Intranuclear Inclusions in Renal Tubular Epithelium in Immunodeficient Mice Stain with Antibodies for Bovine Papillomavirus Type 1 L1 Protein
Abstract
:1. Introduction
2. Methods
2.1. Animals and Necropsy
| Animal | Breed | Sex | Description | Necropsy |
|---|---|---|---|---|
| A | CBySmn.CB17-Prkdcscid/J | F | Ex-breeder | Cerberus |
| B | CBySmn.CB17-Prkdcscid/J | F | Young | Cerberus |
| C | NOD.CB17-Prkdcscid | F | Ex-breeder | ARC |
| D | NOD.CB17-Prkdcscid | M | Ex-breeder | ARC |
| E | NOD.CB17-Prkdcscid | F | Ex-breeder | ARC |
| F | B6.129S7-Rag1tm1M°m/J | F | Ex-breeder | ARC |
2.2. Serology
2.3. Electron Microscopy
2.4. Polymerase Chain Reaction
2.5. Immunohistochemistry (IHC)
3. Results
3.1. Histopathology
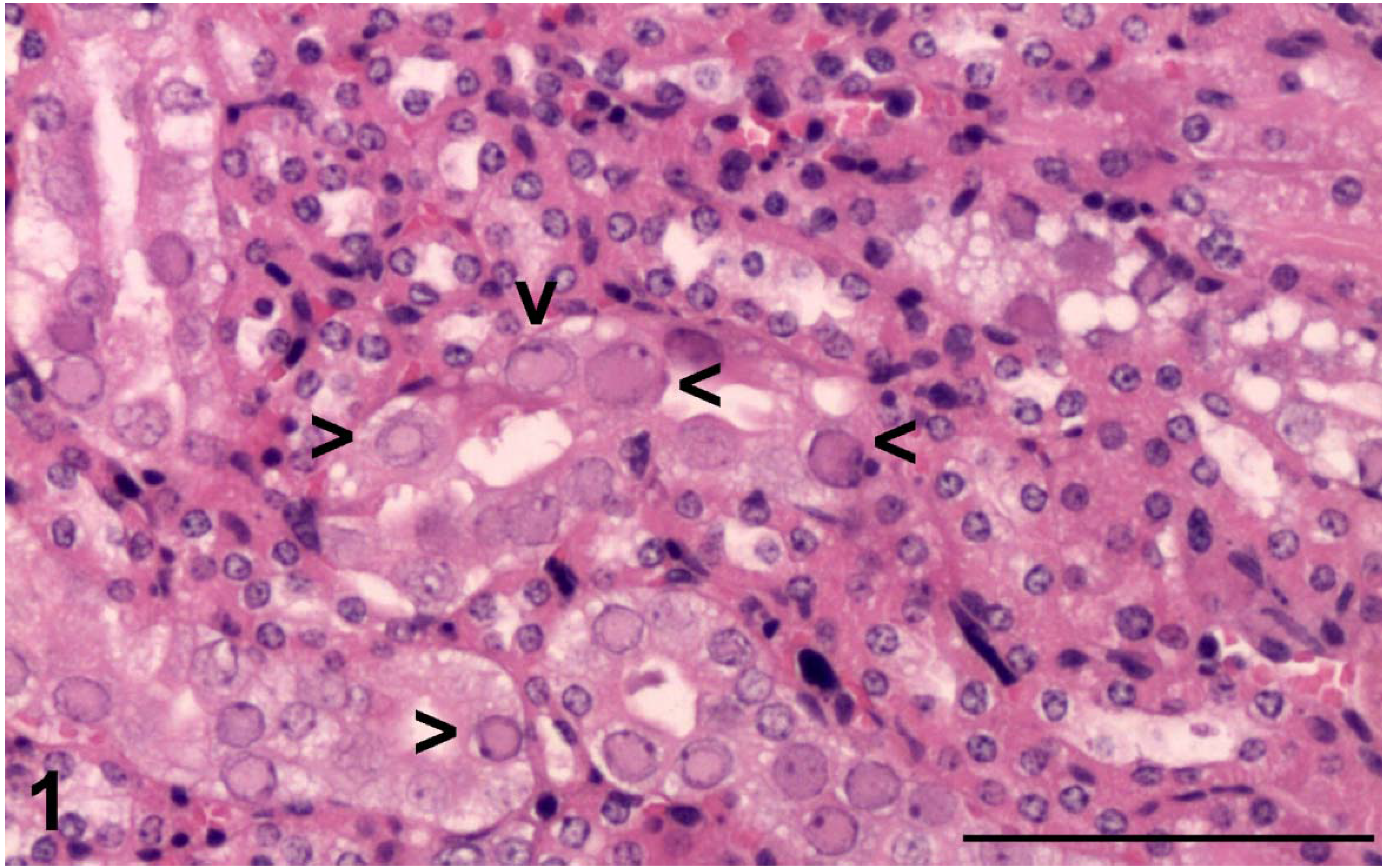
3.2. Electron Microscopy
3.3. Laboratory Tests

3.4. Histochemistry and Immunohistochemistry
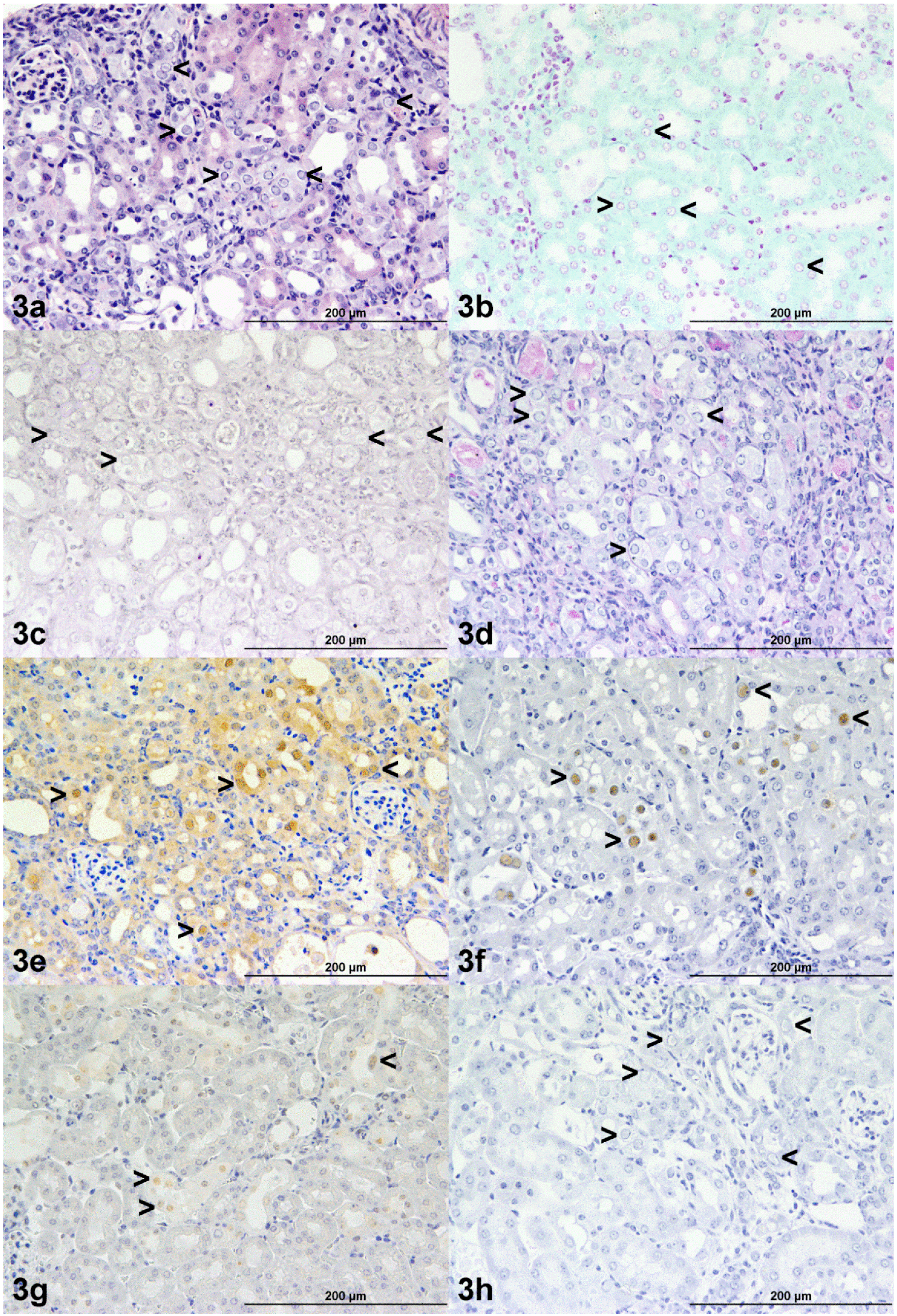
| Animal | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| H&E | + | + | + | + | + | + |
| Mixed monoclonal anti-BPV-1 L1/1H8 + Camvir | + | + | + | + | + | + |
| Rabbit polyclonal anti-bovine papillomavirus L1 antibody | + | + | + | + | + | + |
| Camvir monoclonal anti-papillomavirus antibody | – | – | – | – | – | – |
| Rabbit monoclonal anti-Hsc70 | cyto > nuc | cyto > nuc | weak + | weak + | weak + | weak + |
| PAS | – | – | – | – | – | – |
| Feulgen | – | – | – | – | – | – |
| Methyl Green Pyronin | – | – | – | – | – | – |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Besselsen, D.G.; Franklin, C.L.; Livingston, R.S.; Riley, L.K. Lurking in the shadows: Emerging rodent infectious diseases. ILAR J. 2008, 49, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Baze, W.B.; Steinbach, T.J.; Fleetwood, M.L.; Blanchard, T.W.; Barnhart, K.F.; McArthur, M.J. Karyomegaly and intranuclear inclusions in the renal tubules of sentinel ICR mice (Mus musculus). Comp. Med. 2006, 56, 435–438. [Google Scholar] [PubMed]
