The Effects of Various Weather Conditions as a Potential Ischemic Stroke Trigger in Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Canine Data Collection
2.2. Meteorological Exposures
2.3. Statistical Analysis
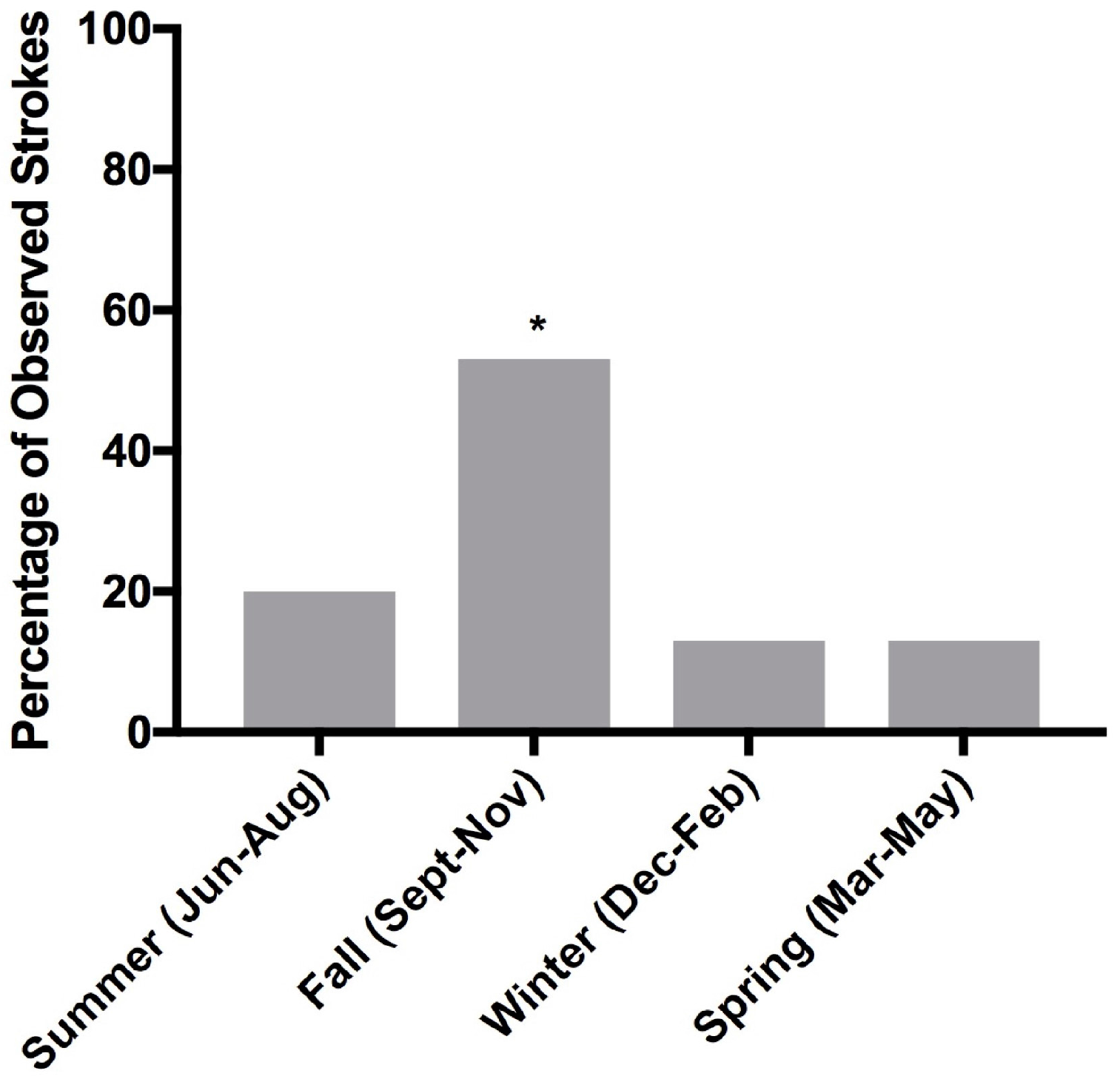
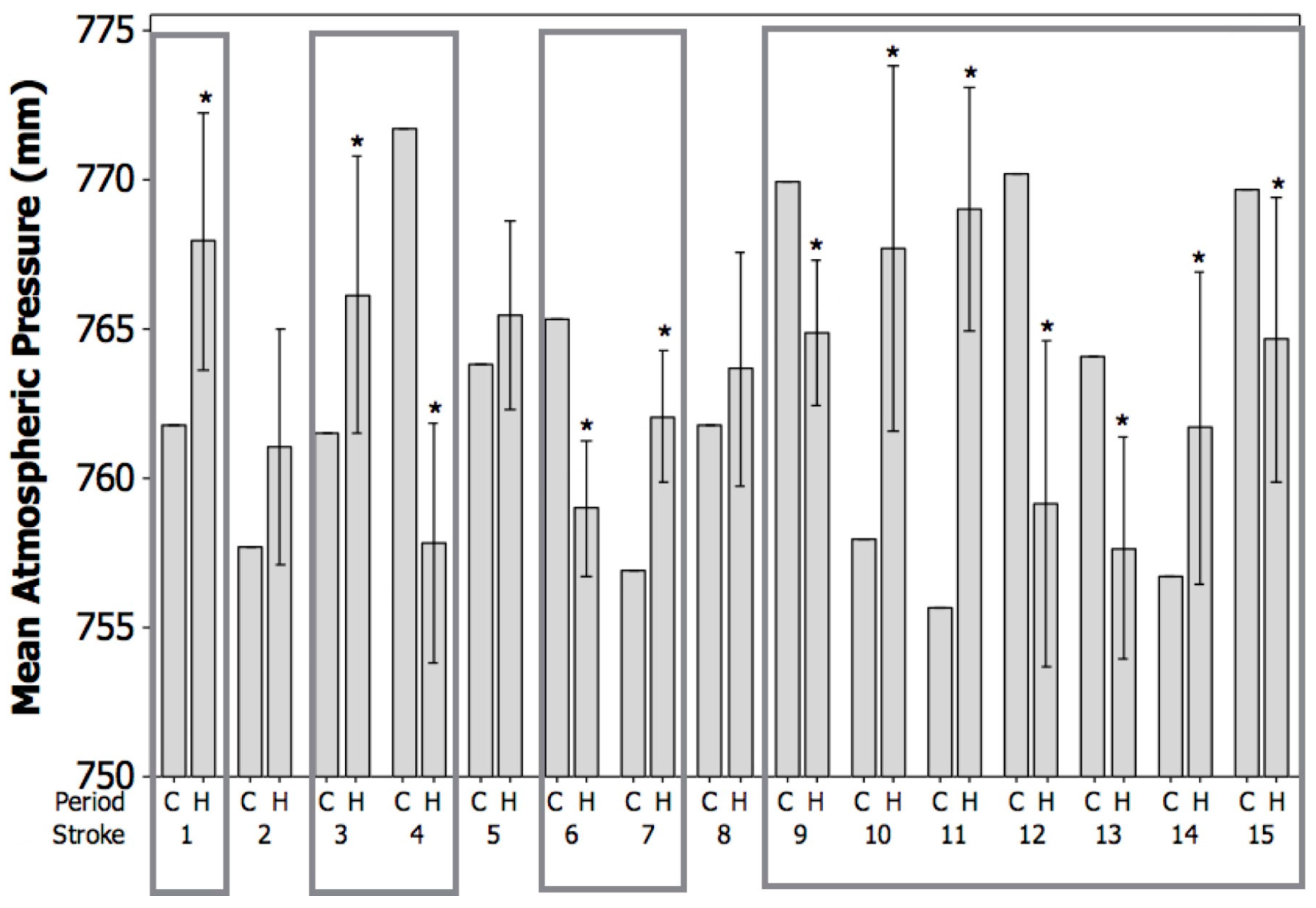
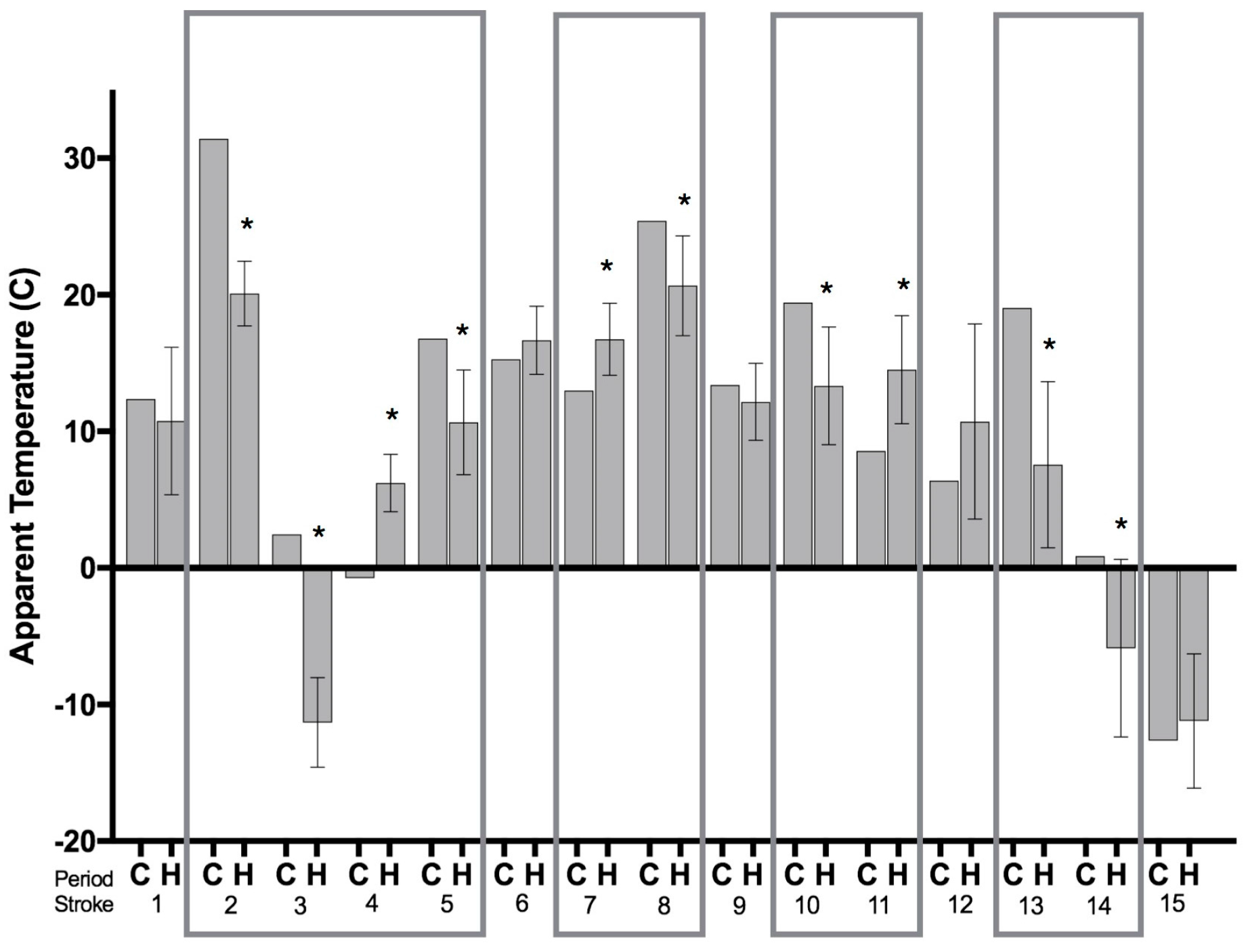
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Broussalis, E.; Killer, M.; McCoy, M.; Harrer, A.; Trinka, E.; Kraus, J. Current therapies in ischemic stroke. Part A. Recent developments in acute stroke treatment and in stroke prevention. Drug Discov. Today 2012, 17, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, A.A.; Rabie, T.; Sutherland, B.A.; Papadakis, M.; Hadley, G.; Cai, R.; Buchan, A.M. Importance of preclinical research in the development of neuroprotective strategies for ischemic stroke. JAMA Neurol. 2014, 71, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Durukan, A.; Tatlisumak, T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav. 2007, 87, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Altay, U.M.; Skerritt, G.C.; Hilbe, M.; Ehrensperger, F.; Steffen, F. Feline cerebrovascular disease: Clinical and histopathologic findings in 16 cats. J. Am. Anim. Hosp. Assoc. 2011, 47, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Garosi, L.S. Cerebrovascular Disease in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Veterinary Information Network (VIN)—For Veterinarians, by Veterinarians. Available online: http://www.vin.com/ (accessed on 30 April 2016).
