Influence of Different Alcohol Reduction Technologies on the Volatile Composition of La Mancha Tempranillo Rosé Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Wines
2.2.1. Control Wine
2.2.2. Partially Fermented Wines
2.2.3. Total Dealcoholized Wines by SCC
2.3. Conventional Analysis of Wines
2.4. Analysis of Major Volatile Compounds
2.5. Analysis of Minor Volatile Compounds
2.6. Odor Activity Values (OAV)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Conventional Analysis
3.2. Volatile Compounds
3.3. Varietal Compounds
3.3.1. C6 Compounds
3.3.2. Terpene and C13-Noirsoprenoids Compounds
3.3.3. Benzene Compounds
3.4. Volatile Compounds Formed Principally during Alcoholic Fermentation
3.4.1. Aldehydes
3.4.2. Alcohols
3.4.3. Esters
3.4.4. Acids
3.4.5. Lactones
3.5. Odor Activity Values (OAV)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deroover, K.; Siegrist, M.; Brain, K.; McIntyre, J.; Bucher, T. A scoping review on consumer behaviour related to wine and health. Trends Food Sci. Technol. 2021, 112, 559–580. [Google Scholar] [CrossRef]
- Ubeda, C.; Hornedo-Ortega, R.; Cerezo, A.B.; Garcia-Parrilla, M.C.; Troncoso, A.M. Chemical hazards in grapes and wine, climate change and challenges to face. Food Chem. 2020, 314, 126222. [Google Scholar] [CrossRef] [PubMed]
- Raineau, Y.; Giraud-Héraud, É.; Lecocq, S.; Pérès, S.; Pons, A.; Tempère, S. When health-related claims impact environmental demand: Results of experimental auctions with Bordeaux wine consumers. Ecol. Econ. 2023, 204 Pt A, 107663. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.M.; Fernández-Fernández, E.; Palacio, L.; Carmona, F.J.; Hernández, A.; Prádanos, P. Application of pervaporation and nanofiltration membrane processes for the elaboration of full-flavored low alcohol white wines. Food Bioprod. Process. 2017, 101, 11–21. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; Martínez de Toda, F. Current viticultural techniques to mitigate the effects of global warming on grape and wine quality: A comprehensive review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef]
- Lu, H.C.; Hu, L.; Liu, Y.; Cheng, C.F.; Chen, W.; Li, S.; He, F.; Duan, C.Q.; Wang, J. Reducing the source/sink ratio of grapevine to face global warming in a semi-arid climate: Effects on volatile composition of Cabernet Sauvignon grapes and wines. Food Chem. 2022, 15, 100449. [Google Scholar] [CrossRef]
- Labanda, J.; Vichi, S.; Llorens, J.; López, E. Membrane separation technology for the reduction of alcoholic degree of a white model wine. J. Food Sci. Technol. 2009, 42, 1390–1395. [Google Scholar] [CrossRef]
- Catarino, M.; Mendes, A. Dealcoholizing wine by membrane separation processes. Innov. Food Sci. Emerg. Technol. 2011, 12, 330–337. [Google Scholar] [CrossRef]
- Bui, K.; Dick, R.; Moulin, G.; Galzy, P. A reverse osmosis for the production of low ethanol content wine. Am. J. 1986, 37, 4–7. Available online: http://ajevonline.org/content/37/4/297.short (accessed on 11 April 2023). [CrossRef]
- Meillon, S.; Urbano, C.; Schlich, P. Contribution of the Temporal Dominance of Sensations (TDS) Method to the Sensory Description of Subtle Differences in Partially Dealcoholized RedWines. Food Qual. Prefer. 2009, 20, 490–499. [Google Scholar] [CrossRef]
- Hogan, P.A.; Canning, R.P.; Peterson, P.A.; Johnson, R.A.; Michaels, A.S. A New Option: Osmotic Distillation. Chem. Eng. Prog. 1998, 94, 49–61. [Google Scholar]
