 and S3).
4. Discussion
To the best of our knowledge, this is the first study to evaluate the safety and effectiveness of DEBs versus DESs in young patients with AMI. The present study showed that as compared with DESs, DEBs achieved a numerically lower rate of DOCEs mainly driven by the need for TLR during the mid- to long-term follow-up in the young AMI population. DEBs might be a feasible, safe, and effective alternative to DESs for the treatment of young patients with AMI.
To date, there are no specific recommendations on the management of young individuals with AMI. According to current guidelines, PCI with implantation of a permanent DES was the preferred reperfusion strategy in all patients presenting with AMI irrespective of the patient’s age [9]. However, younger age has been identified by previous studies as an independent predictor of LST and VLST after DES treatment, leading to a higher risk of repeat revascularization [11,12,19]. Consistently, the present study showed that the total rate of TLR due to LST and VLST and in-stent restenosis was relatively higher in the DES group than in the DEB group. Moreover, among the total of 12 patients who had LST and VLST, eight patients received DAPT at the time of the event. These findings suggested that younger AMI patients might have a less favorable vascular response to stent implantation. One of the possible explanations is that younger AMI patients have specific pathophysiologic characteristics and atherosclerotic plaque features compared with older cohorts with AMI [20]. Indeed, angiographic studies that used intravascular ultrasound (IVUS) and optical coherence tomography (OCT) have demonstrated that compared with older patients, younger patients are more likely to have eroded plaques, characterized by a lower percentage of dense calcium volume, necrotic core, and greater percentage of fibrous tissue volume [21,22,23]. Hu et al. suggested that eroded plaque was independently associated with less favorable healing following DES implantation at 6 months in patients with acute coronary syndrome [24]. Hong et al. found that absolute fibrous tissue volume was positively and independently associated with the development of plaque prolapse after DES implantation [25]. Similarly, Haine et al. confirmed that the fibrous tissue volume was independently related to an in-stent late luminal loss on angiography and to maximal percentage area stenosis and percentage volume intima hyperplasia on IVUS [26].
Combaret et al. proposed a two-stage management strategy in young AMI patients [27]. Based on the results of the second angiography which was performed 2 to 7 days after achieving optimal epicardial reperfusion (TIMI flow ≥ 2) by the initial strategy, patients were assigned to receive a bioresorbable vascular scaffold (BVS) in the case of stenosis greater than 70% or plaque prolapse or to receive medical treatment alone in other cases. Among the forty-five patients enrolled, only one patient in the medical group encountered recurrent MI at six months. This finding indicated that management by limiting the implantation of durable intracoronary devices was a potential treatment option in the young population. Nevertheless, the results should be interpreted with caution due to the small sample size in this “proof of concept” study, and the safety and effectiveness of bioresorbable vascular scaffolds in young AMI patients remains controversial because there was a lack of comparisons with patients managed by stenting.
DEBs are a semicompliant angioplasty balloon coated with a homogeneous distribution of the antiproliferative drug, which could be rapidly released into the target vessel wall in high concentrations [14,15]. Theoretically, DEBs could provide superiority over DESs in young AMI patients because they could avoid stent thrombosis, decrease the chronic inflammatory response, reduce delayed healing as well as maintain the coronary vasomotor response and vessel geometry with proven positive remodeling. Moreover, the ability to leave nothing behind and allow a shorter duration of DAPT might reduce mental and life stress in younger AMI patients.
Consistent with previous trials which have shown favorable clinical and angiographic outcomes of the DEB-only strategy in the general population with AMI [28,29], the present study showed that DEBs had a favorable safety and effectiveness in the treatment of the young AMI population. Indeed, in the present study, only two patients in the DEB-only arm encountered TLR during hospitalization, including one patient who required bailout stenting due to a type D dissection (National Heart, Lung, and Blood Institute classification) after DEB angioplasty and one patient who received an additional DES due to acute vessel closure which may have been caused by a delayed dissection occurring one day after the index procedure. After discharge, neither these two patients nor the remaining sixty-five patients suffered a DOCE. These findings indicated that the occurrence of coronary dissections and acute vessel closure might be the major concern of DEB use instead of implanting DESs in young AMI patients. Lin et al. demonstrated that the independent predictors of dissection after DEB treatment for patients with native coronary artery disease were women, higher DEB-to-reference vessel ratio, and longer lesion length [30]. Cortese et al. showed that patients had more severe–moderate calcification, a larger diameter of the predilation balloon, and DEBs in the dissection group than in the nondissection group [31]. In the current study, the higher DEB-to-reference vessel ratio and the longer lesion might also be the major contributors to the dissection after DEB treatment in these two patients. In addition, optimal lesion pretreatment is essential for the success of the DEB-only strategy. Iijima et al. showed that the use of a nonslip element balloon was effective for optimal lesion preparation before the use of the DEB due to its ability to reduce elastic recoil, traumatic vessel injury, and dissection [32].