- DeLahunta, A.; Glass, E.; Kent, M. Veterinary Neuroanatomy and Clinical Neurology, 4th ed.; Elsevier: St. Louis, MO, USA, 2015. [Google Scholar]
- Henderson, V.W.; Lobo, R.A. Hormone therapy and the risk of stroke: Perspectives 10 years after the Women’s Health Initiative trials. Climact. J. Int. Menopause Soc. 2012, 15, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [PubMed]
- Kelly-Hayes, M.; Wolf, P.A.; Kase, C.S.; Brand, F.N.; McGuirk, J.M.; D’Agostino, R.B. Temporal patterns of stroke onset the Framingham Study. Stroke 1995, 26, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, E.; Wilker, E.H.; Schwartz, J.; Zanobetti, A.; Gold, D.R.; Wellenius, G.A.; Mittleman, M.A. Short-term changes in ambient temperature and risk of ischemic stroke. Cerebrovasc. Dis. Extra 2014, 4, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtman, J.H.; Leifheit-Limson, E.C.; Jones, S.B.; Wang, Y.; Goldstein, L.B. Average Temperature, Diurnal Temperature Variation, and Stroke Hospitalizations. J. Stroke Cerebrovasc. Dis. 2016, 25, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Ruan, Y.; Liang, R.; Liu, X.; Fan, Z. Short-Term Effect of Ambient Temperature and the Risk of Stroke: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2015, 12, 9068–9088. [Google Scholar] [CrossRef] [PubMed]
- Cowperthwaite, M.C.; Burnett, M.G. An analysis of admissions from 155 United States hospitals to determine the influence of weather on stroke incidence. J. Clin. Neurosci. 2011, 18, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Awano, T.; Johnson, G.S.; Wade, C.M.; Katz, M.L.; Johnson, G.C.; Taylor, J.F.; Perloski, M.; Biagi, T.; Baranowska, I.; Long, S.; et al. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.R.; Wininger, F.A. Canine Degenerative Myelopathy. Vet. Clin. North Am. Small Anim. Pract. 2010, 40, 929–950. [Google Scholar] [CrossRef] [PubMed]
- Crisp, M.J.; Beckett, J.; Coates, J.R.; Miller, T.M. Canine degenerative myelopathy: Biochemical characterization of superoxide dismutase 1 in the first naturally occurring non-human amyotrophic lateral sclerosis model. Exp. Neurol. 2013, 248, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gredal, H.; Skerritt, G.C.; Gideon, P.; Arlien-Soeborg, P.; Berendt, M. Spontaneous ischaemic stroke in dogs: Clinical topographic similarities to humans. Acta Neurol. Scand. 2013, 128, e11–e16. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Creevy, K.E.; Promislow, D.E.L. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS ONE 2013, 8, e61082. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. (Ed.) Principles of Neural Science, 5th ed.; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Kumar, M.S.A. Clinically Oriented Anatomy of the Dog & Cat; Linus Publications-Incorporated: Ronkonkoma, NY, USA, 2013. [Google Scholar]
- National Oceanic and Atmospheric Administration|U.S. Department of Commerce. Available online: http://www.noaa.gov/ (accessed on 3 May 2016).
