Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes
Abstract
:1. Introduction
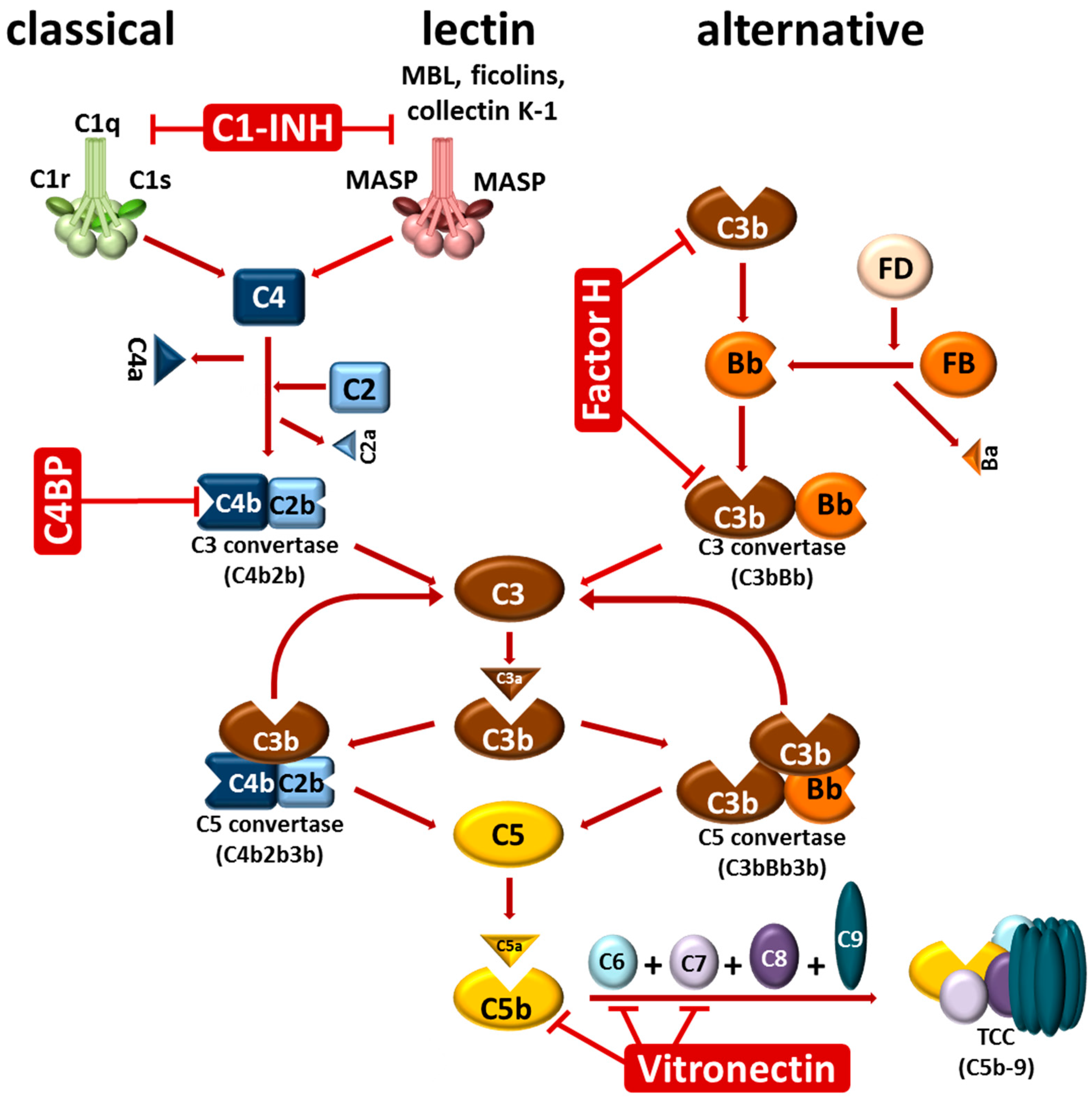
2. The Complement System
3. Contribution of Complement to Host-Specificity and Transmissibility of Lyme Disease Spirochetes
4. Species-Specific Factors Contributing to Host Specificity of Lyme Disease Spirochetes
5. The Role of Factor H-Binding Complement-Acquiring Surface Proteins in Evading Complement of Diverse Hosts
6. Interaction of Borreliae with Additional Complement Proteins
7. Future Perspectives
8. Conclusions
Acknowledgements
Conflicts of Interest
References
- Blom, A.M.; Hallström, T.; Riesbeck, K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol. Immunol. 2009, 46, 2808–2817. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Wurzner, R. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 2006, 43, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Lambris, J.D.; Ricklin, D.; Geisbrecht, B.V. Complement evasion by human pathogens. Nat. Rev. Microb. 2008, 6, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, R.D.; Sievertsen, H.J.; Knobloch, J.; Fischetti, V.A. Antiphagocytic activity of streptococcal m protein: Selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 1988, 85, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Neeleman, C.; Geelen, S.P.; Aerts, P.C.; Daha, M.R.; Mollnes, T.E.; Roord, J.J.; Posthuma, G.; van Dijk, H.; Fleer, A. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor h. Infect. Immunity 1999, 67, 4517–4524. [Google Scholar]
- Ram, S.; Mackinnon, F.G.; Gulati, S.; McQuillen, D.P.; Vogel, U.; Frosch, M.; Elkins, C.; Guttormsen, H.K.; Wetzler, L.M.; Oppermann, M.; et al. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 1999, 36, 915–928. [Google Scholar] [CrossRef]
- Ram, S.; McQuillen, D.P.; Gulati, S.; Elkins, C.; Pangburn, M.K.; Rice, P.A. Binding of complement factor H to loop 5 of porin protein 1A: A molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 1998, 188, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Biedzka-Sarek, M.; Jarva, H.; Hyytiainen, H.; Meri, S.; Skurnik, M. Characterization of complement factor H binding to Yersinia enterocolitica serotype O:3. Infect. Immunity 2008, 76, 4100–4109. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Hellwage, J.; Artiushin, S.; Zipfel, P.F.; Kraiczy, P.; Timoney, J.F.; Stevenson, B. LFHA, a novel factor H-binding protein of Leptospira interrogans. Infect. Immunity 2006, 74, 2659–2666. [Google Scholar] [CrossRef] [PubMed]
- McDowell, J.V.; Frederick, J.; Stamm, L.; Marconi, R.T. Identification of the gene encoding the FHBB protein of Treponema denticola, a highly unique factor H-like protein 1 binding protein. Infect. Immunity 2007, 75, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Stevenson, B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick Borne Dis. 2013, 4, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Poltermann, S.; Kunert, A.; Rupp, S.; Zipfel, P.F. Immune evasion of the human pathogenic yeast Candida albicans: PRA1 is a factor H, FHL-1 and plasminogen binding surface protein. Mol. Immunol. 2009, 47, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Meri, T.; Amdahl, H.; Lehtinen, M.J.; Hyvarinen, S.; McDowell, J.V.; Bhattacharjee, A.; Meri, S.; Marconi, R.; Goldman, A.; Jokiranta, T.S. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog. 2013, 9, e1003308. [Google Scholar] [CrossRef]
