The Canine Vaginal Flora: A Large-Cohort Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Qualitative Analysis
3.2. Number of Species Per Sample
3.3. Qualitative Analysis of Monocultures
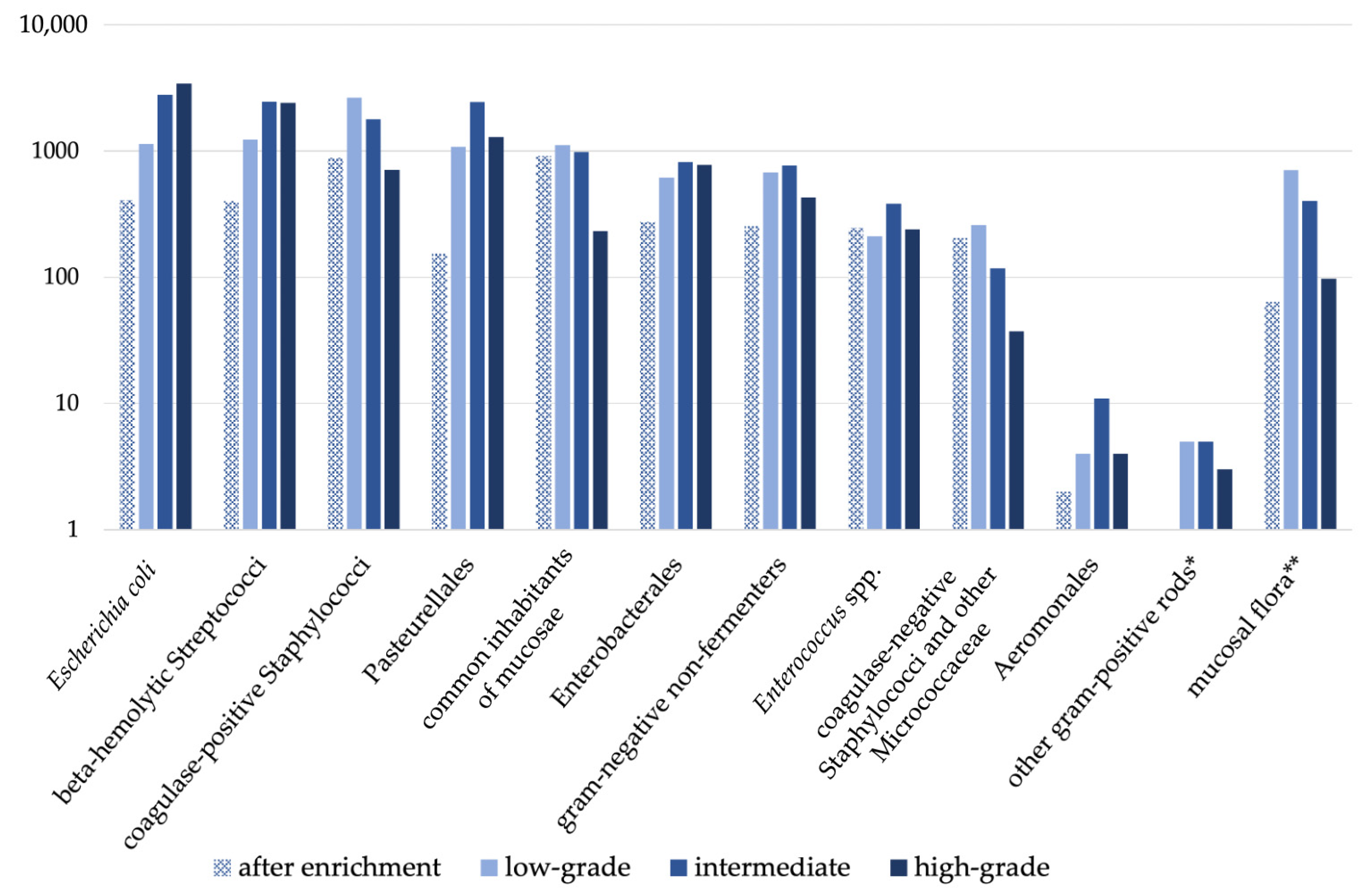
3.4. Semiquantitative Analysis
3.5. Comparative Annual Qualitative and Semiquantitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milani, C.; Corro, M.; Drigo, M.; Rota, A. Antimicrobial resistance in bacteria from breeding dogs housed in kennels with differing neonatal mortality and use of antibiotics. Theriogenology 2012, 78, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Milani, C.; Corro, M.; Drigo, I.; Borjesson, S. Misuse of antimicrobials and selection of methicillin-resistant Staphylococcus pseudintermedius strains in breeding kennels: Genetic characterization of bacteria after a two-year interval. Reprod. Domest. Anim. 2013, 48, 1–6. [Google Scholar] [CrossRef]
- Groppetti, D.; Pecile, A.; Barbero, C.; Martino, P.A. Vaginal bacterial flora and cytology in proestrous bitches: Role on fertility. Theriogenology 2012, 77, 1549–1556. [Google Scholar] [CrossRef]
- Bjurström, L.; Linde-Forsberg, C. Long-term study of aerobic bacteria of the genital tract in breeding bitches. Am. J. Vet. Res. 1992, 53, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Osbaldiston, G.W.; Nuru, S.; Mosier, J.E. Vaginal Cytology and Microflora of Infertile Bitches. J. Am. Anim. Hosp. Assoc. 1972, 8, 93–101. [Google Scholar]
- Hirsh, D.C.; Wiger, N. The bacterial flora of the normal canine vagina compared with that of vaginal exudates. J. Small Anim. Pract. 1977, 18, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Baba, E.; Hata, H.; Fukata, T.; Arakawa, A. Vaginal and uterine microflora of adult dogs. Am. J. Vet. Res. 1983, 44, 606–609. [Google Scholar]
- Olson, P.N.; Mather, E.C. Canine vaginal and uterine bacterial flora. J. Am. Vet. Med. Assoc. 1978, 172, 708–711. [Google Scholar]
- Allen, W.E.; Dagnall, G.J.R. Some observations on the aerobis bacterial flora of the genital tract of the dog and bitch. J. Small Anim. Pract. 1982, 23, 325–335. [Google Scholar] [CrossRef]
- Watts, J.R.; Wright, P.J.; Whithear, K.C. Uterine, cervical and vaginal microflora of the normal bitch throughout the reproductive cycle. J. Small Anim. Pract. 1996, 37, 54–60. [Google Scholar] [CrossRef]
