1. Introduction
Mammalian hearts are complex in development, structure and function. From single heart tubes spatiotemporal gene expression patterns, cytoplasmatic gradients, signaling of various origin, cellular migration, transformations and even epigenetic influences as local hemodynamics add to the ultimate heart design. The assignment of circulatory demand even in the developing heart requires a work sharing of cellular functions assembling into tissue generating cohorts.
In adult hearts, syncytial cardiomyocytes slide along fibrous septations, electrical signals travel in preformed specialized cells and valves close and open ejecting blood according to contractile forces and circulatory demand. It is therefore not surprising that this unique organ needs cellular homeostasis to guarantee longlasting adaptive function. The consequence of this “complex” architecture and functional ability however is that a “scarless” regenerative potential known from other more “primitive” species is lost or at least dormant and disabled in adult mammalian hearts [
1]. What is little appreciated is the fact that even in this tight structural straightjacket, 50% of cardiomyocytes are renewed during a lifespan of individual humans showing that the basic principles of regeneration per se persist in adult hearts [
2]. It may very likely be, that paracrine signals originating from the “non cardiomyocytes” and extracellular matrix might be the “controller” of coordinated repair [
1,
2,
3]. Even arrested developmental pathways as “Hippo signaling” or the fact that cardiomyocyte proliferation is by and large aborted as shown in newborn rats, illustrates that “myocardial growth signals” are present in mammals however actively suppressed for a better good of organ homeostasis [
4]. The question however remains how to facilitate, support and assist these conserved mechanisms in myocardial jeopardy?
Several concepts spanning from genetic engineering, cellular reprogramming and stem cell transplantation have been tried, some of them reaching the clinical arena under scrutiny of randomized trials [
5].
1.1. An Embryonic Developmental Process as Impulse for Regeneration
Alternatively, another important regenerative principle has obviously less been appreciated in the past. Is a re-activation of imprinted developmental processes per se able to restart programs able to recover the dys-functioning adult heart [
6]? This implicates that we reopen the door of an embryonic process in a very early stage, so that signals originating from this procedure rewire developmental steps regaining structural integrity. It is a semantic consideration whether or not reiterated signals may be transduced in their original “embryonic” fashion or whether there are deviations because of the adult environment of jeopardized myocardium and ultimately whether their functional ability persists in a different adult environment.
1.2. Hemodynamic Force Sculpturing the Developing Heart
The role of mechantransduction and the sculpturing force of embryonic hemodynamics have long been known and regain scientific focus reconnecting the “mechanistic” stimulus to molecular cascades [
7,
8]. On about day 22 the human heart starts beating. If we consider the developmental process as a stepwise approach, certain achievements in tissue generation have to be sensed to allow an organized triggering of subsequent gene expression and signal transduction. This “biophysical” testing is claimed by our hypothesis as core control module checking the actual output of a developmental phase. To achieve robust phenotypes, the developing heart probably passes several of these modules to allow a stepwise approach from the primitive heart tube to 4 chambered hearts. Deviations of this body plan and its stepwise tactic are frozen in disease and dysfunction [
9].
As early as in heart tube formation an important gain of function is reached. At this stage the primordial heart beat has to be sensed regulating subsequent cardiogenesis. As mentioned above core control modules are important to produce robust phenotypes and controlling the achievement of heart beat in embryonic life, as proposed by our hypothesis, might be one of these key regulators. Many parts of the puzzle of cardiac development and the underlying principles have not been deciphered yet, although special core control modules and “Acupoints” have been predicted [
10,
11].
1.3. The Term “Embryonic Recall”
The central idea of the “embryonic recall” hypothesis is that the onset of heart beat resembles an important and therefore “sensible” gain of function and that this achievement in function initiates a multitude of signals for cardiogenesis [
12].
This time dependent monitoring of a pending process might be an “Acupoint” opening a window between functional achievements on an environmental meta-level and the genome. Because of the quantity of signals originating from this process, any deviation or lack of timely sensing can be considered as a lethal situation or at least a deviation from a normal “body plan” for the developing organism.
It is the central dogma of our hypothesis that a gain of function sensing of the embryonic heart beat by the developing endocardium can be reiterated in the adult and failing heart restarting developmental pathways by echoing the same mechanism on cellular descendants involved in primary sensing namely vascular endothelium in cardiac veins.