- Hong, Y.-C.; Rha, J.-H.; Lee, J.-T.; Ha, E.-H.; Kwon, H.-J.; Kim, H. Ischemic stroke associated with decrease in temperature. Epidemiology 2003, 14, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Rakers, F.; Schiffnerm, R.; Rupprecht, S.; Brandstadt, A.; Witte, O.W.; Walther, M.; Schlattmann, P.; Schwab, M. Rapid weather changes are associated with increased ischemic stroke risk: A case-crossover study. Eur. J. Epidemiol. 2015, 31, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, P.; Wolf, L.E.; Segal, M.R.; McCulloch, C.E. Ethics and sample size. Am. J. Epidemiol. 2005, 161, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.D.; Karlawish, J.H.T.; Berlin, J.A. The continuing unethical conduct of underpowered clinical trials. JAMA 2002, 288, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Ethics in Quantitative Methodology, 1st ed.; Panter, A.T.; Sterba, S.K. (Eds.) Routledge: New York, NY, USA, 2011. [Google Scholar]
- Gomes, J.; Damasceno, A.; Carrilho, C.; Lobo, V.; Lopes, H.; Madede, T.; Pravinrai, P.; Silva-Matos, C.; Diogo, D.; Azevedo, A.; et al. Triggering of stroke by ambient temperature variation: A case-crossover study in Maputo, Mozambique. Clin. Neurol. Neurosurg. 2015, 129, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gunes, H.; Kandis, H.; Saritas, A.; Dikici, S.; Buyukkaya, R. The relationship between ischemic stroke and weather conditions in Duzce, Turkey. World J. Emerg. Med. 2015, 6, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Brandstätter, E. Confidence intervals as an alternative to significance testing. Methods Psychol. Res. Online 1999, 4, 33–46. [Google Scholar]
- Fidler, F.; Thomason, N.; Cumming, G.; Finch, S.; Leeman, J. Editors can lead researchers to confidence intervals, but can’t make them think: Statistical reform lessons from medicine. Psychol. Sci. 2004, 15, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, R. Scientific method: Statistical errors. Nature 2014, 506, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.G.; Curran-Everett, D.; Vowler, S.L.; Drummond, G.B. The fickle P value generates irreproducible results. Nat. Methods 2015, 12, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Krstić, G. Apparent temperature and air pollution vs. elderly population mortality in Metro Vancouver. PLoS ONE 2011, 6, e25101. [Google Scholar] [CrossRef] [PubMed]
- Kalkstein, L.S.; Valimont, K.M. An Evaluation of Summer Discomfort in the United State Using a Relative Climatological Index. Bull. Am. Meteorol. Soc. 1986, 67, 842–848. [Google Scholar] [CrossRef]
- Chiriboga, D.E.; Ma, Y.; Li, W.; Stanek, E.J.; Hebert, J.R.; Merriam, P.A.; Rawson, E.S.; Ockene, I.S. Seasonal and Sex Variation of High-Sensitivity C-Reactive Protein in Healthy Adults: A Longitudinal Study. Clin. Chem. 2009, 55, 313–321. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.; Formica, M.; Schmid, C.H.; Fletcher, J. Changes in barometric pressure and ambient temperature influence osteoarthritis pain. Am. J. Med. 2007, 120, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.R.; Bauserman, R.L.; Ballas, S.K.; McCarthy, W.F.; Steinberg, M.H.; Swerdlow, P.S.; Waclawiw, M.A.; Barton, B.A.; The Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Climatic and geographic temporal patterns of pain in the Multicenter Study of Hydroxyurea. Pain 2009, 146, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Houck, P.D.; Lethen, J.E.; Riggs, M.W.; Gantt, D.S.; Dehmer, G.J. Relation of atmospheric pressure changes and the occurrences of acute myocardial infarction and stroke. Am. J. Cardiol. 