Although a bailout stenting could be used as a remedial treatment for dissection, the combined use of DEBs and DESs in patients with dissection remains a matter of debate. Mitomo et al. found that the TLR rate at 2 years was up to 14.5% in patients receiving bailout stenting with DESs due to suboptimal DEB results [33]. Additionally, the second stent placement may also be psychologically stressful for younger patients. Therefore, it would be better to avoid bailout stenting as far as possible. In fact, it is sometimes possible to overestimate and underestimate the severity of the dissection using angiographic findings alone [34,35]. Spiral longitudinal dissection is usually diagnosed as type D dissection on angiography, whereas IVUS or OCT sometimes reveals less severe dissection. Conversely, hematoma as the major cause of acute occlusion sometimes cannot be identified on angiography but is clearly observed on IVUS or OCT. Intriguingly, a recent study conducted by Yamamot et al. showed that the dissection index, a new dissection grading system according to intravascular imaging findings which indicated the extent of coronary dissection after DEB treatment, was positively related to both chronic lumen and vessel enlargement [36]. A possible explanation was that dissection could help transmural diffusion of the drug and additionally force paclitaxel to be delivered in high doses near the adventitia, such as in intrapericardial paclitaxel delivery. Evidence showed that intrapericardial delivery of paclitaxel in large doses could lead to an increase in vessel enlargement and a decrease in neointimal mass by apoptotic cells. Therefore, to precisely detect the severity of dissection and optimal treatment for coronary dissection and the related prognosis after DEB angioplasty for younger AMI patients, further larger trials using IVUS or OCT should be conducted.
5. Limitations
The present study had several important limitations. First, it was a retrospective, single-center study with observational analysis; thus, the inherent biases cannot be completely avoided. Second, this study might be underpowered to detect statistical significance between groups due to the small sample size, especially for the DEB group. Moreover, meaningful subgroup analysis according to gender could not be performed given the limited number of patients in the female group. Third, angiographic or intravascular ultrasound follow-up could provide additional insights into the assessment of DEBs. However, due to the inherent limitations of the study design, we were unable to obtain follow-up angiographic data in all study populations. Likewise, comparative data on the prevalence of stress disorder between the two groups were also unavailable. Future prospective studies designed to assess coronary angiographic outcomes and mental health in these particular populations are required.
6. Conclusions
The present study demonstrated that for the young AMI population, DEBs could achieve a numerically lower rate of DOCEs mainly driven by the need for TLR compared with DESs. DEBs might be a feasible, safe, and effective treatment alternative for young patients with AMI. Further large-scale, randomized controlled trials are required to prove the benefits of the DEB strategy in this setting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcdd10010029/s1, Figure S1: Influence of the 10 pre-specified variables on the risk of MACE between the two treatment groups; Table S1: Multivariate regression analysis of the predictors for major bleeding events; Table S2: Clinical outcomes during 1-year of follow-up; Table S3: Clinical outcomes beyond 1-year of follow-up.
Author Contributions
Conceptualization, L.-F.W.; Methodology, Y.-X.Y.; Software, K.-Z.H.; Validation, J.-Y.L.; Formal Analysis, Y.F.; Investigation, C.L.; Resources, X.-M.L.; Data Curation, H.-J.W.; Writing—Original Draft Preparation, Y.-X.Y.; Writing—Review and Editing, L.X.; Visualization, M.-L.C.; Supervision, P.-X.S.; Project Administration, L.-F.W.; Funding Acquisition, L.-F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was funded by the Clinical Incubation Program of Beijing Chaoyang Hospital (CYFH202204) and Beijing Municipal Administration of Hospitals (XXZ0607).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Beijing Chaoyang Hospital (2022-S-275 and approved on 5 November 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Le-Feng Wang, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.M.; Qamar, A.; Vaduganathan, M.; Pandey, A.; Mph, D.L.B.; Caughey, M.C. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Behfar, A.; Narula, J.; Kanwar, A.; Lerman, A.; Cooper, L.; Singh, M. Acute Myocardial Infarction in Young Individuals. Mayo Clin. Proc. 2020, 95, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Rallidis, L.S.; Xenogiannis, I.; Brilakis, E.S.; Bhatt, D.L. Causes, Angiographic Characteristics, and Management of Premature Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2431–2449. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Collins, B.L.; Singh, A.; Biery, D.W.; Fatima, A.; Qamar, A.; Berman, A.N.; Gupta, A.; Cawley, M.; Wood, M.J.; et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: The Mass General Brigham YOUNG-MI registry. Eur. Heart J. 2020, 41, 4127–4137. [Google Scholar] [CrossRef]
- Kerola, A.M.; Palomäki, A.; Rautava, P.; Kytö, V. Less revascularization in young women but impaired long-term outcomes in young men after myocardial infarction. Eur. J. Prev. Cardiol. 2022, 29, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.-P.; Blessberger, H.; Alimohammadi, A.; Pavo, N.; Huber, K.; Wojta, J.; Lang, I.M.; Wiesbauer, F.; Goliasch, G. Long-term outcome and risk assessment in premature acute myocardial infarction: A 10-year follow-up study. Int. J. Cardiol. 2017, 240, 37–42. [Google Scholar] [CrossRef]
- Minhas, A.M.K.; Awan, M.U.; Raza, M.; Virani, S.S.; Sharma, G.; Blankstein, R.; Blaha, M.J.; Al-Kindi, S.G.; Kaluksi, E.; Nasir, K.; et al. Clinical and Economic Burden of Percutaneous Coronary Intervention in Hospitalized Young Adults in the United States, 2004–2018. Curr. Probl. Cardiol. 2022, 47, 101070. [Google Scholar] [CrossRef]
- Wu, M.; Wang, W.; Zhang, X.; Li, J. The prevalence of acute stress disorder after acute myocardial infarction and its psychosocial risk factors among young and middle-aged patients. Sci. Rep. 2022, 12, 7675. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Kuramitsu, S.; Sonoda, S.; Ando, K.; Otake, H.; Natsuaki, M.; Anai, R.; Honda, Y.; Kadota, K.; Kobayashi, Y.; Kimura, T. Drug-eluting stent thrombosis: Current and future perspectives. Cardiovasc. Interv. Ther. 2021, 36, 158–168. [Google Scholar] [CrossRef]
- Park, K.W.; Hwang, S.-J.; Kwon, D.-A.; Oh, B.-H.; Park, Y.-B.; Chae, I.-H.; Gwon, H.-C.; Park, S.-J.; Seung, K.B.; Ahn, T.; et al. Characteristics and predictors of drug-eluting stent thrombosis: Results from the multicenter ‘Korea Stent Thrombosis (KoST)’ registry. Circ. J. 2011, 75, 1626–1632. [Google Scholar] [CrossRef]
- Waksman, R.; Kirtane, A.J.; Torguson, R.; Cohen, D.J.; Ryan, T.; Räber, L.; Applegate, R.; Waxman, S.; Gordon, P.; Kaneshige, K.; et al. Correlates and outcomes of late and very late drug-eluting stent thrombosis: Results from DESERT (International Drug-Eluting Stent Event Registry of Thrombosis). JACC Cardiovasc. Interv. 2014, 7, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Study of Anxiety/Depression in Patients with Coronary Heart Disease After Percutaneous Coronary Intervention. Cell Biochem. Biophys. 2015, 72, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Eccleshall, S.; Wan Ahmad, W.A.; Ge, J.; Poerner, T.C.; Shin, E.-S.; Alfonso, F.; Latib, A.; Ong, P.J.; Rissanen, T.T.; et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, D.; Lombardo, R.M.; Cortese, B. Drug-coated balloons for coronary artery disease: Current concepts and controversies. Future Cardiol. 2019, 15, 437–454. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.-A.; Van Es, G.-A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.; Anker, S.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Schulman, S.; Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Kuramitsu, S.; Ohya, M.; Shinozaki, T.; Otake, H.; Horie, K.; Kawamoto, H.; Yamanaka, F.; Natsuaki, M.; Shiomi, H.; Nakazawa, G.; et al. Risk Factors and Long-Term Clinical Outcomes of Second-Generation Drug-Eluting Stent Thrombosis. Circ. Cardiovasc. Interv. 2019, 12, e007822. [Google Scholar] [CrossRef]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2022, 118, 2281–2292. [Google Scholar] [CrossRef]
- Fang, C.; Dai, J.; Zhang, S.; Wang, Y.; Wang, J.; Li, L.; Wang, Y.; Yu, H.; Wei, G.; Zhang, X.; et al. Culprit lesion morphology in young patients with ST-segment elevated myocardial infarction: A clinical, angiographic and optical coherence tomography study. Atherosclerosis 2019, 289, 94–100. [Google Scholar] [CrossRef]