- Noguchi, K.; Tsukumi, K.; Urano, T. Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp. Med. 2003, 53, 404–412. [Google Scholar] [PubMed]
- Maksimović, A.; Maksimović, Z.; Filipović, S.; Beširović, H.; Rifatbegović, M. Vaginal and uterine bacteria of healthy bitches during different stages of their reproductive cycle. Vet. Rec. 2012, 171, 375. [Google Scholar] [CrossRef]
- Golińska, E.; Sowińska, N.; Tomusiak-Plebanek, A.; Szydło, M.; Witka, N.; Lenarczyk, J.; Strus, M. The vaginal microflora changes in various stages of the estrous cycle of healthy female dogs and the ones with genital tract infections. BMC Vet. Res. 2021, 17, 8. [Google Scholar] [CrossRef]
- Pretzer, S.D. Bacterial and protozoal causes of pregnancy loss in the bitch and queen. Theriogenology 2008, 70, 320–326. [Google Scholar] [CrossRef]
- Guardabassi, L.; Apley, M.; Olsen, J.E.; Toutain, P.L.; Weese, S. Optimization of Antimicrobial Treatment to Minimize Resistance Selection. Microbiol. Spectr. 2018, 6, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Bjurstrom, L. Aerobic bacteria occurring in the vagina of bitches with reproductive disorders. Acta Vet. Scand. 1993, 34, 29–34. [Google Scholar] [CrossRef]
- Grundy, S.A.; Feldman, E.; Davidson, A. Evaluation of infertility in the bitch. Clin. Tech. Small Anim. Pract. 2002, 17, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.M.; Taylor, D.J. Bacterial reproductive pathogens of cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 561–582. [Google Scholar] [CrossRef]
- Tavares Pereira, M.; Nowaczyk, R.; Aslan, S.; Ay, S.S.; Kowalewski, M.P. Utero-Placental Immune Milieu during Normal and Aglepristone-Induced Parturition in the Dog. Animals 2021, 11, 3598. [Google Scholar] [CrossRef]
- Tavares Pereira, M.; Nowaczyk, R.; Payan-Carreira, R.; Miranda, S.; Aslan, S.; Kaya, D.; Kowalewski, M.P. Selected Uterine Immune Events Associated With the Establishment of Pregnancy in the Dog. Front. Vet. Sci. 2020, 7, 625921. [Google Scholar] [CrossRef]
- Robertson, S. Control of the immunological environment of the uterus. Rev. Reprod. 2000, 5, 164–174. [Google Scholar] [CrossRef]
- Ström, B.; Linde-Forsberg, C. Effects of ampicillin and trimethoprim-sulfamethoxazole on the vaginal bacterial flora of bitches. Am. J. Vet. Res. 1993, 54, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2021; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2022.