1.4. Gain of Function Sensing Sculpturing Tissue Maturation
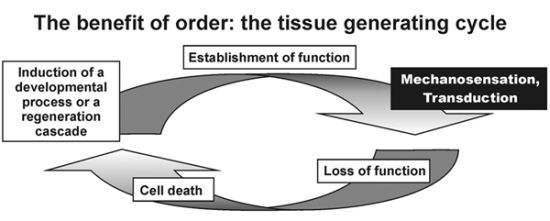
In 1979 we described, as we called it then, a digital integrative model of organogenesis, including gain of function and loss of function as information and signaling mediators. This concept developed over the years to a tissue-generating clock describing a process with “biophysical” sensing able to produce spatiotemporal differences in developing tissues [
13]. In this study we analyzed the similarity of structural malformations of inherited and sporadic atrial septal defects which led to the conception that robustness in sculpturing phenotypes needs a process like a tissue generating cycle involving gain of function sensing on as well as loss of function as an organizational meta-level and a key element in cardiogenesis [
10,
12]. The distinct phenotypes in congenital malformations of the heart with multifactorial causes from hereditable changes to environmental influences predict that signal transduction is time sensitive and that knock out of signaling involves not only the signal threshold, but as well as the time slot of its function. Therefore we claim that certain “windows of opportunity” connect the meat-level of achievements in building structure according to the body plan and its function. Recent findings of miRNA expression as consequence of the initiation of heart beat in the primitive embryonic heart seems to be this predicted burst of signaling as result of a gain of function sensing as an epimorphic sculpturing event prospering further development [
7].
Figure 1.
The tissue generating clock depicts Mechanosensation and Transduction as core control module checking realization of a new level of morphogenesis or equally “gain of function” on the path to developing a mature phenotype. Reviving this biophysical process is the central dogma of the hypothesis “embryonic recall”. This developmental “toll gate” or “biophysical monitor” represents an “Acupoint” as predicted by Sasai [
10].
Figure 1.
The tissue generating clock depicts Mechanosensation and Transduction as core control module checking realization of a new level of morphogenesis or equally “gain of function” on the path to developing a mature phenotype. Reviving this biophysical process is the central dogma of the hypothesis “embryonic recall”. This developmental “toll gate” or “biophysical monitor” represents an “Acupoint” as predicted by Sasai [
10].
As depicted in
Figure 1, gain of function sensing is an important element in the above mentioned tissue generating process. The systemic biology approach of this principle allows a better understanding of this important process from differential expressing genomic information to produce a mature phenotype. The first part of our hypothesis “embryonic recall” therefore involves the hitherto undescribed principle that gene expression is translated into somatic function and the achieved biophysical function is sensed by molecular networks thriving further discriminant morphogenetic evolution.
The gold standard in stem cell research claims that initiation of regeneration mainly involves transplantation of molecules or pluripotent cells. Unlike these present concepts the main principle of our hypothesis “embryonic recall” means that structure defines function and function designs structure involving an epigenetic phenomenon as key element.
Therefore we conclude in the first part of our hypothesis that “a process rather than a transplantable element” can be used to re-institute developmental processes and therefore regeneration of some kind. The second part in this hypothesis is that this process, namely the gain of function sensing can be achieved in the adult failing heart by a temporarily elevating hemodynamic pressure in cardiac veins.
Re-expression of fetal proteins in myocardium due to pressure overload in hypertension are well known for decades and may be considered as surrogate for the principle of the potential to reactivate embryonic pathways also in the adult heart induced by hemodynamic force [
14].
If we consider the endothelium in cardiac veins as descendants of endocardial layers in the primordial heart tube, one can appreciate that the same signaling event may cause the same structural consequence. Knowing the controversy about the origin of venous endothelium in the coronary circulation, we have to substantiate the claim whether a developmental process on endocardium can be revived via adult cardiac veins. Wu and colleagues found that venous endothelium in cardiac veins are descendants from endothelium, although at a very small amount. Whether or not this implies a different perspective to our hypothesis remains to be elucidated. The proposed transfer of hemodynamic action on pericytes in the microcirculation via mechanotransduction of periodic increased pressure in cardiac veins, might also be involved in the initiation of structural recovery [
15,
16].