- Rector, A.; Tachezy, R.; Van Doorslaer, K.; MacNamara, T.; Burk, R.D.; Sundberg, J.P.; Van Ranst, M. Isolation and cloning of a papillomavirus from a North American porcupine by using multiply primed rolling-circle amplification: The Erethizon dorsatum papillomavirus type 1. Virology 2005, 331, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Fredenburgh, J.L.; Grizzle, W.E.; Myers, R.B. Proteins and nucleic acids. In Theory and Practice of Histological Techniques, 5th ed.; Bancroft, J.D., Stevens, M., Eds.; Churchill Livingstone: Edinburgh, UK, 2002; pp. 217–233. [Google Scholar]
- Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed. 2013. Available online: http://www.webcitation.org/6YwpUvaz3 (accessed on 5 June 2015).
- McInnes, E.F.; Rasmussen, L.; Fung, P.; Auld, A.M.; Alvarez, L.; Lawrence, D.A.; Quinn, M.E.; del Fierro, G.M.; Vassallo, B.A.; Stevenson, R. Prevalence of viral, bacterial and parasitological diseases in rats and mice used in research environments in Australasia over a 5-y period. Lab. Anim. (NY). 2011, 40, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Carty, A.J.; Franklin, C.L.; Riley, L.K.; Besch-Williford, C. Diagnostic polymerase chain reaction assays for identification of murine polyomaviruses in biological samples. Comp. Med. 2001, 51, 145–149. [Google Scholar] [PubMed]
- Forslund, O.; Antonsson, A.; Nordin, P.; Stenquist, B.; Hansson, B.G. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999, 80, 2437–2443. [Google Scholar] [PubMed]
- Rector, A.; Tachezy, R.; Van Ranst, M. A sequence-independent strategy for detection and cloning of circular DNA virus genomes by using multiply primed rolling-circle amplification. J. Virol. 2004, 78, 4993–4998. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.S.; Churcher, M.J.; Meinke, J.; Smith, G.L.; Higgins, G.; Stanley, M.; Minson, A.C. Production and characterisation of a monoclonal antibody to human papillomavirus type 16 using recombinant vaccinia virus. J. Clin. Pathol. 1990, 43, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Whitesell, L.; Katsanis, E. Molecular chaperones: Biology and prospects for pharmacological intervention. Pharmacol. Rev. 1998, 50, 493–514. [Google Scholar] [PubMed]
- Liu, T.; Daniels, C.K.; Cao, S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol. Ther. 2012, 136, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Ghadially, F.N. Intranuclear inclusions. In Ultrastructural Pathology of the Cell and Matrix, 3rd ed.; Ghadially, F.N., Ed.; Butterworths: London, UK, 1988; pp. 1–180. [Google Scholar]
