Alternative Vaccination Routes against Paratuberculosis Modulate Local Immune Response and Interference with Tuberculosis Diagnosis in Laboratory Animal Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccination and Challenge Experiment
2.1.1. Experimental Design
2.1.2. Tissue Gene Extraction and MAP PCR
2.1.3. PPA-3 Enzyme Linked Immunosorbent Assay (ELISA)
2.1.4. Histopathology
2.1.5. Immunohistochemistry
2.2. Interference with bTB Diagnosis
2.3. Statistical Analysis
3. Results
3.1. Vaccination and Challenge Experiment
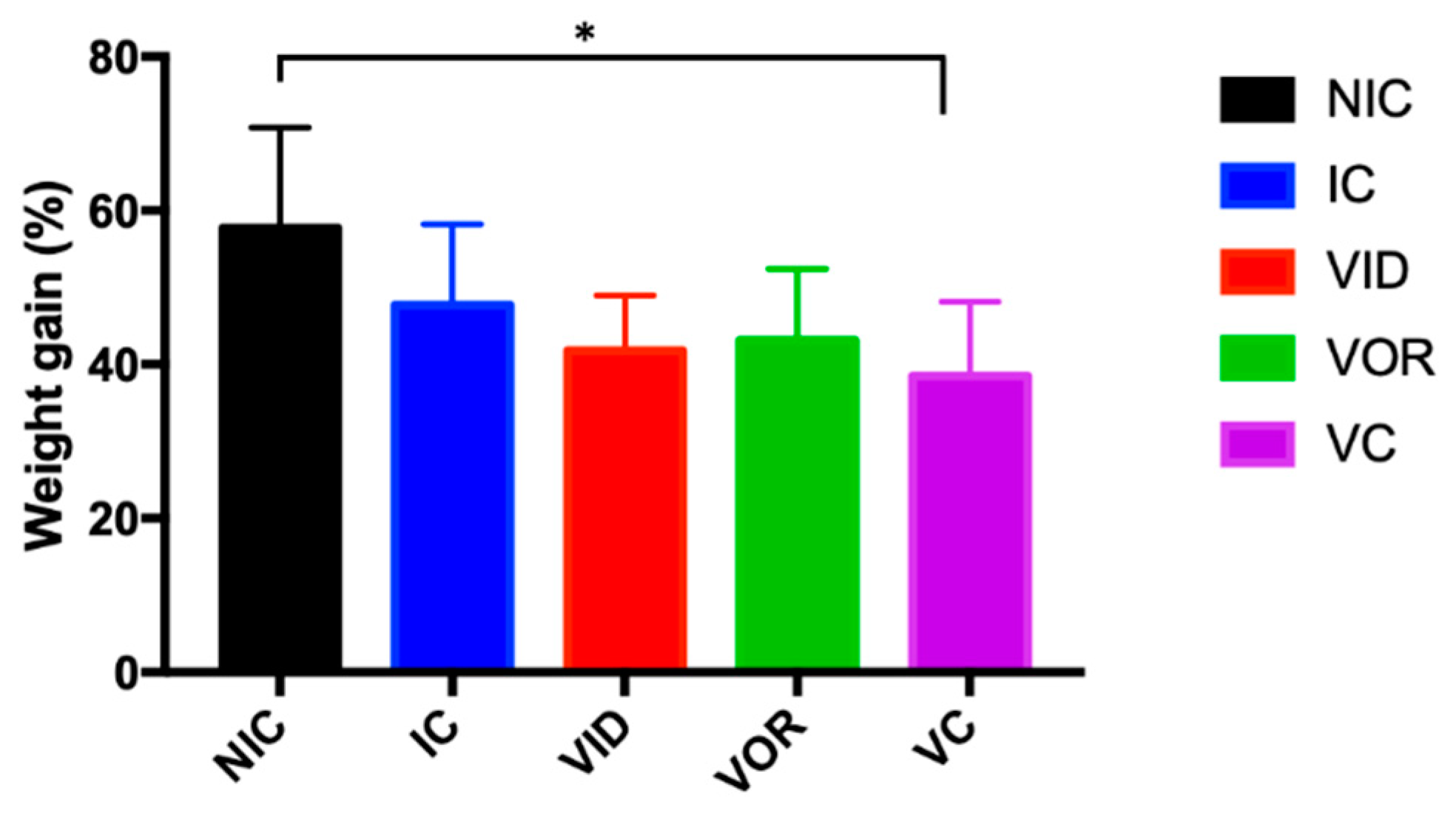
3.1.1. Weight Gain, Gross Pathology and MAP Detection
3.1.2. Humoral Response
3.1.3. Histology
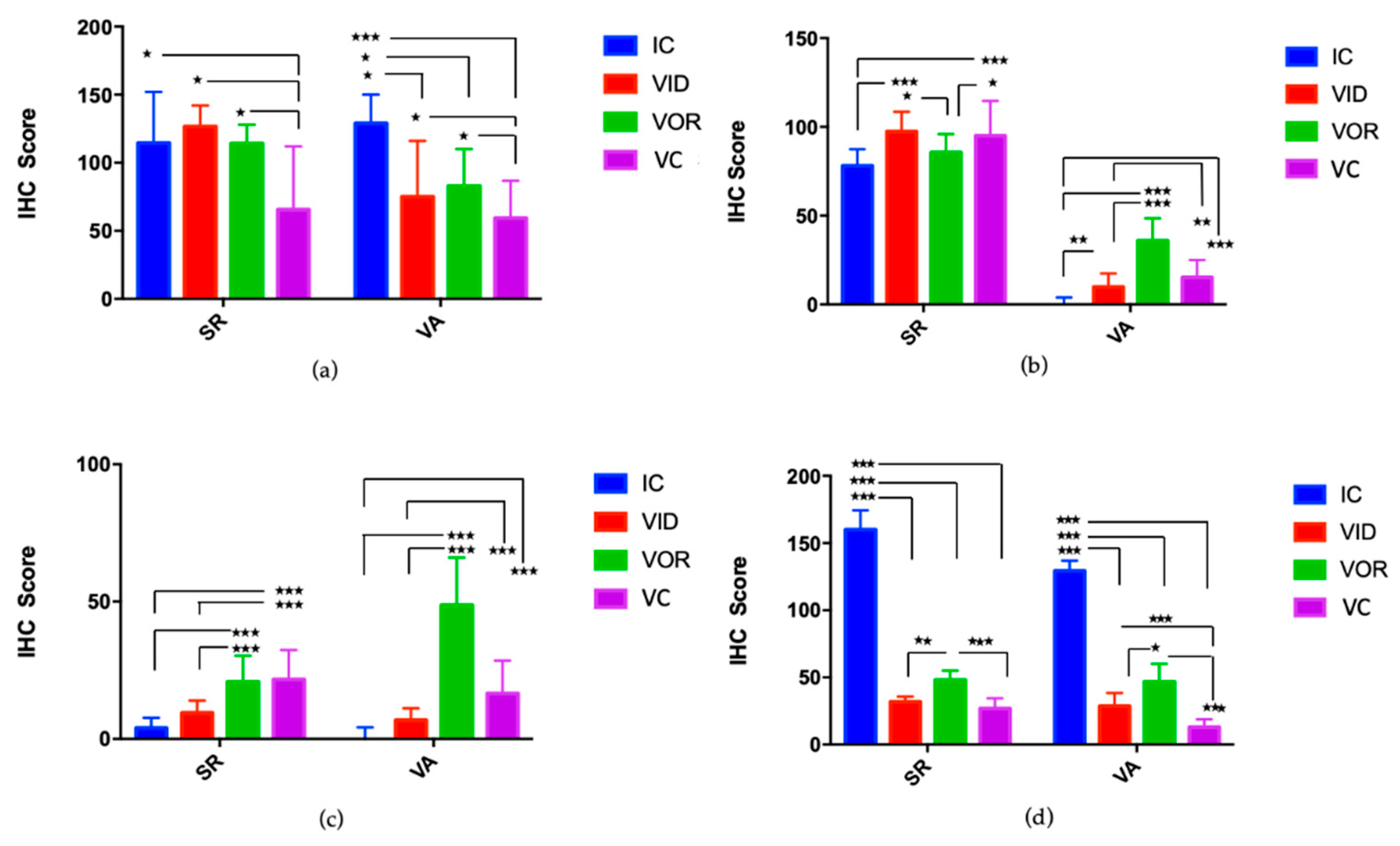
3.1.4. Cell Subsets on Granulomatous Lesions
3.1.5. Interference with bTB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carta, T.; Álvarez, J.; Pérez de la Lastra, J.M.; Gortázar, C. Wildlife and paratuberculosis: A review. Res. Vet. Sci. 2013, 94, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Beard, P.M.; Daniels, M.J.; Henderson, D.; Pirie, A.; Rudge, K.; Buxton, D.; Rhind, S.; Greig, A.; Hutchings, M.R.; McKendrick, I.; et al. Paratuberculosis infection of nonruminant wildlife in Scotland. J. Clin. Microbiol. 2001, 39, 1517–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiodini, R.J.; Van Kruiningen, H.J.; Merkal, R.S. Ruminant paratuberculosis (Johne’s disease): The current status and future prospects. Cornell Vet. 1984, 74, 218–262. [Google Scholar] [PubMed]
- Grant, I.R. Zoonotic potential of Mycobacterium avium ssp. paratuberculosis: The current position. J. Appl. Microbiol. 2005, 98, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Bull, T.J.; McMinn, E.J.; Sidi-Boumedine, K.; Skull, A.; Durkin, D.; Neild, P.; Rhodes, G.; Pickup, R.; Hermon-Taylor, J. Detection and Verification of Mycobacterium avium subsp. paratuberculosis in Fresh Ileocolonic Mucosal Biopsy Specimens from Individuals with and without Crohn’s Disease. J. Clin. Microbiol. 2003, 41, 2915–2923. [Google Scholar] [CrossRef] [Green Version]