The onset of primitive heart pulsations is known to be sensed by changes in the cytoskeleton of cells and transduced into a multitude of signals including the secretion of miRNA 143. Several candidate microRNAs like hsa-miR-590 and hsa-miR-199a, miR-143 and 145, miRNA 1, 133, 208, and 499 have been promoted as capable of inducing direct cellular reprogramming of fibroblasts to cardiomyocyte-like cells [
17,
18,
19,
20]. It is therefore part of our hypothesis that a burst of miRNA might be involved in this process.
Although many signals as impact of mechanical forces in heart development have been described recently, most of the signaling potentially reactivated by periodic pressure elevations in cardiac veins and reinstituting the same signaling cascade remain unknown.
1.5. Pressure Controlled Coronary Sinus Occlusion (PICSO)
Periodic occlusion of the coronary sinus and therefore the majority of cardiac veins draining the myocardium, elevates the pressure in veins reaching a systolic pressure plateau after a few seconds. In PICSO obstruction to venous flow is limited to this plateau not to impede coronary arterial inflow and therefore nutrition [
21].
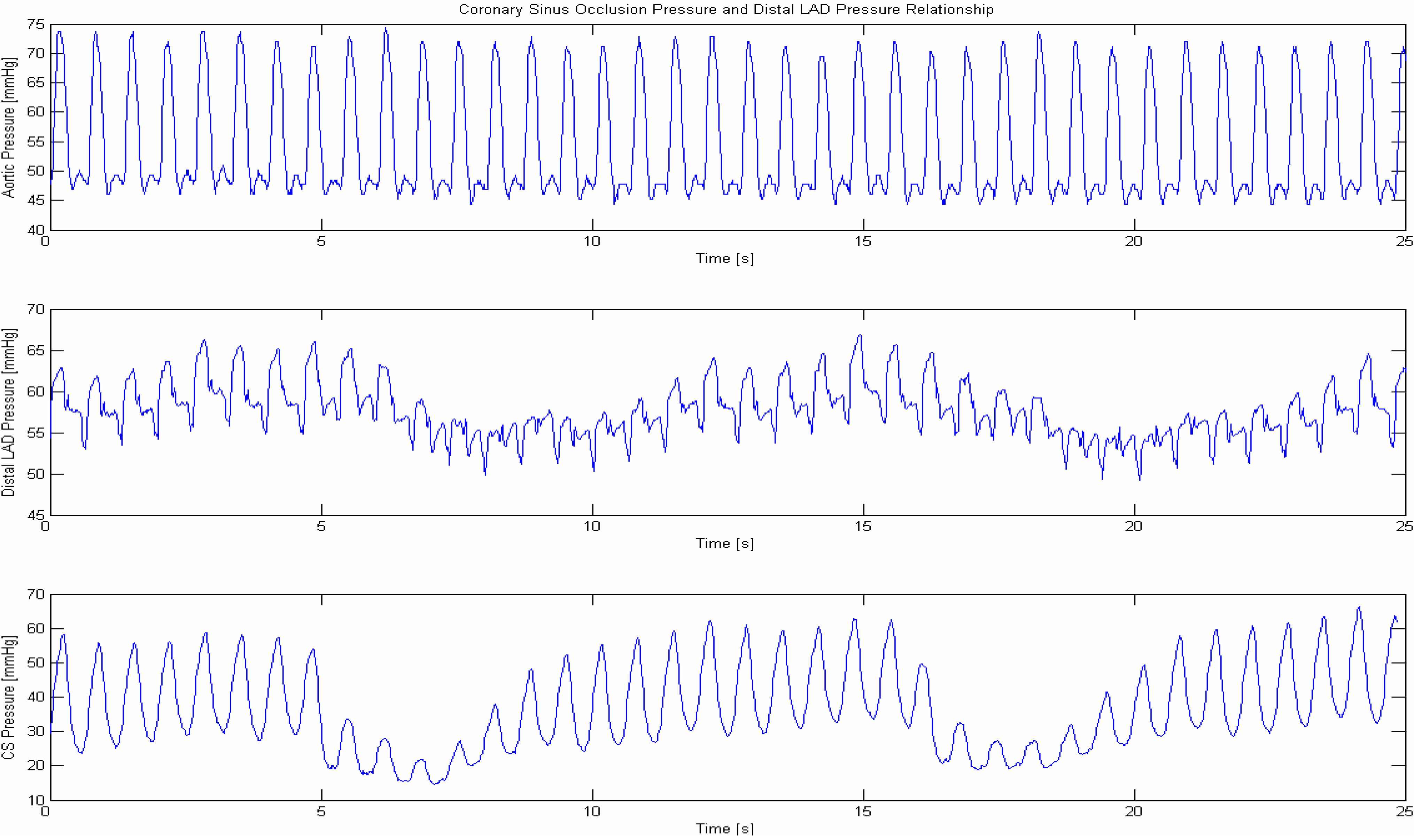
Figure 2 shows a pressure reading in cardiac veins. It is hypothesized that the systolic pulsations within cardiac veins as well as the shear stress of the reflowing blood resemble the same “biophysical signal” as the primordial heart beat within the primitive heart tube [
22,
23,
24,
25,
26].