- Lim, P.S.; Jenson, A.B.; Cowsert, L.; Nakai, Y.; Lim, L.Y.; Jin, X.W.; Sundberg, J.P. Distribution and specific identification of papillomavirus major capsid protein epitopes by immunocytochemistry and epitope scanning of synthetic peptides. J. Infect. Dis. 1990, 162, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, S.; Wood, L.V.; Roby, G.; Ryder, C.; Steinberg, S.M.; Zheng, Z.M. Could human papillomaviruses be spread through blood? J. Clin. Microbiol. 2005, 43, 5428–5434. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Keleher, A.; Kedda, M.A.; Spurdle, A.B.; McMillan, N.A.; Antonsson, A. Human pa pillomavirus DNA detected in peripheral blood samples from healthy Australian male blood donors. J. Med. Virol. 2009, 81, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; Borzacchiello, G.; Esposito, I.; Riccardi, M.; Urraro, C.; Lucà, R.; Corteggio, A.; Tatè, R.; Cermola, M.; Paciello, O.; Roperto, F. Productive infection of bovine papillomavirus type 2 in the placenta of pregnant cows affected with urinary bladder tumors. PLoS ONE 2012, 7, e33569. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; Comazzi, S.; Ciusani, E.; Paolini, F.; Borzacchiello, G.; Esposito, I.; Lucà, R.; Russo, V.; Urraro, C.; Venuti, A.; Roperto, F. PBMCs are additional sites of productive infection of bovine papillomavirus type 2. J. Gen. Virol. 2011, 92, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Nafz, J.; Schäfer, K.; Chen, S.F.; Bravo, I.G.; Ibberson, M.; Nindl, I.; Stockfleth, E.; Rösl, F. A novel rodent papillomavirus isolated from anogenital lesions in its natural host. Virology 2008, 374, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Ghim, S.; Joh, J.; Chepkoech, I.; Bennett Jenson, A.; Sundberg, J.P. Novel laboratory mouse papillomavirus (MusPV) infection. Vet. Pathol. 2011, 48, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Maeda, H.; Kameyama, Y.; Moriyama, M.; Kanai, S.; Kurata, T. Presence of a novel hamster oral papillomavirus in dysplastic lesions of hamster lingual mucosa induced by application of dimethylbenzanthracene and excisional wounding: molecular cloning and complete nucleotide sequence. J. Gen. Virol. 1997, 78, 1087–1093. [Google Scholar] [PubMed]