- Guardabassi, L.; Butaye, P.; Dockrell, D.H.; Fitzgerald, J.R.; Kuijper, E.J.; On Behalf of the ESCMID Study Group for Veterinary. One Health: A multifaceted concept combining diverse approaches to prevent and control antimicrobial resistance. Clin. Microbiol. Infect. 2020, 26, 1604–1605. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Comission Notice. Guidelines for the Prudent Use of Antimicrobials in Veterinary Medicine; Comission notice (2015/C 299/04) 2015; European Comission: Brussels, Belgium, 2015.
- Weese, J.S.; Giguere, S.; Guardabassi, L.; Morley, P.S.; Papich, M.; Ricciuto, D.R.; Sykes, J.E. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J. Vet. Intern. Med. 2015, 29, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Jessen, L.; Damborg, P.; Spohr, A.; Goericke-Pesch, S.; Langhorn, R.; Houser, G.; Willesen, J.; Schjaerff, M.; Søerensen, T.; Eriksen, T.; et al. Antibiotic Use Guidelines for Companion Animal Practice, 2nd ed.; Companion Animal Group, Danish Veterinary Association: Frederiksberg, Denmark, 2018. [Google Scholar]
- Wilborn, R.R.; Maxwell, H.S. Clinical approaches to infertility in the bitch. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Wieser, A.; Schneider, L.; Jung, J.; Schubert, S. MALDI-TOF MS in microbiological diagnostics-identification of microorganisms and beyond (mini review). Appl. Microbiol. Biotechnol. 2012, 93, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Lyman, C.C.; Holyoak, G.R.; Meinkoth, K.; Wieneke, X.; Chillemi, K.A.; DeSilva, U. Canine endometrial and vaginal microbiomes reveal distinct and complex ecosystems. PLoS ONE 2019, 14, e0210157. [Google Scholar] [CrossRef] [PubMed]
- Theron, J.; Cloete, T.E. Molecular techniques for determining microbial diversity and community structure in natural environments. Crit. Rev. Microbiol. 2000, 26, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Greninger, A.L. The challenge of diagnostic metagenomics. Expert Rev. Mol. Diagn. 2018, 18, 605–615. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Simner, P.J. Next-Generation Sequencing in Clinical Microbiology: Are We There Yet? Clin. Lab. Med. 2019, 39, 405–418. [Google Scholar] [CrossRef]
- Hilt, E.E.; Ferrieri, P. Next Generation and Other Sequencing Technologies in Diagnostic Microbiology and Infectious Diseases. Genes 2022, 13, 1566. [Google Scholar] [CrossRef]
- Bizzini, A.; Durussel, C.; Bille, J.; Greub, G.; Prod’hom, G. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 2010, 48, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, B.; Marin, M.; Sanchez-Carrillo, C.; Cercenado, E.; Ruiz, A.; Rodriguez-Creixems, M.; Bouza, E. Improvement of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of difficult-to-identify bacteria and its impact in the workflow of a clinical microbiology laboratory. Diagn. Microbiol. Infect. Dis. 2014, 79, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chalker, V.J. Canine mycoplasmas. Res. Vet. Sci. 2005, 79, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Doig, P.A.; Ruhnke, H.L.; Bosu, W.T. The genital Mycoplasma and Ureaplasma flora of healthy and diseased dogs. Can. J. Comp. Med. 1981, 45, 233–238. [Google Scholar]