Figure 2.
Coronary sinus pressure during occlusion of the LAD (occlusion of the left anterior descending coronary artery results in a large area deprived from nutritive blood flow and is the major cause for a subseuent myocardial infarction, therefore any means to increase flow into the area with a perfusion deficit salvages ischemic myocardium). Systolic pressure peaks produced by ventricular contraction retroperfuses blood into the deprived ischemic microcirculation and elevates the coronary artery pressure concomittantlyuntil reaching a plateau after a few heart beats. Filling of the deprived ischemic microvasculature is seen by an elevation of the pressure in the occluded coronary artery. After pressure resumes by reopening the temporary occluded venous drainage, blood is squeezed out from zones dependent from occluded coronary arteries washing out toxic debris [
27]. Mechanosensation of pressure peaks as well as distortion of cilia within cardiac veins by the force of the retroperfued blood and transduction via the cytosceleton of vascular cells initiate the process of “embryonic recall”. The putative element as we hypothesize is (1) Sensing of the elevated venous pressure in cardiac veins mimicking the equivalent mechanotransductory procedure during the first heartbeat in the embryo, re-opens a core control module for functional achievement initiating a molecular pathway similar to the developing heart. It corresponds to a desired reopening of a cascade of developmental events now deliberately initiated by biomechanical force using principles of normal organogenesis by restarting the biophysical initiation leading to re-establishment of dormant developmental molecular pathways similar to the initiation of the first heart beats in the primordial heart tube; (2) Re-establishment of monitoring the biophysical process with its molecular consequences of this core control module is the central dogma of the “embryonic recall hypothesis”.
Figure 2.
Coronary sinus pressure during occlusion of the LAD (occlusion of the left anterior descending coronary artery results in a large area deprived from nutritive blood flow and is the major cause for a subseuent myocardial infarction, therefore any means to increase flow into the area with a perfusion deficit salvages ischemic myocardium). Systolic pressure peaks produced by ventricular contraction retroperfuses blood into the deprived ischemic microcirculation and elevates the coronary artery pressure concomittantlyuntil reaching a plateau after a few heart beats. Filling of the deprived ischemic microvasculature is seen by an elevation of the pressure in the occluded coronary artery. After pressure resumes by reopening the temporary occluded venous drainage, blood is squeezed out from zones dependent from occluded coronary arteries washing out toxic debris [
27]. Mechanosensation of pressure peaks as well as distortion of cilia within cardiac veins by the force of the retroperfued blood and transduction via the cytosceleton of vascular cells initiate the process of “embryonic recall”. The putative element as we hypothesize is (1) Sensing of the elevated venous pressure in cardiac veins mimicking the equivalent mechanotransductory procedure during the first heartbeat in the embryo, re-opens a core control module for functional achievement initiating a molecular pathway similar to the developing heart. It corresponds to a desired reopening of a cascade of developmental events now deliberately initiated by biomechanical force using principles of normal organogenesis by restarting the biophysical initiation leading to re-establishment of dormant developmental molecular pathways similar to the initiation of the first heart beats in the primordial heart tube; (2) Re-establishment of monitoring the biophysical process with its molecular consequences of this core control module is the central dogma of the “embryonic recall hypothesis”.