- Walport, M.J. Complement—Second of two parts. N. Engl. J. Med. 2001, 344, 1140–1144. [Google Scholar] [PubMed]
- Walport, M.J. Complement—First of two parts. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [PubMed]
- Zipfel, P.F. Complement and immune defense: From innate immunity to human diseases. Immunol. Lett. 2009, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Trouw, L.A.; Daha, M.R. Role of complement in innate immunity and host defense. Immunol. Lett. 2011, 138, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.F.; Heinen, S.; Jozsi, M.; Skerka, C. Complement and diseases: Defective alternative pathway control results in kidney and eye diseases. Mol. Immunol. 2006, 43, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Reiter, M. The expanding lyme Borrelia complex-clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J. Relapsing fever—A forgotten disease revealed. J. Appl. Microbiol. 2010, 108, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.; Hildebrandt, A.; Dorn, W. Exploring gaps in our knowledge on lyme borreliosis spirochaetes—Updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick Borne Dis. 2013, 4, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Diza, E.; Papa, A.; Vezyri, E.; Tsounis, S.; Milonas, I.; Antoniadis, A. Borrelia valaisiana in cerebrospinal fluid. Emerg. Infect. Dis. 2004, 10, 1692–1693. [Google Scholar] [CrossRef] [PubMed]
- Rijpkema, S.G.; Tazelaar, D.J.; Molkenboer, M.J.; Noordhoek, G.T.; Plantinga, G.; Schouls, L.M.; Schellekens, J.F. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin. Microbiol. Infect. 1997, 3, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.; Golovchenko, M.; Mokracek, A.; Piskunova, N.; Ruzek, D.; Mallatova, N.; Grubhoffer, L. Detection of Borrelia bissettii in cardiac valve tissue of a patient with endocarditis and aortic valve stenosis in the Czech Republic. J. Clin. Microbiol. 2008, 46, 3540–3543. [Google Scholar] [CrossRef] [PubMed]
- Gern, L.; Estrada-Pena, A.; Frandsen, F.; Gray, J.S.; Jaenson, T.G.; Jongejan, F.; Kahl, O.; Korenberg, E.; Mehl, R.; Nuttall, P.A. European reservoir hosts of Borrelia burgdorferi sensu lato. Int. J. Med. Microbiol. 1998, 287, 196–204. [Google Scholar] [CrossRef]
- Humair, P.F.; Postic, D.; Wallich, R.; Gern, L. An avian reservoir (Turdus merula) of the lyme borreliosis spirochetes. Int. J. Med. Microbiol. 1998, 287, 521–538. [Google Scholar]
- Marie-Angele, P.; Lommano, E.; Humair, P.F.; Douet, V.; Rais, O.; Schaad, M.; Jenni, L.; Gern, L. Prevalence of Borrelia burgdorferi sensu lato in ticks collected from migratory birds in Switzerland. Appl. Environ. Microbiol. 2006, 72, 976–979. [Google Scholar] [PubMed]
- Humair, P.F.; Rais, O.; Gern, L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: Differential transmission pattern and overwintering maintenance. Parasitology 1999, 118, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; Rijpkema, S.G.; Hoodless, A.N.; Nuttall, P.A.; Randolph, S.E. Borrelia burgdorferi sensu latoin the vertebrate host. In Lyme borreliosis: Biology of the Infectious Agents and Epidemiology of Disease; Gray, S.L., Kahl, O., Lane, R.S., Stanek, G., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 117–148. [Google Scholar]
- Kurtenbach, K.; Carey, D.; Hoodless, A.N.; Nuttall, P.A.; Randolph, S.E. Competence of pheasants as reservoirs for lyme disease spirochetes. J. Med. Entomol. 1998, 35, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; Peacey, M.; Rijpkema, S.G.; Hoodless, A.N.; Nuttall, P.A.; Randolph, S.E. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in england. Appl. Environ. Microbiol. 1998, 64, 1169–1174. [Google Scholar] [PubMed]