- Diban, N.; Arruti, A.; Barceló, A.; Puxeu, M.; Urtiaga, A.; Ortiz, I. Membrane dealcoholization of different wine varieties reducing aroma losses modeling and experimental validation. Innov. Food Sci. Emerg. 2013, 20, 259–268. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Zhao, L.; Zheng, C.; Ma, F. Changes in volatile organic compounds and differential expression of aroma-related genes during flowering of Rosa rugosa Shanxian. Hort. Environ. Biotechnol. 2019, 60, 741–751. [Google Scholar] [CrossRef]
- Taran, N.; Stoleicova, S.; Soldatenco, O.; Morari, B. The Influence of Pressure on Chemical and Physical Parametres of White and Red Wines Obtained by Dealcoholization Method. J. Agroaliment. Proc. Technol. 2014, 20, 215–219. [Google Scholar]
- Belisario-Sánchez, Y.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; López-Gómez, A. Dealcoholized wines by spinning cone column distillation: Phenolic compounds and antioxidant activity measured by the 1,1-Diphenyl-2-pic-rylhydrazyl method. J. Agric. Food Chem. 2009, 57, 6770–6778. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2014; pp. 154–196. [Google Scholar]
- Sánchez-Palomo, E.; Pérez-Coello, M.S.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Cabezudo, M.D. Contribution of free and glicosidically bound volatile compounds to the aroma of Muscat “a petit grains” wines and effect of skin contact. Food Chem. 2006, 95, 279–289. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Etiévant, P.X. Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different WhiteWine Varieties. J. Agric. Food Chem. 1997, 8, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Ma, T.; Sam, F.E.; Didi, D.A.; Atuna, R.A.; Amagloh, F.K.; Zhang, B. Contribution of edible flowers on the aroma profile of dealcoholized pinot noir rose wine. LWT 2022, 170, 114034. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Petrozziello, M.; Ciambotti, A.; Panero, L.; Solomita, M.; Bosso, A. Comparison of the physicochemical and volatile composition of wine fractions obtained by two different dealcoholization techniques. Food Chem. 2017, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2021/2117 of the European Parliament and of the Council of 2 December 2021 amending Regulations (EU) No 1308/2013 Establishing a Common Organisation of the Markets in Agricultural Products, (EU) No 1151/2012 on Quality Schemes for Agricultural Products and Foodstuffs, (EU) No 251/2014 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Aromatised Wine Products and (EU) No 228/2013 Laying Down Specific Measures for Agriculture in the Outermost Regions of the Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32021R2117 (accessed on 15 April 2023).
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine—Stabilization and Treatments; Wiley: London, UK, 2000. [Google Scholar]
- Aznar, M.; Lopez, R.; Cacho, J.F.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition. Partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Sam, F.E.; Ma, T.-Z.; Wang, J.; Liang, Y.; Sheng, W.; Li, J.; Jiang, Y.; Zhang, B. Aroma improvement of dealcoholized Merlot red wine using edible flowers. Food Chem. 2023, 404 Pt B, 134711. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Lisanti, M.T.; Gambuti, A.; Piombino, P.; Moio, L. Relationship between sensory perception and aroma compounds of monovarietal red wines. Acta Hortic. 2007, 754, 549–556. [Google Scholar] [CrossRef]
- Flanzy, C. Enología: Fundamentos Científicos y Tecnológicos; AMV-Mundi Prensa: Madrid, Spain, 2003; pp. 137–168. [Google Scholar]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar]
- Antalick, G.; Perello, M.C.; Revel, G. Characterization of Fruity Aroma Modifications in Red Wines during Malolactic Fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. A comparative study of partial dealcoholisation versus early harvest: Effects on wine volatile and sensory profiles. J. Food Chem. 2018, 26, 21–29. [Google Scholar] [CrossRef]