2005, 96, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kyobutungi, C.; Grau, A.; Stieglbauer, G.; Becher, H. Absolute temperature, temperature changes and stroke risk: A case-crossover study. Eur. J. Epidemiol. 2005, 20, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.M.S.; Santos, B.F.C.D.; Neto, M.C.; Lisboa, L.F.; Cypriano, A.S.; Lopes, T.O.; de Miranda, M.J.; Avila, M.H.; Alonso, J.B.; Pinto, H.S. Temperature variation in the 24 hours before the initial symptoms of stroke. Arq. Neuropsiquiatr. 2010, 68, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Ma, W.-P.; Zhao, N.-Q.; Wang, X.-L. Time series analysis of the association between ambient temperature and cerebrovascular morbidity in the elderly in Shanghai, China. Sci. Rep. 2016, 6, 19052. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Damasceno, A.; Carriho, C.; Lobo, V.; Lopes, H.; Madede, T.; Pravinrai, P.; Silca-Matos, C.; Diogo, D.; Azevedo, A.; et al. The effect of season and temperature variation on hospital admissions for incident stroke events in Maputo, Mozambique. J. Stroke Cerebrovasc. Dis. 2014, 23, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.; Silva, M.C.; Correia, M.; Bailey, T. Are stroke occurrence and outcome related to weather parameters? Results from a population-based study in northern portugal. Cerebrovasc. Dis. 2011, 32, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Çevik, Y.; Doğan, N.Ö.; Daş, M.; Ahmedali, A.; Kul, S.; Bayram, H. The association between weather conditions and stroke admissions in Turkey. Int. J. Biometeorol. 2015, 59, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Goggins, W.B.; Woo, J.; Ho, S.; Chan, E.Y.Y.; Chau, P.H. Weather, season, and daily stroke admissions in Hong Kong. Int. J. Biometeorol. 2012, 56, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Halonen, J.I.; Zanobetti, A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Outdoor temperature is associated with serum HDL and LDL. Environ. Res. 2011, 111, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Arima, H.; Heeley, E.; Karpin, A.; Yang, J.; Chalmers, J.; Anderson, C.S.; The INTERACT investigators. Ambient temperature and volume of perihematomal edema in acute intracerebral haemorrhage: The INTERACT1 study. Int. J. Stroke 2015, 10, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.H.; Jones, S.B.; Wang, Y.; Leifheit-Limson, E.C.; Goldstein, L.B. Seasonal variation in 30-day mortality after stroke: Teaching versus nonteaching hospitals. Stroke J. Cereb. Circ. 2013, 44, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Roberson, S.; Dutton, M.; Macdonald, M.; Odoi, A. Does Place of Residence or Time of Year Affect the Risk of Stroke Hospitalization and Death? A Descriptive Spatial and Temporal Epidemiologic Study. PLoS ONE 2016, 11, e0145224. [Google Scholar] [CrossRef] [PubMed]
- Kanikowska, D.; Sato, M.; Iwase, S.; Shimizu, Y.; Nishimura, N.; Inukai, Y.; Sugenoya, J. Effects of living at two ambient temperatures on 24-h blood pressure and neuroendocrine function among obese and non-obese humans: A pilot study. Int. J. Biometeorol. 2013, 57, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Kanikowska, D.; Sugenoya, J.; Satom, M.; Shimizu, Y.; Inukai, Y.; Nishimura, N.; Iwase, S. Influence of season on plasma antidiuretic hormone, angiotensin II, aldosterone and plasma renin activity in young volunteers. Int. J. Biometeorol. 