- Craine, N.G.; Nuttall, P.A.; Marriott, A.C.; Randolph, S.E. Role of grey squirrels and pheasants in the transmission of Borrelia burgdorferi sensu lato, the lyme disease spirochaete, in the UK. Folia Parasitol. 1997, 44, 155–160. [Google Scholar] [PubMed]
- Matuschka, F.R.; Heiler, M.; Eiffert, H.; Fischer, P.; Lotter, H.; Spielman, A. Diversionary role of hoofed game in the transmission of lyme disease spirochetes. Am. J. Trop. Med. Hyg. 1993, 48, 693–699. [Google Scholar] [PubMed]
- Mather, T.N.; Fish, D.; Coughlin, R.T. Competence of dogs as reservoirs for lyme disease spirochetes (Borrelia burgdorferi). J. Am. Vet. Med. Assoc. 1994, 205, 186–188. [Google Scholar] [PubMed]
- Peavey, C.A.; Lane, R.S. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J. Parasitol. 1995, 81, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Rand, P.W.; Lacombe, E.H.; Smith, R.P., Jr.; Rich, S.M.; Kilpatrick, C.W.; Dragoni, C.A.; Caporale, D. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J. Med. Entomol. 1993, 30, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Matuschka, F.R.; Fischer, P.; Heiler, M.; Richter, D.; Spielman, A. Capacity of European animals as reservoir hosts for the lyme disease spirochete. J. Infect. Dis. 1992, 165, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.F.; Wilson, M.L.; Spielman, A. Mice as reservoirs of the lyme disease spirochete. Am. J. Trop. Med. Hyg. 1985, 34, 355–360. [Google Scholar] [PubMed]
- Richter, D.; Schlee, D.B.; Matuschka, F.R. Reservoir competence of various rodents for the lyme disease spirochete Borrelia spielmanii. Appl. Environ. Microbiol. 2011, 77, 3565–3570. [Google Scholar] [CrossRef] [PubMed]
- Norte, A.C.; Alves da Silva, A.; Alves, J.; da Silva, L.P.; Nuncio, M.S.; Escudero, R.; Anda, P.; Ramos, J.A.; Lopes de Carvalho, I. The importance of lizards and small mammals as reservoirs for Borrelia lusitaniae in Portugal. Environ. Microbiol. Rep. 2015, 7, 188–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanincova, K.; Schafer, S.M.; Etti, S.; Sewell, H.S.; Taragelova, V.; Ziak, D.; Labuda, M.; Kurtenbach, K. Association of Borrelia afzelii with rodents in Europe. Parasitology 2003, 126, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hanincova, K.; Taragelova, V.; Koci, J.; Schafer, S.M.; Hails, R.; Ullmann, A.J.; Piesman, J.; Labuda, M.; Kurtenbach, K. Association of Borrelia garinii and B. Valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 2003, 69, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Dsouli, N.; Younsi-Kabachii, H.; Postic, D.; Nouira, S.; Gern, L.; Bouattour, A. Reservoir role of lizard Psammodromus algirus in transmission cycle of Borrelia burgdorferi sensu lato (Spirochaetaceae) in Tunisia. J. Med. Entomol. 2006, 43, 737–742. [Google Scholar] [CrossRef]
- Bhide, M.R.; Travnicek, M.; Levkutova, M.; Curlik, J.; Revajova, V.; Levkut, M. Sensitivity of Borrelia genospecies to serum complement from different animals and human: A host-pathogen relationship. FEMS Immunol. Med. Microbiol. 2005, 43, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; De Michelis, S.; Etti, S.; Schafer, S.M.; Sewell, H.S.; Brade, V.; Kraiczy, P. Host association of Borrelia burgdorferi sensu lato-the key role of host complement. Trends Microbiol. 2002, 10, 74–79. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Sewell, H.-S.; Ogden, N.H.; Randolph, S.E.; Nuttall, P.A. Serum complement sensitivity as a key factor in lyme disease ecology. Infect. Immunity 1998, 66, 1248–1251. [Google Scholar]