![Jcdd 01 00073 g002]()
2. Periodic Elevation of Coronary Sinus Pressure
ICSO, PICSO (intermittent and pressure controlled) was primarily developed to salvage ischemic hearts and was tested in a multitude of experimental and clinical studies [
27,
28,
29]. Since 1978 this intervention was analyzed in a series of animal models mainly on its anti-ischemic effects as well as the hemodynamic consequences on regional and global hemodynamics. In other animals molecular changes within the myocardium were analyzed.
In clinical trials, reduction of infarct size and long term results in patients with acute coronary syndromes as well as in heart failure were investigated. Ongoing trials explore the preconditioning potential and compare them with already completed trials with PICSO applications during reperfusion.
3. Results and Discussion
There are mainly two distinct mechanisms of PICSO, the first leading to a reduction of myocardial ischemia. The basis is a redistribution of flow towards underperfused zones and clearing of occluded ischemic/reperfused microcirculation. This leads to a dose dependent reduction of infarct size, which was corroborated by several groups in different forms of ischemia and animal models. A meta-analysis showed significant reduction in infarct size of about 30% (
p < 0.001). A relevant statistical significant correlation (r = −0.92,
p < 0.007) between developed coronary sinus pressure and infarct size underscores the dose dependence of the elevated coronary sinus pressure forcing the blood into underperfused zones and washout of thrombotic corpuscles thus clearing the occluded microcirculation [
30].
This mechanism on ischemia is supported by a second separate mechanism, which is based on the activation of vascular cells by the mechanic and hemodynamic stretch and shear stress of the retroperfused blood and its pulsations in the cardiac venous vasculature. Mechanotransduction via the cytoskeleton of vascular cells initiates a molecular cascade leading to angiogenetic, antiatherosclerotic gene expression changes corresponding with a 4 fold increase of hemeoxygenase-1 gene expression (
p < 0.001) in the center of infarction and a 2.5 fold increase of vascular endothelial growth factor (VEGF) (
p < 0.002) in border zones whereas “Hypoxia induced factor” (HIF) activity remains unchanged suggesting an independent regenerative stimulus [
31].
Furthermore we found a marked upregulation of VEGF and VEGF-receptor 2 protein in the capillary endothelial cells in remote areas in 86% of the PICSO treated hearts and only in 43% of the non PICSO hearts in an animal model of myocardial infarction [
32]. This has been confirmed clinically by significant salvage and event free survival in patients with acute myocardial infarction and PICSO and a risk reduction for event free survival 60 months after the acute event (
p < 0.0001). Similar observations were made in heart failure patients showing a risk reduction of 72% and a favorable patient survival up to 5 years endorsing our hypothesis and preclinical experience that PICSO via hemodynamic power activates regenerative pulses also in adult human hearts [
33,
34,
35].
Structure Defines Function and Function Creates Structure
It is now well accepted that mechanical force sensed and transduced at the right time slot and local environment creates signals transduced to initiate gene expression and new impulses for cardiogenesis. We anticipate that robustness in phenotypic development needs core control modules as first proposed in our tissue generating clock connecting genome and environment. Some of the same signals found during development and induced by biophysical force are evidently re-expressed by systolic pressure pulsations in cardiac veins supporting our hypothesis that a reactivation of a dormant developmental process is possible to restart an adult analogue of cardiogenesis. Whether or not this reestablished impulse has the same magnitude, developmental direction and potential or may be deviated by a different “adult and diseased” environment of a failing heart remains to be proven by scientific scrutiny.
4. Conclusions
Temporal pressure elevation within cardiac veins has two distinct mechanisms reducing the amount of ischemic burden, which is dose dependent, and inducing threshold contingent changes, reiterating developmental processes by linking epigenetic phenomena like “gain of function sensing” of hemodynamic force with molecular cascades defining structure. These data support our hypothesis “embryonic recall” which might be a game changer how regeneration can be perceived. The reestablishment of a process similar, but not identical to the normally evolving vertebrate hearts due to the different environment in the adult jeopardized heart, might therefore be an attractive alternative to cellular therapies for structural myocardial recovery.