- Diban, N.; Athes, V.; Bes, M.; Souchon, I. Ethanol and aroma compounds transfer study for partial dealcoholization of wine using membrane contactor. J. Membr. Sci. 2008, 311, 136–146. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef]
- Rocha, M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Saha, B.; Torley, P.; Blackmann, J.; Schmidtke, L.M. Review of processing technology to reduce alcohol levels in wines. In Proceedings of the 1st International Symposium Alcohol Level Reduction in Wine-Oenoviti International Network, Bordeauxq, France, 6 September 2013. [Google Scholar]
- Gómez-Míguez, M.J.; Gómez-Míguez, M.; Vicario, I.M.; Heredía, F.J. Assessment of colour and aroma in white wines vi-nifications: Effects of grape maturity and soil type. J. Food Eng. 2007, 79, 758–764. [Google Scholar] [CrossRef]
- Lorenzo, C.; Pardo, F.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Differentiation of co-winemaking wines by their aroma composition. Eur. Food Res. Technol. 2008, 227, 777–787. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Gómez García-Carpintero, E.; Alonso-Villegas, R.; González-Viñas, M.A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Fragr. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Charles, M.; Martin, B.; Ginies, C.; Etievant, P.; Coste, G.; Guichard, E. Potent aroma compounds of two red wine vinegars. J. Agric. Food Chem. 2000, 48, 70–77. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.; Medina, M. Aroma series as fingerprints for biological ageing in fino sherry-type wines. J. Sci. Food Agric. 2007, 87, 2319–2326. [Google Scholar] [CrossRef]
- Gürbüz, O.; Rouseff, J.M.; Rouseff, R.L. Comparison of aroma volatiles in commercial Merlot and Cabernet Sauvignon wines using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J. Agric. Food Chem. 2006, 54, 3990–3996. [Google Scholar] [CrossRef]
| % Alcohol (v/v) | Volatile Acidity (g/L Acetic Acid) | Total Acidity (g/L Tartaric Acid) | pH | Residual Sugars (g/L) | Free SO2 (mg/L) | Total SO2 (mg/L) | |
|---|---|---|---|---|---|---|---|
| Control wine | 11.44 a | 0.20 a | 4.99 a | 3.30 a | 3.95 a | 23.33 a | 42.67 a |
| (0.04) | (0.01) | (0.02) | (0.01) | (0.21) | (1.53) | (1.15) | |
| Partially fermented | 7.90 b | 0.20 a | 4.93 a | 3.35 b | 56.63 b | 34.33 b | 63.33 b |
| wine | (0.17) | (0.00) | (0.16) | (0.01) | (1.77) | (1.15) | (1.53) |
| Total dealcoholized | 0.52 c | 0.23 a | 4.91 a | 3.34 a | 66.73 c | 28.67 c | 55.33 c |
| wine | (0.03) | (0.03) | (0.04) | (0.01) | (0.40) | (1.15) | (0.58) |
| Source * | RI A | Compound | Control Wine | Partially Fermented Wine | Total Dealcoholized Wine | |||
|---|---|---|---|---|---|---|---|---|
| Fluka | 1282 | 1-hexanol | 1035.49 b | (4.37) | 1458.66 c | (3.93) | 106.46 a | (0.96) |
| Sigma-Aldrich | 1286 | (E)-3-hexen-1-ol | 13.70 a | (8.52) | 25.03 b | (3.98) | 24.47 b | (1.73) |
| Sigma-Aldrich | 1296 | (Z)-3-hexen-1-ol | 327.37 c | (7.11) | 184.16 b | (5.69) | 5.72 a | (2.85) |
| Sigma-Aldrich | 1300 | (E)-2-hexen-1-ol | n.d. | n.d. | 0.40 a | (0.03) | ||
| Sigma-Aldrich | 1394 | 2-ethyl-1-hexanol | 6.79 a | (4.17) | n.d. | n.d. | ||
| Sigma-Aldrich | 1197 | 2-hexanol | 37.22 b | (5.02) | n.d.a | 44.27 c | (2.96) | |
| C6 alcohols | 1420.56 | 1667.84 | 181.31 | |||||
| Tentatively identified | 1455 | cis-linalool oxyde furanic | 5.50 b | (3.99) | 0.32 a | (0.01) | 0.82 a | (0.01) |
| Tentatively identified | 1483 | trans-linaool oxyde furanic | 3.67 c | (3.28) | Tr | 0.30 b | (0.24) | |
| Fluka | 1529 | Linalool | 0.21 a | (0.01) | 8.65 c | (0.57) | 3.71 b | (3.43) |