- Ullmann, A.J.; Lane, R.S.; Kurtenbach, K.; Miller, M.; Schriefer, M.E.; Zeldner, N.; Piesman, J. Bacteriolytic activity of selected vertebrate sera for Borrelia burgdorferi sensu stricto and Borrelia bissettii. J. Parasitol. 2003, 89, 1256–1257. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.M.; Lane, R.S.; Giclas, P.C. A comparative study of mammalian and reptilian alternative pathway of complement-mediated killing of the lyme disease spirochete (Borrelia burgdorferi). J. Parasitol. 2000, 86, 1223–1228. [Google Scholar] [CrossRef]
- Isogai, E.; Kamewaka, Y.; Isogai, H.; Kimura, K.; Fujii, N.; Nishikawa, T. Complement-mediated killing of Borrelia garinii—Bactericidal activity of wild deer serum. Microbiol. Immunol. 1994, 38, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Matuschka, F.R. Elimination of lyme disease spirochetes from ticks feeding on domestic ruminants. Appl. Environ. Microbiol. 2010, 76, 7650–7652. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; Schafer, S.M.; Sewell, H.S.; Peacey, M.; Hoodless, A.; Nuttall, P.A.; Randolph, S.E. Differential survival of lyme borreliosis spirochetes in ticks that feed on birds. Infect. Immunity 2002, 70, 5893–5895. [Google Scholar] [CrossRef]
- Breitner-Ruddock, S.; Würzner, R.; Schulze, J.; Brade, V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. 1997, 185, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Hunfeld, K.P.; Breitner-Ruddock, S.; Wurzner, R.; Acker, G.; Brade, V. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology 2000, 201, 406–419. [Google Scholar] [CrossRef]
- Van Burgel, N.D.; Kraiczy, P.; Schuijt, T.J.; Zipfel, P.F.; van Dam, A.P. Identification and functional characterisation of complement regulator acquiring surface protein-1 of serum resistant Borrelia garinii ospa serotype 4. BMC Microbiol. 2010, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, A.P.; Oei, A.; Jaspars, R.; Fijen, C.; Wilske, B.; Spanjaard, L.; Dankert, J. Complement-mediated serum sensitivity among spirochetes that cause lyme disease. Infect. Immunity 1997, 65, 1228–1236. [Google Scholar]
- Lachmann, P.J. Preparing serum for functional complement assays. J. Immunol. Methods 2010, 352, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, A.; Meri, T.; Ramo, L.; Jokiranta, T.S.; Heikkila, T.; Seppala, I.J.T.; Oksi, J.; Viljanen, M.; Meri, S. Complement evasion by Borrelia burgdorferi: Serum-resistant strains promote C3B inactivation. Infect. Immunity 2001, 69, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Hellwage, J.; Meri, T.; Heikkila, T.; Alitalo, A.; Panelius, J.; Lahdenne, P.; Seppala, I.J.T.; Meri, S. The complement regulator factor H binds to the surface protein ospe of Borrelia burgdorferi. J. Biol. Chem. 2001, 276, 8427–8435. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Skerka, C.; Brade, V.; Zipfel, P.F. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immunity 2001, 69, 7800–7809. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Skerka, C.; Kirschfink, M.; Brade, V.; Zipfel, P.F. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 2001, 31, 1674–1684. [Google Scholar] [CrossRef]
- Kraiczy, P.; Skerka, C.; Kirschfink, M.; Zipfel, P.F.; Brade, V. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Intern. Immunopharmacol. 2001, 1, 393–401. [Google Scholar] [CrossRef]