- Maksimović, Z.; Maksimović, A.; Halilbašić, A.; Rifatbegović, M. Genital mycoplasmas of healthy bitches. J. Vet. Diagn. Investig. 2018, 30, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Pesch, S.; Weiss, R.; Hoffmann, B. Zur bakteriellen Flora im Rüdenejakulat. Tierärztliche Prax. Ausg. K Kleintiere/Heimtiere 2006, 34, 17–21. [Google Scholar]
- Hagman, R. Pyometra in Small Animals 2.0. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 631–657. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Weiss, R.; Wehrend, A. Bacteriological findings in different fractions of canine ejaculates showing normospermia, teratozoospermia or azoospermia. Aust. Vet. J. 2011, 89, 318–322. [Google Scholar] [CrossRef]
- Weese, J.S.; van Duijkeren, E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 2010, 140, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Root Kustritz, M.V. Collection of tissue and culture samples from the canine reproductive tract. Theriogenology 2006, 66, 567–574. [Google Scholar] [CrossRef]
- Schäfer-Somi, S. Analysis of factors contributing to the individual change in the vaginal flora of bitches in estrus. In Proceedings of the 21st EVSSAR Congress, Venice, Italy, 22–23 June 2018; p. 227. [Google Scholar]
- Poulsen, C.S.; Kaas, R.S.; Aarestrup, F.M.; Pamp, S.J. Standard Sample Storage Conditions Have an Impact on Inferred Microbiome Composition and Antimicrobial Resistance Patterns. Microbiol. Spectr. 2021, 9, e0138721. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, T. Erhebungen zur Situation der Caninen Reproduktionsmedizin bei Tierärzten und Züchtern: Ein Beitrag zur Erhebung des Status quo und zur Verbesserung der Lehre Auf Diesem Gebiet; Universitätsbibliothek: Gießen, Germany, 2008. [Google Scholar]
- Sant’Anna, M.C.; Kelliton Fabretti, A.; Mello Martins, M.I. Clinical approach to canine vaginitis. Semin. Ciências Agrárias 2012, 33, 1543–1553. [Google Scholar] [CrossRef]

| Group Name | Species |
|---|---|
| Escherichia coli | Escherichia coli |
| Beta-hemolytic Streptococci | e.g., Strep. canis, Strep. equi ssp. zooepidemicus, Strep. equisimilis, Strep. dysgalactiae |
| Coagulase-positive Staphylococci | Staph. aureus, Staph. intermedius, Staph. pseudintermedius, Staph. delphini |
| Pasteurellales | Pasteurella spp., Neisseria spp., Haemophilus spp., Mannheimia spp. |
| Common inhabitants of mucosae | Bacillus spp., Streptococcus spp. (except beta-hemolytic), Aerococcus spp., Weissella spp., Lactobacillus spp., Lactococcus spp., Carnobacterium spp. |
| Enterobacterales | Citrobacter spp., Cronobacter spp., Enterobacter spp., Erwinia spp., Klebsiella spp., Kosakonia spp., Leclercia spp., Lelliottia spp., Pantoea spp., Pluralibacter spp., Providencia spp., Proteus spp., Rahnella spp., Raoultella spp., Serratia spp., Morganella spp., Hafnia spp., Escherichia spp. (except Escherichia coli), Kluyvera spp., Salmonella spp., Yersinia spp., Pseudescherichia spp., Buttiauxiella spp., Mixta spp. |
| Gram-negative non-fermenters | Pseudomonas spp., Burkholderia spp., Acinetobacter spp., Achromobacter spp., Bergeyella spp., Psychrobacter spp., Stenotrophomonas spp., Empedobacter spp., Sphingobacterium spp., Sphingomonas spp., Pseudoacidovorax spp., Ralstonia spp. Ochrobacterium spp., Moraxella spp., Flavimonas spp., Chryseobacterium spp., Elizabethkingae spp., Advenella spp., Comamonas spp., Alcaligenes spp. |