- Xie, J.; Qi, J.; Mao, H.; Wang, N.; Ye, X.; Zhou, L.; Tong, G.; Yang, J.; Pan, H.; Huang, J. Coronary plaque tissue characterization in patients with premature coronary artery disease. Int. J. Cardiovasc. Imaging 2020, 36, 1003–1011. [Google Scholar] [CrossRef]
- Ruiz-García, J.; Lerman, A.; Weisz, G.; Maehara, A.; Mintz, G.S.; Fahy, M.; Xu, K.; Lansky, A.J.; Cristea, E.; Farah, T.G.; et al. Age- and gender-related changes in plaque composition in patients with acute coronary syndrome: The PROSPECT study. EuroIntervention 2012, 8, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, C.; Zhe, C.; Zhu, Y.; Yonetsu, T.; Jia, H.; Hou, J.; Zhang, S.; Jang, I.-K.; Yu, B. Plaque erosion delays vascular healing after drug eluting stent implantation in patients with acute coronary syndrome: An In Vivo Optical Coherence Tomography Study. Catheter. Cardiovasc. Interv. 2017, 89, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Jeong, M.H.; Kim, S.W.; Choi, Y.H.; Ma, E.H.; Ko, J.S.; Lee, M.G.; Park, K.H.; Sim, D.S.; Yoon, N.S.; et al. Relation between plaque components and plaque prolapse after drug-eluting stent implantation—Virtual histology-intravascular ultrasound. Circ. J. 2010, 74, 1142–1151. [Google Scholar] [CrossRef]
- Haine, S.; Wouters, K.; Miljoen, H.; Vandendriessche, T.; Claeys, M.; Bosmans, J.; Vrints, C. A higher volume of fibrotic tissue on virtual histology prior to coronary stent implantation predisposes to more pronounced neointima proliferation. Acta Cardiol. 2018, 73, 171–178. [Google Scholar] [CrossRef]
- Combaret, N.; Souteyrand, G.; Barber-Chamoux, N.; Malcles, G.; Amonchot, A.; Pereira, B.; Le Bivic, L.; Eschalier, R.; Trésorier, R.; Motreff, P. Management of ST-elevation myocardial infarction in young patients by limiting implantation of durable intracoronary devices and guided by optical frequency domain imaging: “proof of concept” study. EuroIntervention 2017, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Vos, N.S.; Fagel, N.D.; Amoroso, G.; Herrman, J.-P.R.; Patterson, M.S.; Piers, L.H.; van der Schaaf, R.J.; Slagboom, T.; Vink, M.A. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction: The Revelation Randomized Trial. JACC Cardiovasc. Interv. 2019, 12, 1691–1699. [Google Scholar] [CrossRef]
- Scheller, B.; Ohlow, M.-A.; Ewen, S.; Kische, S.; Rudolph, T.K.; Clever, Y.P.; Wagner, A.; Richter, S.; El-Garhy, M.; Böhm, M.; et al. Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: The randomised PEPCAD NSTEMI trial. EuroIntervention 2020, 15, 1527–1533. [Google Scholar] [CrossRef]
- Hui, L.; Shin, E.-S.; Jun, E.J.; Bhak, Y.; Garg, S.; Kim, T.-H.; Sohn, C.-B.; Choi, B.J.; Kun, L.; Yuan, S.L.; et al. Impact of Dissection after Drug-Coated Balloon Treatment of De Novo Coronary Lesions: Angiographic and Clinical Outcomes. Yonsei Med. J. 2020, 61, 1004–1012. [Google Scholar] [CrossRef]
- Cortese, B.; Orrego, P.S.; Agostoni, P.; Buccheri, D.; Piraino, D.; Andolina, G.; Seregni, R.G. Effect of Drug-Coated Balloons in Native Coronary Artery Disease Left with a Dissection. JACC Cardiovasc. Interv. 2015, 8, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Iijima, R.; Kougame, N.; Hara, H.; Moroi, M.; Nakamura, M. Clinical Outcomes of Drug-Coated Balloons in Coronary Artery Disease Unsuitable for Drug-Eluting Stent Implantation. Circ. J. 2018, 82, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Mitomo, S.; Jabbour, R.J.; Mangieri, A.; Ancona, M.; Regazzoli, D.; Tanaka, A.; Giannini, F.; Carlino, M.; Montorfano, M.; Chieffo, A.; et al. Mid-term clinical outcomes after bailout drug-eluting stenting for suboptimal drug-coated balloon results: Insights from a Milan registry. Int. J. Cardiol. 2018, 263, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.; Sandoval, J.; Lennie, V.; Dutary, J.; Medina, M.; Gonzalo, N.; Jimenez-Quevedo, P.; Escaned, J.; Bañuelos, C.; Hernandez, R.; et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc. Imaging 2013, 6, 830–832. [Google Scholar] [CrossRef]
- Schroeder, S.; Baumbach, A.; Mahrholdt, H.; Haase, K.; Oberhoff, M.; Herdeg, C.; Athanasiadis, A.; Karsch, K. The impact of untreated coronary dissections on acute and long-term outcome after intravascular ultrasound guided PTCA. Eur. Heart J. 2000, 21, 137–145. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sawada, T.; Uzu, K.; Takaya, T.; Kawai, H.; Yasaka, Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int. J. Cardiol. 2020, 321, 30–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).