- McDowell, J.V.; Wolfgang, J.; Tran, E.; Metts, M.S.; Hamilton, D.; Marconi, R.T. Comprehensive analysis of the factor h binding capabilities of Borrelia species associated with lyme disease: Delineation of two distinct classes of factor H binding proteins. Infect. Immunity 2003, 71, 3597–3602. [Google Scholar] [CrossRef]
- Herzberger, P.; Siegel, C.; Skerka, C.; Fingerle, V.; Schulte-Spechtel, U.; van Dam, A.; Wilske, B.; Brade, V.; Zipfel, P.F.; Wallich, R.; et al. Human pathogenic Borrelia spielmanii sp. Nov. Resists complement-mediated killing by direct binding of immune regulators factor h and factor H-like protein 1. Infect. Immunity 2007, 75, 4817–4825. [Google Scholar]
- Dieterich, R.; Hammerschmidt, C.; Richter, D.; Skerka, C.; Wallich, R.; Matuschka, F.R.; Zipfel, P.F.; Kraiczy, P. Inadequate binding of immune regulator factor H is associated with sensitivity of Borrelia lusitaniae to human complement. Infect. Immunity 2010, 78, 4467–4476. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.; Hammerschmidt, C.; Richter, D.; Skerka, C.; Matuschka, F.R.; Wallich, R.; Zipfel, P.F.; Kraiczy, P. Borrelia valaisiana resist complement-mediated killing independently of the recruitment of immune regulators and inactivation of complement components. PLoS ONE 2013, 8, e53659. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, C.; Klevenhaus, Y.; Koenigs, A.; Hallstrom, T.; Fingerle, V.; Skerka, C.; Pos, K.M.; Zipfel, P.F.; Wallich, R.; Kraiczy, P. BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol. Microbiol. 2016, 99, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, A.; Meri, T.; Chen, T.; Lankinen, H.; Cheng, Z.-Z.; Jokiranta, T.S.; Seppala, I.J.T.; Lahdenne, P.; Hefty, P.S.; Akins, D.R.; et al. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 2004, 172, 6195–6201. [Google Scholar] [CrossRef] [PubMed]
- Bhide, M.R.; Escudero, R.; Camafeita, E.; Gil, H.; Jado, I.; Anda, P. Complement factor H binding by different lyme disease and relapsing fever Borrelia in animals and human. BMC Res. Notes 2009, 2, 134. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Kraiczy, P.; Wallich, R.; Brade, V.; Skerka, C.; Zipfel, P. Binding of human factor h-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis. 2007, 196, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hovis, K.M.; Tran, E.; Sundy, C.M.; Buckles, E.; McDowell, J.V.; Marconi, R.T. Selective binding of Borrelia burgdorferi ospe paralogs to factor H and serum proteins from diverse animals: Possible expansion of the role of ospe in lyme disease pathogenesis. Infect. Immunity 2006, 74, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Kisova-Vargova, L.; Cernanska, D.; Bhide, M. Comparative study of binding of ovine complement factor h with different Borrelia genospecies. Folia Microbiol. 2012, 57, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.; El-Hage, N.; Hines, M.A.; Miller, J.C.; Babb, K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi erp surface proteins: A possible mechanism underlying the expansive host range of lyme disease spirochetes. Infect. Immunity 2002, 70, 491–497. [Google Scholar] [CrossRef]