- Juste, R.A.; Elguezabal, N.; Pavón, A.; Garrido, J.M.; Geijo, M.; Sevilla, I.; Cabriada, J.L.; Tejada, A.; García-Campos, F.; Casado, R.; et al. Association between Mycobacterium avium subsp. paratuberculosis DNA in blood and cellular and humoral immune response in inflammatory bowel disease patients and controls. Int. J. Infect. Dis. 2009, 13, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Kuenstner, J.T.; Naser, S.; Chamberlin, W.; Borody, T.; Graham, D.Y.; McNees, A.; Hermon-Taylor, J.; Hermon-Taylor, A.; Thomas Dow, C.; Thayer, W.; et al. The consensus from the Mycobacterium avium ssp. paratuberculosis (MAP) conference 2017. Front. Public Health 2017. [Google Scholar] [CrossRef]
- Sweeney, R.W. Transmission of Paratuberculosis. Vet. Clin. Food Anim. Pract. 1996, 12, 305–312. [Google Scholar] [CrossRef]
- Bastida, F.; Juste, R.A. Paratuberculosis control: A review with a focus on vaccination. J. Immune Based Ther. Vaccines 2011, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Juste, R.A.; Perez, V. Control of Paratuberculosis in Sheep and Goats. Vet. Clin. Food Anim. Pract. 2011, 27, 127–138. [Google Scholar] [CrossRef]
- Alonso-Hearn, M.; Molina, E.; Geijo, M.; Vazquez, P.; Sevilla, I.A.; Garrido, J.M.; Juste, R.A. Immunization of adult dairy cattle with a new heat-killed vaccine is associated with longer productive life prior to cows being sent to slaughter with suspected paratuberculosis. J. Dairy Sci. 2012, 95, 618–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewari, D.; Hovingh, E.; Linscott, R.; Martel, E.; Lawrence, J.; Wolfgang, D.; Griswold, D. Mycobacterium avium subsp. paratuberculosis antibody response, fecal shedding, and antibody cross-reactivity to Mycobacterium bovis in M. avium subsp. paratuberculosis-infected cattle herds vaccinated against Johne’s disease. Clin. Vaccine Immunol. 2014, 21, 698–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juste, R.A.; Alonso-Hearn, M.; Molina, E.; Geijo, M.; Vazquez, P.; Sevilla, I.A.; Garrido, J.M. Significant reduction in bacterial shedding and improvement in milk production in dairy farms after the use of a new inactivated paratuberculosis vaccine in a field trial. BMC Res. Notes 2009, 2, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.; Elguezabal, N.; Sevilla, I.A.; Geijo, M.V.; Molina, E.; Arrazuria, R.; Urkitza, A.; Jones, G.J.; Vordermeier, M.; Garrido, J.M.; et al. Tuberculosis detection in paratuberculosis vaccinated calves: New alternatives against interference. PLoS ONE 2017, 12, e0169735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannantine, J.P.; Everman, J.L.; Rose, S.J.; Babrak, L.; Katani, R.; Barletta, R.G.; Talaat, A.M.; Gröhn, Y.T.; Chang, Y.F.; Kapur, V.; et al. Evaluation of eight live attenuated vaccine candidates for protection against challenge with virulent Mycobacterium avium subspecies paratuberculosis in mice. Front. Cell. Infect. Microbiol. 2014, 4, 88. [Google Scholar] [CrossRef] [Green Version]