Acknowledgments
We acknowledge the support of the Society of coronary sinus interventions (
www.coronarysinus.com) in the development and execution of several studies helping to define our hypothesis.
Author Contributions
W.M. and D.M. wrote and edited the publication and W.M. created the hypothesis; D.M., T.A., A.J. and A.M. did experiments and helped to analyze experimental data; F.R. supervised the analysis of data and contributed in defining the hypothesis.
Conflicts of Interest
WM is the inventor of the ICSO & PICSO concept and Founder of Miracor medical systems investigating PICSO in clinical settings. The other authors declare no conflict of interest.
References and Notes
- Schneider, M.D. Development. A cardiac nonproliferation treaty. Science 2011, 332, 426–427. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; Jovinge, S.; Frisén, J. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Noseda, M.; Schneider, M.D. Fibroblasts inform the heart: Control of cardiomyocyte cycling and size by age-dependent paracrine signals. Dev. Cell. 2009, 16, 161–162. [Google Scholar] [CrossRef]
- Morrisey, E.E. Not too large and not too small—Just the right size: A hippo-sized heart. Circ. Res. 2011, 109, 614–615. [Google Scholar] [CrossRef]
- Leri, A.; Anversa, P.; Frishman, W.H. Cardiovascular Regeneration and Stem Cell Therapy. Blackwell Futura: Malden, MA, USA, 2007. [Google Scholar]
- Mohl, W.; Mina, S.; Milasinovic, D.; Kasahara, H.; Wei, S.; Maurer, G. Is activation of coronary venous cells the key to cardiac regeneration? Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 528–530. [Google Scholar] [CrossRef]
- Miyasaka, K.Y.; Kida, Y.S.; Banjo, T.; Ueki, Y.; Nagayama, K.; Matsumoto, T.; Sato, M.; Ogura, T. Heartbeat regulates cardiogenesis by suppressing retinoic acid signaling via expression of miR-143. Mech. Dev. 2011, 128, 18–28. [Google Scholar] [CrossRef]
- Granados-Riveron, J.T.; Brook, J.D. The impact of mechanical forces in heart morphogenesis. Circ. Cardiovasc. Genet. 2012, 5, 132–142. [Google Scholar] [CrossRef]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.G.; Seidman, C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef]
- Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 2013, 493, 318–326. [Google Scholar] [CrossRef]
- Sperling, S.R. Systems biology approaches to heart development and congenital heart disease. Cardiovasc. Res. 2011, 91, 269–278. [Google Scholar] [CrossRef]
- Mohl, W. Embryonic recall: Myocardial regeneration beyond stem cell transplantation. Wien. Klin. Wochenschr. 2007, 119, 333–336. [Google Scholar] [CrossRef]
- Mohl, W.; Mayr, W.R.; Hauser, G.; Reuer, E.; Wimmer, M.; Herbich, J. Mechanisms common to the development of malformation in congenital and sporadic forms of atrial septal defect (type II) (in German). Wien. Klin. Wochenschr. 1979, 91, 307–314. [Google Scholar]
- Schluter, K.D.; Piper, H.M. Regulation of growth in the adult cardiomyocytes. FASEB J. 1999, 13, S17–S22. [Google Scholar]
- Wu, B.; Zhang, Z.; Lui, W.; Chen, X.; Wang, Y.; Chamberlain, A.A.; Moreno-Rodriguez, R.A.; Markwald, R.R.; O’Rourke, B.P.; Sharp, D.J.; Zheng, D.; Lenz, J.; Baldwin, H.S.; Chang, C.P.; Zhou, B. Endocardial Cells Form the Coronary Arteries by Angiogenesis through Myocardial-Endocardial VEGF Signaling. Cell 2012, 151, 1083–1096. [Google Scholar]