- O’Banion, M.K.; Reichmann, M.E.; Sundberg, J.P. Cloning and characterization of a papillomavirus associated with papillomas and carcinomas in the European harvest mouse (Micromys minutus). J. Virol. 1988, 62, 226–233. [Google Scholar] [PubMed]
- Van Doorslaer, K.; Rector, A.; Jenson, A.B.; Sundberg, J.P.; Van Ranst, M.; Ghim, S.J. Complete genomic characterization of a murine papillomavirus isolated from papillomatous lesions of a European harvest mouse (Micromys minutus). J. Gen. Virol. 2007, 88, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tachezy, R.; Van Ranst, M.; Chan, S.Y.; Bernard, H.U.; Burk, R.D. The Mastomys natalensis papillomavirus: Nucleotide sequence, genome organization, and phylogenetic relationship of a rodent papillomavirus involved in tumorigenesis of cutaneous epithelia. Virology 1994, 198, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.; Jenson, A.B.; Proctor, M.; Ingle, A.; Silva, K.A.; Potter, C.S.; Sundberg, J.P.; Ghim, S.J. Molecular diagnosis of a laboratory mouse papillomavirus (MusPV). Exp. Mol. Pathol. 2012, 93, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P.; Stearns, T.M.; Joh, J.; Proctor, M.; Ingle, A.; Silva, K.A.; Dadras, S.S.; Jenson, A.B.; Ghim, S.J. Immune status, strain background, and anatomic site of inoculation affect mouse papillomavirus (MmuPV1) induction of exophytic papillomas or endophytic trichoblastomas. PLoS ONE 2014, 9, e113582. [Google Scholar] [CrossRef] [PubMed]
- Tilbrook, P.A.; Greenoak, G.E.; Reeve, V.E.; Canfield, P.J.; Gissmann, L.; Gallagher, C.H.; Kulski, J.K. Identification of papillomaviral DNA sequences in hairless mouse tumours induced by ultraviolet irradiation. J. Gen. Virol. 1989, 70, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Percy, D.H.; Barthold, S.W. Mouse. In Pathology of Laboratory Rodents and Rabbits, 3rd ed.; Percy, D.H., Barthold, S.W., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 15–46. [Google Scholar]