- Koets, A.; Hoek, A.; Langelaar, M.; Overdijk, M.; Santema, W.; Franken, P.; Van Eden, W.; Rutten, V. Mycobacterial 70 kD heat-shock protein is an effective subunit vaccine against bovine paratuberculosis. Vaccine 2006, 24, 2550–2559. [Google Scholar] [CrossRef]
- Click, R.E. Successful treatment of asymptomatic or clinically terminal bovine Mycobacterium avium subspecies paratuberculosis infection (Johne’s disease) with the bacterium Dietzia used as a probiotic alone or in combination with dexamethasone: Chronic human diarrheal diseases. Virulence 2011, 2, 131–143. [Google Scholar]
- Park, S.-U.; Kathaperumal, K.; McDonough, S.; Akey, B.; Huntley, J.; Bannantine, J.P.; Chang, Y.-F. Immunization with a DNA vaccine cocktail induces a Th1 response and protects mice against Mycobacterium avium subsp. paratuberculosis challenge. Vaccine 2008, 26, 4329–4337. [Google Scholar] [CrossRef]
- Kathaperumal, K.; Park, S.-U.; McDonough, S.; Stehman, S.; Akey, B.; Huntley, J.; Wong, S.; Chang, C.-F.; Chang, Y.-F. Vaccination with recombinant Mycobacterium avium subsp. paratuberculosis proteins induces differential immune responses and protects calves against infection by oral challenge. Vaccine 2008, 26, 1652–1663. [Google Scholar] [CrossRef]
- Thakur, A.; Andrea, A.; Mikkelsen, H.; Woodworth, J.S.; Andersen, P.; Jungersen, G.; Aagaard, C. Targeting the Mincle and TLR3 receptor using the dual agonist cationic adjuvant formulation 9 (CAF09) induces humoral and polyfunctional memory T cell responses in calves. PLoS ONE 2018, 13, e0201253. [Google Scholar] [CrossRef]
- Garrido, J.M.; Sevilla, I.A.; Beltrán-Beck, B.; Minguijón, E.; Ballesteros, C.; Galindo, R.C.; Boadella, M.; Lyashchenko, K.P.; Romero, B.; Geijo, M.V.; et al. Protection against tuberculosis in eurasian wild boar vaccinated with heat-inactivated mycobacterium bovis. PLoS ONE 2011, 6, e24905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.J.; Steinbach, S.; Sevilla, I.A.; Garrido, J.M.; Juste, R.; Vordermeier, H.M. Oral vaccination of cattle with heat inactivated Mycobacterium bovis does not compromise bovine TB diagnostic tests. Vet. Immunol. Immunopathol. 