- del Monte, G.; Harvey, R.P. An Endothelial Contribution to Coronary Vessels. Cell 2012, 151, 932–934. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Wang, X. Regulation of Cellular miRNA Expression by Human Papillomaviruses. Biochim. Biophys. Acta 2011, 1809, 668–677. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Dal Ferro, M.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Bauersachs, J.; Thum, T. Biogenesis and Regulation of Cardiovascular microRNAs. Circ. Res. 2011, 109, 334–347. [Google Scholar] [CrossRef]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef]
- Mohl, W.; Kajgana, I.; Bergmeister, H.; Rattay, F. Intermittent pressure elevation of the coronary venous system as a method to protect ischemic myocardium. Interact. Cardiovasc. Thorac. Surg. 2005, 4, 66–69. [Google Scholar] [CrossRef]
- Campàs, O.; Mammoto, T.; Hasso, S.; Sperling, R.A.; O’Connell, D.; Bischof, A.G.; Maas, R.; Weitz, D.A.; Mahadevan, L.; Ingber, D.E. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Meth. 2014, 11, 183–189. [Google Scholar]
- Mammoto, A.; Mammoto, T.; Ingber, D.E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012, 125, 3061–3073. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Gittenberger-de Groot, A.C.; Mentink, M.M.; Bökenkamp, R.; Hogers, B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ. Res. 1993, 73, 559–568. [Google Scholar] [CrossRef]
- Poelmann, R.E.; van der Heiden, K.; Groot, A.G.; Hierck, B.P. Deciphering the Endothelial Shear Stress Sensor. Circulation 2008, 117, 1124–1126. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Hierck, B.P.; Vrolijk, J.; Baiker, M.; Pourquie, M.J.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Changes in Shear Stress-Related Gene Expression after Experimentally Altered Venous Return in the Chicken Embryo. Circ. Res. 2005, 96, 1291–1298. [Google Scholar] [CrossRef]
- Mohl, W. The momentum of coronary sinus interventions clinically. Circulation 1988, 77, 6–12. [Google Scholar] [CrossRef]
- Mohl, W. The development and rationale of pressure-controlled intermittent coronary sinus occlusion—A new approach to protect ischemic myocardium. Wien. Klin. Wochenschr. 1984, 96, 20–25. [Google Scholar]
- Mohl, W. Coronary sinus interventions: from concept to clinics. J. Card. Surg. 1987, 2, 467–493. [Google Scholar] [CrossRef]
- Syeda, B.; Schukro, C.; Heinze, G.; Modaressi, K.; Glogar, D.; Maurer, G.; Mohl, W. The salvage potential of coronary sinus interventions: Meta-analysis and pathophysiologic consequences. J. Thorac. Cardiovasc. Surg. 2004, 127, 1703–1712. [Google Scholar] [CrossRef]
- Weigel, G.; Kajgana, I.; Bergmeister, H.; Riedl, G.; Glogar, H.D.; Gyöngyösi, M.; Blasnig, S.; Heinze, G.; Mohl, W. Beck and back: A paradigm change in coronary sinus interventions—Pulsatile stretch on intact coronary venous endothelium. J. Thorac. Cardiovasc. Surg. 2007, 133, 1581–1587. [Google Scholar] [CrossRef]
- de Jonge, M.; Gittenberger-de Groot, A.C.; Wisse, L.J.; Mina, S.; Kasahara, H.; Mohl, W. Mechanical activation of coronary venous endothelium causes a rapid angiogenic impulse after induced myocardial infarction. Eur. Heart J. 2008, 29, 686. [Google Scholar] [CrossRef]
- Khatami, N.; Wadowski, P.; Wagh, V.; Hescheler, J.; Sachinidis, A.; Mohl, W. Pressure-controlled intermittent coronary sinus occlusion (PICSO) study on mechanical control of cardiac tissue morphogenesis. Cardiovasc. Res. 2012, 93 (Suppl. 1), S56. [Google Scholar]
- Mohl, W.; Komamura, K.; Kasahara, H.; Heinze, G.; Glogar, D.; Hirayama, A.; Kodama, K. Myocardial protection via the coronary sinus. Circ. J. 2008, 72, 526–533. [Google Scholar]
- Mohl, W.; Mina, S.; Milasinovic, D.; Kasahara, H.; Wei, S. The legacy of coronary sinus interventions: Endogenous cardioprotection and regeneration beyond stem cell research. J. Thorac. Cardiovasc. Surg. 2008, 136, 1131–1135. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).