- McDowell, J.V.; Hovis, K.M.; Zhang, H.; Tran, E.; Lankford, J.; Marconi, R.T. Evidence that the BBA68 protein (BBCRASP-1) of the lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immunity 2006, 74, 3030–3034. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Q.; Chen, X.Z. Detecting protein-protein interactions by far western blotting. Nat. Protoc. 2007, 2, 3278–3284. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.; Hallström, T.; Skerka, C.; Eberhardt, H.; Uzonyi, B.; Beckhaus, T.; Karas, M.; Wallich, R.; Stevenson, B.; Zipfel, P.F.; et al. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated crasp proteins of Borrelia burgdorferi. PLoS ONE 2010, 5, e13519. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, C.; Hallstrom, T.; Skerka, C.; Wallich, R.; Stevenson, B.; Zipfel, P.F.; Kraiczy, P. Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ERPC) to complement resistance of Borrelia burgdorferi. Clin. Dev. Immunol. 2012, 2012, 349657. [Google Scholar] [CrossRef] [PubMed]
- Herzberger, P.; Siegel, C.; Skerka, C.; Fingerle, V.; Schulte-Spechtel, U.; Wilske, B.; Brade, V.; Zipfel, P.F.; Wallich, R.; Kraiczy, P. Identification and characterization of the factor H and FHL-1 binding complement regulator-acquiring surface protein 1 of the lyme disease spirochete Borrelia spielmanii sp. Nov. Int. J. Med. Microbiol. 2009, 299, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kenedy, M.R.; Akins, D.R. The ospe-related proteins inhibit complement deposition and enhance serum resistance of Borrelia burgdorferi, the lyme disease spirochete. Infect. Immunity 2011, 79, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Kenedy, M.R.; Vuppala, S.R.; Siegel, C.; Kraiczy, P.; Akins, D.R. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect. Immunity 2009, 77, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Hellwage, J.; Skerka, C.; Becker, H.; Kirschfink, M.; Simon, M.M.; Brade, V.; Zipfel, P.F.; Wallich, R. Complement resistance of Borrelia burgdorferi correlates with the expression of BBCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to ERP proteins. J. Biol. Chem. 2004, 279, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Wallich, R. Borrelial Complement-Binding Proteins; Springer: New York, NY, USA, 2012. [Google Scholar]
- Wallich, R.; Pattathu, J.; Kitiratschky, V.; Brenner, C.; Zipfel, P.F.; Brade, V.; Simon, M.M.; Kraiczy, P. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immunity 2005, 73, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Seling, A.; Siegel, C.; Fingerle, V.; Jutras, B.L.; Brissette, C.A.; Skerka, C.; Wallich, R.; Zipfel, P.F.; Stevenson, B.; Kraiczy, P. Functional characterization of Borrelia spielmanii outer surface proteins that interact with distinct members of the human factor H protein family and with plasminogen. Infect. Immunity 2010, 78, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Corvey, C.; Skerka, C.; Kirschfink, M.; Karas, M.; Brade, V.; Miller, J.C.; Stevenson, B.; Wallich, R.; Zipfel, P.F.; et al. Functional characterization of BBCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 2006, 61, 1220–1236. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.S.; Vuppala, S.R.; Jett, A.M.; Alitalo, A.; Meri, S.; Akins, D.R. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 2005, 175, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, C.; Koenigs, A.; Siegel, C.; Hallstrom, T.; Skerka, C.; Wallich, R.; Zipfel, P.F.; Kraiczy, P. Versatile roles of CspA orthologs in complement inactivation of serum-resistant lyme disease spirochetes. Infect. Immunity 2014, 82, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Bhide, M.; Bhide, K.; Pulzova, L.; Madar, M.; Mlynarcik, P.; Bencurova, E.; Hresko, S.; Mucha, R. Variable regions in the sushi domains 6–7 and 19–20 of factor h in animals and human lead to change in the affinity to factor H binding protein of Borrelia. J. Proteom. 2012, 75, 4520–4528. [Google Scholar] [CrossRef] [PubMed]