2016, 182, 85–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Risalde, M.A.; Casal, C.; Romero, B.; de Juan, L.; Menshawy, A.M.; Díez-Guerrier, A.; Juste, R.A.; Garrido, J.M.; Sevilla, I.A.; et al. Oral vaccination with heat-inactivated Mycobacterium bovis does not interfere with the antemortem diagnostic techniques for tuberculosis in goats. Front. Vet. Sci. 2017, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, M.E.; Turnquist, S.E.; Ilha, M.R.S.; Rajeev, S.; Jones, A.L.; Whittington, L.; Bannantine, J.P.; Barletta, R.G.; Gröhn, Y.T.; Katani, R.; et al. Evaluation of novel oral vaccine candidates and validation of a caprine model of Johne’s disease. Front. Cell. Infect. Microbiol. 2014, 4, 26. [Google Scholar] [CrossRef]
- Nisbet, D.I.; Gilmour, N.J.; Brotherson, J.G. Quantitative studies of Mycobacterium johnei in tissues of sheep. III. Intestinal histopathology. J. Comp. Pathol. Ther. 1962, 72, 80–91. [Google Scholar] [CrossRef]
- Gilmour, N.J.L.; Angus, K.W. Absence of immunogenicity of an oral vaccine against Mycobacterium johnei in sheep. Res. Vet. Sci. 1974, 6, 269–270. [Google Scholar] [CrossRef]
- Holland, D.; Booy, R.; Looze, F.D.; Eizenberg, P.; McDonald, J.; Karrasch, J.; McKeirnan, M.; Salem, H.; Mills, G.; Reid, J.; et al. Intradermal Influenza Vaccine Administered Using a New Microinjection System Produces Superior Immunogenicity in Elderly Adults: A Randomized Controlled Trial. J. Infect. Dis. 2008, 198, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Propst, T.; Propst, A.; Lhotta, K.; Vogel, W.; Konig, P. Reinforced intradermal hepatitis B vaccination in hemodialysis patients is superior in antibody response to intramuscular or subcutaneous vaccination. Am. J. Kidney Dis. 1998, 32, 1041–1045. [Google Scholar] [CrossRef]
- Diebold, S.S. Determination of T-cell fate by dendritic cells. Immunol. Cell Biol. 2008, 86, 389–397. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Maattanen, P.; Daigle, J.; Potter, A.; Griebel, P.; Napper, S. From mouth to macrophage: Mechanisms of innate immune subversion by Mycobacterium avium subsp. paratuberculosis. Vet. Res. 2014, 45, 54. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Singh, V.K.; Hunter, R.L.; Jagannath, C. Macrophage heterogeneity and plasticity in tuberculosis. J. Leukoc. Biol. 2019, 106, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Arrazuria, R.; Molina, E.; Mateo-Abad, M.; Arostegui, I.; Garrido, J.M.; Juste, R.A.; Elguezabal, N. Effect of various dietary regimens on oral challenge with Mycobacterium avium subsp. paratuberculosis in a rabbit model. Res. Vet. Sci. 2015, 101, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, I.A.; Garrido, J.M.; Molina, E.; Geijo, M.V.; Elguezabal, N.; Vázquez, P.; Juste, R.A. Development and evaluation of a novel multicopy-element-targeting triplex PCR for detection of Mycobacterium avium subsp. paratuberculosis in feces. Appl. Environ. Microbiol. 2014, 80, 3757–3768. [Google Scholar] [CrossRef] [Green Version]
- Arrazuria, R.; Molina, E.; Garrido, J.M.; Pérez, V.; Juste, R.A.; Elguezabal, N. Vaccination sequence effects on immunological response and tissue bacterial burden in paratuberculosis infection in a rabbit model. Vet. Res. 2016, 47, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, M.; Benavides, J.; Castaño, P.; Elguezabal, N.; Fuertes, M.; Muñoz, M.; Royo, M.; Ferreras, M.C.; Pérez, V. Macrophage Subsets Within Granulomatous Intestinal Lesions in Bovine Paratuberculosis. Vet. Pathol. 2017, 54, 82–93. [Google Scholar] [CrossRef]
- Elguezabal, N.; Bastida, F.; Sevilla, I.A.; González, N.; Molina, E.; Garrido, J.M.; Juste, R.A. Estimation of Mycobacterium avium subsp. paratuberculosis growth parameters: Strain characterization and comparison of methods. Appl. Environ. Microbiol. 2011, 77, 8615–8624. [Google Scholar] [CrossRef] [Green Version]
- Begg, D.J.; de Silva, K.; Carter, N.; Plain, K.M.; Purdie, A.; Whittington, R.J. Does a th1 over th2 dominancy really exist in the early stages of Mycobacterium avium subspecies paratuberculosis infections? Immunobiology 2011, 216, 840–846. [Google Scholar] [CrossRef]
- Achkar, J.M.; Casadevall, A. Antibody-mediated immunity against tuberculosis: Implications for vaccine development. Cell Host Microbe 2013, 13, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Balu, S.; Reljic, R.; Lewis, M.J.; Pleass, R.J.; McIntosh, R.; van Kooten, C.; van Egmond, M.; Challacombe, S.; Woof, J.M.; Ivanyi, J. A Novel Human IgA Monoclonal Antibody Protects against Tuberculosis. J. Immunol. 2011, 186, 3113–3119. [Google Scholar] [CrossRef] [Green Version]