| Enterococcus spp. | Enterococcus spp. |
| Coagulase-negative Staphylococci and other Micrococcaceae | Staph. spp. (except for above listed), Rothia spp., Micrococcus spp., Macrococcus spp., Arthrobacter spp., Glutamibacter spp. |
| Aeromonales | Aeromonas spp. |
| Gram-positive rods * | Actinomyces spp., Streptomyces spp., Paenarthrobacter spp., Rhodococcus spp., Trueperella spp., Pseudarthrobacter spp., Corynebacterium spp. |
| Number of Bacterial Species Per Sample | n | Percentage |
|---|---|---|
| 1 | 10,158 | 43.7% |
| 2 | 8302 | 35.7% |
| 3 | 2326 | 10.0% |
| 4 | 323 | 1.4% |
| 5 | 13 | 0.1% |
| 6 | 3 | 0.01% |
| not specified * | 1263 | 5.4% |
| No growth | 866 | 3.7% |
| Group of Bacterial Species | n | Percentage of Overall Monocultures | Percentage of Monocultures within a Group |
|---|---|---|---|
| Escherichia coli | 2976 | 29.3% | 38.3% |
| Pasteurellales | 1779 | 17.5% | 35.8% |
| Coagulase-positive Staphylococci | 1695 | 16.7% | 28.4% |
| Beta-hemolytic Streptococci | 1583 | 15.6% | 24.2% |
| Enterobacterales | 674 | 6.6% | 27.2% |
| Gram-negative non-fermenters | 552 | 5.4% | 25. 9% |
| Common inhabitants of mucosae | 519 | 5.1% | 16.0% |
| Enterococcus spp. | 191 | 1.9% | 17.7% |
| Coagulase-negative Staphylococci and other Micrococcaceae | 186 | 1.8% | 26.7% |
| Aeromonales | 2 | 0.02% | 9.5% |
| Other gram-positive rods* | 1 | 0.01% | 7.7% |
| Group of Bacteria | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|
| Escherichia coli | 21.70 | 21.36 | 18.72 | 22.85 | 23.40 | 23.34 | 23.35 |
| Beta-hemolytic Streptococci | 19.07 | 18.43 | 18.23 | 18.87 | 19.14 | 19.48 | 17.78 |
| Coagulase-positive Staphylococci | 14.95 | 16.32 | 17.03 | 17.17 | 17.12 | 19.22 | 16.90 |
| Pasteurellales | 15.91 | 14.19 | 14.93 | 13.89 | 13.64 | 12.82 | 15.02 |
| Common inhabitants of mucosae | 9.90 | 10.48 | 13.19 | 7.65 | 8.25 | 7.27 | 9.57 |
| Enterobacterales | 6.86 | 7.40 | 6.51 | 7.91 | 6.87 | 6.99 | 7.07 |
| Gram-negative non-fermenters | 6.09 | 5.64 | 5.47 | 6.10 | 6.51 | 6.04 | 6.55 |
| Enterococcus spp. | 2.71 | 3.01 | 3.10 | 3.47 | 3.50 | 3.36 | 2.33 |
| Coagulase-negative Staphylococci and other Micrococcaceae | 2.60 | 3.13 | 2.73 | 2.02 | 1.44 | 1.40 | 1.33 |
| Aeromonales | 0.17 | 0.03 | 0.09 | 0.03 | 0.09 | 0.06 | 0.00 |
| Gram-positive rods * | 0.03 | 0.00 | 0.00 | 0.02 | 0.05 | 0.04 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leps, A.S.; Klein, B.; Schneider, M.; Meyer, C.; Šoba, A.; Simon, C.; Dyachenko, V.; Siesenop, U.; Verspohl, J.; Goericke-Pesch, S. The Canine Vaginal Flora: A Large-Cohort Retrospective Study. Vet. Sci. 2024, 11, 55. https://doi.org/10.3390/vetsci11020055
Leps AS, Klein B, Schneider M, Meyer C, Šoba A, Simon C, Dyachenko V, Siesenop U, Verspohl J, Goericke-Pesch S. The Canine Vaginal Flora: A Large-Cohort Retrospective Study. Veterinary Sciences. 2024; 11(2):55. https://doi.org/10.3390/vetsci11020055
Chicago/Turabian StyleLeps, Anna Sophia, Babette Klein, Marianne Schneider, Cornelia Meyer, Alexandra Šoba, Christine Simon, Viktor Dyachenko, Ute Siesenop, Jutta Verspohl, and Sandra Goericke-Pesch. 2024. "The Canine Vaginal Flora: A Large-Cohort Retrospective Study" Veterinary Sciences 11, no. 2: 55. https://doi.org/10.3390/vetsci11020055