- Hallström, T.; Siegel, C.; Morgelin, M.; Kraiczy, P.; Skerka, C.; Zipfel, P.F. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Bykowski, T.; Woodman, M.E.; Cooley, A.E.; Brissette, C.A.; Brade, V.; Wallich, R.; Kraiczy, P.; Stevenson, B. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the lyme disease spirochete’s mammal-tick infection cycle. Infect. Immunity 2007, 75, 4227–4236. [Google Scholar] [CrossRef] [PubMed]
- Von Lackum, K.; Miller, J.C.; Bykowski, T.; Riley, S.P.; Woodman, M.E.; Brade, V.; Kraiczy, P.; Stevenson, B.; Wallich, R. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect. Immunity 2005, 73, 7398–7405. [Google Scholar] [CrossRef] [PubMed]
- Kisova-Vargova, L.; Mucha, R.; Cernanka, D.; Bhide, M. Host-dependent differential expression of factor H binding proteins, their affinity to factor H and complement evasion by lyme and relapsing fever borreliae. Vet. Microbiol. 2011, 148, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.; Schreiber, J.; Haupt, K.; Skerka, C.; Brade, V.; Simon, M.M.; Stevenson, B.; Wallich, R.; Zipfel, P.F.; Kraiczy, P. Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J. Biol. Chem. 2008, 283, 34855–34863. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.A.; Marconi, R.T. Delineation of species-specific binding properties of the CspZ protein (BBH06) of lyme disease spirochetes: Evidence for new contributions to the pathogenesis of Borrelia spp. Infect. Immunity 2007, 75, 5272–5281. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, A.; Meri, T.; Lankinen, H.; Seppala, I.; Lahdenne, P.; Hefty, P.S.; Akins, D.; Meri, S. Complement inhibitor factor H binding to lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein e paralogs. J. Immunol. 2002, 169, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Hellwage, J.; Skerka, C.; Kirschfink, M.; Brade, V.; Zipfel, P.F.; Wallich, R. Immune evasion of Borrelia burgdorferi: Mapping of a complement-inhibitor factor H-binding site of BBCRASP-3, a novel member of the ERP protein family. Eur. J. Immunol. 2003, 33, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Oeemig, J.S.; Kolodziejczyk, R.; Meri, T.; Kajander, T.; Lehtinen, M.J.; Iwai, H.; Jokiranta, T.S.; Goldman, A. Structural basis for complement evasion by lyme disease pathogen Borrelia burgdorferi. J. Biol. Chem. 2013, 288, 18685–18695. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, A.; Meri, T.; Comstedt, P.; Jeffery, L.; Tornberg, J.; Strandin, T.; Lankinen, H.; Bergström, S.; Cinco, M.; Vuppala, S.R.; et al. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 2005, 35, 3043–3053. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B. Borrelia burgdorferi erp (ospe-related) gene sequences remain stable during mammalian infection. Infect. Immunity 2002, 70, 5307–5311. [Google Scholar] [CrossRef]
- Garcia, B.L.; Zhi, H.; Wager, B.; Hook, M.; Skare, J.T. Borrelia burgdorferi BBK32 inhibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog. 2016, 12, e1005404. [Google Scholar] [CrossRef] [PubMed]