2010, 54, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kirsz, K.; Szczesna, M.; Molik, E.; Misztal, T.; Wojtowicz, A.K.; Zieba, D.A. Seasonal changes in the interactions among leptin, ghrelin, and orexin in sheep. J. Anim. Sci. 2012, 90, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Kruse, H.-J.; Wieczorek, I.; Hecker, H.; Creutzig, A.; Schellong, S.M. Seasonal variation of endothelin-1, angiotensin II, and plasma catecholamines and their relation to outside temperature. J. Lab. Clin. Med. 2002, 140, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kanikowska, D.; Sugenoya, J.; Inukai, Y.; Shimizu, Y.; Nishmura, N.; Iwase, S. Effects of aging on thermoregulatory responses and hormonal changes in humans during the four seasons in Japan. Int. J. Biometeorol. 2011, 55, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Wang, Z.K. Seasonal changes in body mass, serum leptin levels and hypothalamic neuropeptide gene expression in male Eothenomys olitor. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 184, 83–89. [Google Scholar]
- Bacchetti, P. Small sample size is not the real problem. Nat. Rev. Neurosci. 2013, 14, 585. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, P.T. Misuse of power: In defence of small-scale science. Nat. Rev. Neurosci. 2013, 14, 585. [Google Scholar] [CrossRef] [PubMed]


| Demographics | Number of Dogs | Percentage | Mean (Range) |
|---|---|---|---|
| Age (years) | 10 (4–16) | ||
| Sex | |||
| Male (neutered) | 10 (9) | 67% | |
| Female (spayed) | 5 (5) | 33% | |
| Weight (kg) | 25 (5–52) | ||
| Breed | |||
| Mixed Breed | 3 | ||
| Labrador Retriever | 2 | ||
| Rhodesian Ridgeback | 2 | ||
| Boston Terrier | 2 | ||
| Greyhound | 2 | ||
| Australian Cattle Dog | 1 | ||
| Australian Shepherd | 1 | ||
| Bichon Frise | 1 | ||
| Silky Terrier | 1 |
| Stroke Number | Date | Temperature (°C) | Atmospheric Pressure (mmHg) | Humidity (%) | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| 1 | 10 October 2011 | 14.58 | 4.92 | 767.14 | 4.82 | 62.50 | 15.98 |
| 2 | 25 July 2012 | 22.08 | 3.01 | 760.60 | 4.10 | 67.00 | 12.04 |
| 3 | 13 December 2013 | −3.82 | 4.78 | 765.52 | 4.91 | 75.63 | 10.98 |
| 4 | 7 May 2014 | 10.21 | 2.70 | 759.56 | 6.32 | 64.13 | 18.15 |
| 5 | 19 May 2014 | 15.14 | 3.40 | 765.21 | 3.20 | 63.00 | 18.91 |
| 6 | 9 June 2014 | 18.47 | 2.52 | 759.78 | 3.18 | 67.88 | 15.12 |
| 7 | 13 June 2014 | 18.06 | 2.61 | 761.40 | 2.84 | 71.75 | 14.59 |
| 8 | 8 September 2014 | 21.81 | 21.81 | 763.40 | 3.95 | 71.00 | 8.02 |
| 9 | 16 September 2014 | 14.38 | 14.38 | 765.46 | 3.01 | 76.63 | 8.73 |
| 10 | 28 September 2014 | 16.11 | 16.11 | 766.44 | 7.03 | 72.13 | 8.08 |
| 11 | 29 September 2014 | 16.25 | 16.25 | 767.14 | 4.82 | 70.13 | 5.41 |
| 12 | 20 October 2014 | 13.26 | 13.26 | 760.60 | 4.10 | 78.50 | 9.80 |
| 13 | 22 October 2014 | 12.29 | 12.29 | 765.52 | 4.91 | 80.88 | 11.81 |
| 14 | 24 November 2014 | 1.67 | 1.67 | 757.49 | 8.89 | 59.50 | 14.96 |
| 15 | 6 January 2015 | −4.65 | −4.65 | 759.56 | 6.32 | 61.38 | 16.73 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meadows, K.L.; Silver, G.M. The Effects of Various Weather Conditions as a Potential Ischemic Stroke Trigger in Dogs. Vet. Sci. 2017, 4, 56. https://doi.org/10.3390/vetsci4040056
Meadows KL, Silver GM. The Effects of Various Weather Conditions as a Potential Ischemic Stroke Trigger in Dogs. Veterinary Sciences. 2017; 4(4):56. https://doi.org/10.3390/vetsci4040056
Chicago/Turabian StyleMeadows, Kristy L., and Gena M. Silver. 2017. "The Effects of Various Weather Conditions as a Potential Ischemic Stroke Trigger in Dogs" Veterinary Sciences 4, no. 4: 56. https://doi.org/10.3390/vetsci4040056