- Taylor, I. Mouse. In Background Lesions in Laboratory Animals; McInnes, E.F., Ed.; Saunders: Edinburgh, UK, 2011; pp. 45–72. [Google Scholar]
- Dortant, P.M.; Peters-Volleberg, G.W.; Van Loveren, H.; Marquardt, R.R.; Speijers, G.J. Age-related differences in the toxicity of ochratoxin A in female rats. Food Chem. Toxicol. 2001, 39, 55–65. [Google Scholar] [CrossRef]
- Greaves, P. The urinary tract. In Histopathology of Preclinical Toxicity Studies, 3rd ed.; Greaves, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 570–632. [Google Scholar]
- Vicente-Ortega, V.; Martínez-García, A.F.; Cremades-Campos, A.; Rodríguez-Vicente, J.; Calderón-Rubiales, F.; Martínez-Díaz, F. Ultrastructural investigation of lead-induced intranuclear inclusion bodies in mice. Ultrastruct. Pathol. 1996, 20, 263–273. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McInnes, E.; Bennett, M.; O'Hara, M.; Rasmussen, L.; Fung, P.; Nicholls, P.; Slaven, M.; Stevenson, R. Intranuclear Inclusions in Renal Tubular Epithelium in Immunodeficient Mice Stain with Antibodies for Bovine Papillomavirus Type 1 L1 Protein. Vet. Sci. 2015, 2, 84-96. https://doi.org/10.3390/vetsci2020084
McInnes E, Bennett M, O'Hara M, Rasmussen L, Fung P, Nicholls P, Slaven M, Stevenson R. Intranuclear Inclusions in Renal Tubular Epithelium in Immunodeficient Mice Stain with Antibodies for Bovine Papillomavirus Type 1 L1 Protein. Veterinary Sciences. 2015; 2(2):84-96. https://doi.org/10.3390/vetsci2020084
Chicago/Turabian StyleMcInnes, Elizabeth, Mark Bennett, Mandy O'Hara, Lorna Rasmussen, Peony Fung, Philip Nicholls, Michael Slaven, and Robert Stevenson. 2015. "Intranuclear Inclusions in Renal Tubular Epithelium in Immunodeficient Mice Stain with Antibodies for Bovine Papillomavirus Type 1 L1 Protein" Veterinary Sciences 2, no. 2: 84-96. https://doi.org/10.3390/vetsci2020084
APA StyleMcInnes, E., Bennett, M., O'Hara, M., Rasmussen, L., Fung, P., Nicholls, P., Slaven, M., & Stevenson, R. (2015). Intranuclear Inclusions in Renal Tubular Epithelium in Immunodeficient Mice Stain with Antibodies for Bovine Papillomavirus Type 1 L1 Protein. Veterinary Sciences, 2(2), 84-96. https://doi.org/10.3390/vetsci2020084