- Pooley, H.B.; Begg, D.J.; Plain, K.M.; Whittington, R.J.; Purdie, A.C.; De Silva, K. The humoral immune response is essential for successful vaccine protection against paratuberculosis in sheep. BMC Vet. Res. 2019, 15, 223. [Google Scholar] [CrossRef]
- Juste, R.A.; García Marín, J.F.; Peris, B.; Sáez de Ocáriz, C.; Badiola, J.J. Experimental infection of vaccinated and non-vaccinated lambs with Mycobacterium paratuberculosis. J. Comp. Pathol. 1994, 110, 185–194. [Google Scholar] [CrossRef]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Luo, Q.; Guo, Y.; Chen, J.; Xiong, G.; Peng, Y.; Ye, J.; Li, J. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of tuberculous granulomas in vitro. PLoS ONE 2015, 10, e0129744. [Google Scholar] [CrossRef] [PubMed]
- Bekker, L.G.; Moreira, A.L.; Bergtold, A.; Freeman, S.; Ryffel, B.; Kaplan, G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect. Immun. 2000, 68, 6954–6961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, A.M.; DiFazio, R.; Flynn, J.L. IFN- from CD4 T Cells Is Essential for Host Survival and Enhances CD8 T Cell Function during Mycobacterium tuberculosis Infection. J. Immunol. 2013, 190, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soulas, C.; Conerly, C.; Kim, W.-K.; Burdo, T.H.; Alvarez, X.; Lackner, A.A.; Williams, K.C. Recently Infiltrating MAC387+ Monocytes/Macrophages. Am. J. Pathol. 2011, 178, 2121–2135. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.; Fuertes, M.; Elguezabal, N.; Castaño, P.; Royo, M.; Ferreras, M.C.; Benavides, J.; Pérez, V. Immunohistochemical expression of interferon-γ in different types of granulomatous lesions associated with bovine paratuberculosis. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51, 1–8. [Google Scholar] [CrossRef]
- Duluc, D.; Corvaisier, M.; Blanchard, S.; Catala, L.; Descamps, P.; Gamelin, E.; Ponsoda, S.; Delneste, Y.; Hebbar, M.; Jeannin, P. Interferon-γ reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int. J. Cancer 2009, 125, 367–373. [Google Scholar] [CrossRef]
- Hagemann, T.; Lawrence, T.; McNeish, I.; Charles, K.A.; Kulbe, H.; Thompson, R.G.; Robinson, S.C.; Balkwill, F.R. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 2008, 205, 1261–1268. [Google Scholar] [CrossRef]
- Dannenberg, A.M.; Collins, F.M. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs but is due to a continuous host response to mycobacterial products. Tuberculosis 2001, 81, 229–242. [Google Scholar] [CrossRef]