- Grosskinsky, S.; Schott, M.; Brenner, C.; Cutler, S.J.; Simon, M.M.; Wallich, R. Human complement regulators C4B-binding protein and C1 esterase inhibitor interact with a novel outer surface protein of Borrelia recurrentis. PLoS Negl. Trop. Dis. 2010, 4, e698. [Google Scholar] [CrossRef] [PubMed]
- Madar, M.; Bencurova, E.; Mlynarcik, P.; Almeida, A.M.; Soares, R.; Bhide, K.; Pulzova, L.; Kovac, A.; Coelho, A.V.; Bhide, M. Exploitation of complement regulatory proteins by Borrelia and Francisella. Mol. Biosyst. 2015, 11, 1684–1695. [Google Scholar] [CrossRef] [PubMed]
- Pietikainen, J.; Meri, T.; Blom, A.M.; Meri, S. Binding of the complement inhibitor C4B-binding protein to lyme disease borreliae. Mol. Immunol. 2010, 47, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef] [PubMed]
| Species a | B. burgdorferi | B. afzelii | B. bavariensis | B. garinii | B. valaisiana | B. lusitaniae | B. japonica | B. bissettii | B. andersonii |
|---|---|---|---|---|---|---|---|---|---|
| Human | R | R | R | S | S | S | R | I | S |
| Mouse | R | R | R | S | S | ND | R | R | ND |
| Rat | S | R | R | S | ND | ND | ND | ND | ND |
| Hamster | R | R | R | S | S | S | R | ND | ND |
| Squirrel | R | R | R | S | S | ND | R | ND | ND |
| Cat | I | R | R | I | R | ND | R | ND | ND |
| Dog | I | R | R | S/I | I | S | I | R | R |
| Mouflon | I | R | R | R/I | R | R | R | R | I |
| Lynx | I | I | R | I | R | S | S | R | I |
| Wolf | I | S | R | S/I | S | S | S | R | I |
| Sheep | I | S | S | S | S | R | R | S/R | I |
| Horse | I | S | S | S | S | S | S | ND | ND |
| Pig | I | S | S | S | S | S | S | ND | ND |
| Rabbit | I | S | ND | S | ND | ND | ND | I | ND |
| Pheasant | I | S | S | R | R | S | S | ND | ND |
| Blackbird | I | S | S | R | R | S | S | ND | ND |
| Goat | S | S | ND | S | ND | ND | ND | ND | ND |
| Bovine | S | S | S | S | S | S | S | S | S |
| Deer | S | S | S | S | S | S | S | S | S |
| Eur. Bison | S | S | S | S | S | S | S | S | S |
| Lizard | S | S | S | R | R | R | S | S | ND |
| Quail | R | ND | ND | ND | ND | ND | ND | S | ND |
| Species | B. burgdorferi | B. afzelii | B. bavariensis | B. garinii | B. valaisiana | B. lusitaniae | B. japonica | B. bissettii | B. andersonii |
|---|---|---|---|---|---|---|---|---|---|
| Human | + | + | − | − | − | − | (+) | (+) | + |
| Mouse | (+) | + | (+) | − | − | − | + | + | + |
| Rat | − | − | − | − | − | − | + | − | − |
| Cat | − | + | − | − | − | − | + | − | − |
| Dog | − | − | − | − | + | − | + | − | − |
| Sheep | + | − | − | − | − | − | − | + | − |
| Horse | (+) | − | − | − | − | − | − | − | − |
| Cattle | − | − | − | − | − | − | − | − | − |
| Serum Source a | CspABb (BBA68) | CspABa | CspZ (BBH06) | ErpP (BBN38) | ErpC | ErpA (BBL39) |
|---|---|---|---|---|---|---|
| Human | + | + | + | + | + | + |
| Mouse | (+) | (+) | + | (+) | (+) | (+) |
| Rat | − | − | − | − | + | − |
| Rabbit | − | ND | +/− | − | ND | − |
| Cat | − | − | − | − | ND | − |
| Dog | − | − | − | + | ND | − |
| Sheep | − | ND | ND | − | ND | − |
| Horse | − | − | − | − | ND | − |
| Cow | − | − | + | + | ND | − |
| Pig | − | ND | + | + | ND | − |

© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraiczy, P. Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Vet. Sci. 2016, 3, 12. https://doi.org/10.3390/vetsci3020012
Kraiczy P. Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Veterinary Sciences. 2016; 3(2):12. https://doi.org/10.3390/vetsci3020012
Chicago/Turabian StyleKraiczy, Peter. 2016. "Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes" Veterinary Sciences 3, no. 2: 12. https://doi.org/10.3390/vetsci3020012