| Antigen (Clone) | Specificity and Target Cells | Antigen Retreival Solution | Antibody Dilution | Reference |
|---|---|---|---|---|
| Bovine TNF-α (CC327) | Expressed in M1 macrophages. | 6 | 1:200 | [35] |
| Bovine IFN-γ (CC330) | Released by lymphocytes, macrophages and dendritic cells. Induces M1 macrophage polarization. | 6 | 1:100 | [36] |
| Human calprotectin (MAC387) | Expressed in activated, recently recruited macrophages among other cells. | 9 | 1:200 | [35] |
| Human CD163 (EDHu-1) | Expressed in M2 macrophages. | 6 | 1:300 | [35] |
| MAP PCR | Histopathology Score | |||||
|---|---|---|---|---|---|---|
| Group | ID | SR | VA | SR | VA | Gross Pathology Score |
| IC | 37 | + | − | 2 | 2 | 2 |
| 38 | + | − | 1 | 1 | 0 | |
| 40 | − | + | 3 | 3 | 4 | |
| 43 | − | − | 2 | 2 | 4 | |
| 44 | + | + | 2 | 4 | 0 | |
| VID | 16 | − | − | 1 | 2 | 0 |
| 17 | − | − | 2 | 1 | 0 | |
| 31 | + | + | 1 | 1 | 3 | |
| 32 | − | + | 2 | 2 | 0 | |
| 33 | − | − | 2 | 2 | 3 | |
| VOR | 18 | − | − | 2 | 2 | 1 |
| 19 | − | + | 2 | 2 | 1 | |
| 34 | + | + | 1 | 1 | 0 | |
| 35 | + | − | 1 | 2 | 3 | |
| 36 | − | + | 2 | 4 | 4 | |
| VC | 14 | − | − | 1 | 1 | 0 |
| 15 | + | − | 1 | 1 | 2 | |
| 28 | − | + | 1 | 1 | 1 | |
| 29 | + | + | 1 | 1 | 4 | |
| 30 | + | + | 1 | 2 | 3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrazuria, R.; Ladero, I.; Molina, E.; Fuertes, M.; Juste, R.; Fernández, M.; Pérez, V.; Garrido, J.; Elguezabal, N. Alternative Vaccination Routes against Paratuberculosis Modulate Local Immune Response and Interference with Tuberculosis Diagnosis in Laboratory Animal Models. Vet. Sci. 2020, 7, 7. https://doi.org/10.3390/vetsci7010007
Arrazuria R, Ladero I, Molina E, Fuertes M, Juste R, Fernández M, Pérez V, Garrido J, Elguezabal N. Alternative Vaccination Routes against Paratuberculosis Modulate Local Immune Response and Interference with Tuberculosis Diagnosis in Laboratory Animal Models. Veterinary Sciences. 2020; 7(1):7. https://doi.org/10.3390/vetsci7010007
Chicago/Turabian StyleArrazuria, Rakel, Iraia Ladero, Elena Molina, Miguel Fuertes, Ramón Juste, Miguel Fernández, Valentín Pérez, Joseba Garrido, and Natalia Elguezabal. 2020. "Alternative Vaccination Routes against Paratuberculosis Modulate Local Immune Response and Interference with Tuberculosis Diagnosis in Laboratory Animal Models" Veterinary Sciences 7, no. 1: 7. https://doi.org/10.3390/vetsci7010007