| Fluka | 1607 | α-terpineol | 12.42 c | (6.83) | 8.97 b | (4.97) | 4.82 a | (4.40) |
| Fluka | 1755 | β-citronelol | 5.54 c | (2.30) | 3.15 b | (2.47) | Tr | |
| Fluka | 1831 | Geraniol | Tr | Tr | Tr | |||
| Tentatively identified | 1902 | 3,7-dimethyl-1-octen-3,7-diol | 19.34 b | (9.21) | 5.92 a | (3.58) | 21.27 b | (4.02) |
| Terpenic compounds | 46.67 | 26.99 | 30.91 | |||||
| Tentatively identified | 1685 | Trimethyl dihydronaphtalene | 13.22 b | (5.03) | 0.49 a | (0.04) | 1.62 a | (1.31) |
| Sigma-Aldrich | 1703 | 4-oxo-isophorone | 1.69 b | (0.84) | Tr | 8.78 c | (4.27) | |
| Sigma-Aldrich | 1801 | β-Damascenone | 1.36 a | (1.04) | 5.03 b | (2.81) | 6.28 c | (5.30) |
| Tentatively identified | 1907 | 2,3-Dehydro-4-oxo-β-ionol | 18.59 b | (8.75) | Tr | 24.15 c | (2.37) | |
| Tentatively identified | 2875 | 7,8-dihydro-3-oxo-α-ionol | 15.40 a | (3.31) | 42.52 b | (1.76) | n.d. | |
| C13 norisoprenoids | 50.26 | 48.04 | 40.81 | |||||
| Sigma-Aldrich | 1503 | Benzaldehyde | 8.85 c | (2.10) | 1.83 c | (1.55) | 7.72 b | (2.75) |
| Sigma-Aldrich | 1882 | Guaicol | 0.26 a | (0.01) | 6.89 c | (2.77) | 1.25 b | (1.13) |
| Sigma-Aldrich | 1895 | Benzylic alcohol | 85.42 c | (1.91) | 62.55 b | (2.55) | 29.90 a | (8.07) |
| Sigma-Aldrich | 1971 | Phenol | 20.42 c | (9.18) | 9.79 b | (6.72) | 0.98 a | (0.08) |
| Sigma-Aldrich | 2193 | Eugenol | Tr | Tr | Tr | |||
| Sigma-Aldrich | 2219 | 4-vinyl-guaiacol | 5.06 a | (2.10) | 33.29 b | (3.68) | 61.91 c | (7.22) |
| Sigma-Aldrich | 2225 | 2,6-dimetoxy phenol (syringol) | 14.59 b | (4.41) | 31.43 c | (3.80) | 4.52 a | (3.29) |
| Sigma-Aldrich | 2302 | Isoeugenol | Tr | Tr | Tr | |||
| Panreac | 2511 | Vanillin | 1.69 a | (1.67) | 3.61 b | (2.94) | 4.06 b | (1.04) |
| Sigma-Aldrich | 2543 | Methyl vanillate | 0.88 a | (0.06) | 1.59 c | (1.34) | 1.27 b | (1.11) |
| Sigma-Aldrich | 2676 | Ethyl vanillate | 2.02 a | (1.75) | 4.80 b | (0.74) | 2.08 a | (1.36) |
| Sigma-Aldrich | 2685 | Acetovanillone | 0.99 b | (0.08) | 1.20 b | (1.18) | Tr | |
| Sigma-Aldrich | 2936 | Zingerone | 35.21 b | (8.03) | 6.45 a | (4.28) | 7.28 a | (5.63) |
| Tentatively identified | 3030 | Methyl vanillyl eter | 5.78 a | (2.20) | 6.75 b | (2.93) | 12.97 c | (0.93) |
| Benzenic compounds | 181.14 | 170.14 | 133.92 | |||||
| Source * | RI A | Compound | Control Wine | Partially Fermented Wine | Total Dealcoholized Wine | |||
|---|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 800 | Acetaldehyde | 84,200.00 b | (1.18) | 74,150.00 a | (1.58) | 89,000.00 c | (1.75) |
| Aldehydes | 84,200.00 | 74,150.00 | 89,000.00 | |||||
| Sigma-Aldrich | 879 | Methanol | 85,200.00 a | (2.49) | 90,200.00 a | (10.19) | 90,200.00 a | (5.49) |
| Sigma-Aldrich | 1060 | 1-Propanol | 32,700.00 c | (6.49) | 2415.00 b | (2.05) | 15,930.00 a | (5.15) |
| Sigma-Aldrich | 1190 | 2-methyl-1-propanol | 460.57 c | (3.15) | 398.65 b | (3.36) | 290.35 a | (7.67) |
| Merck | 1214 | Isobutanol | 34,690.00 b | (10.19) | 23,605.00 a | (2.73) | 20,965.00 a | (4.42) |
| Sigma-Aldrich | 1221 | 2-methyl-1-butanol | 50,000.00 a | (14.14) | 3820.00 a | (1.48) | 34,000.00 a | (2.91) |
| Sigma-Aldrich | 1221 | 3-methyl-1-butanol | 198,800.00 a | (3.20) | 192,695.0 a | (0.25) | 188,280.00 a | (0.72) |
| Sigma-Aldrich | 1301 | 2-Methyl-2-butanol | 4.01 a | (1.41) | 8.04 b | (6.68) | 7.46 b | (4.74) |
| Fluka | 1545 | 2,3-Butanediol (levo) | 65.96 b | (5.18) | 43.40 a | (7.23) | 46.37 a | (4.78) |
| Fluka | 1585 | 2,3-Butanediol (meso) | 3.29 a | (2.11) | 4.82 b | (2.81) | 7.68 c | (3.02) |
| Sigma-Aldrich | 1725 | 3-(methylthio)-1-propanol | 129.62 a | (1.06) | 160.68 b | (5.93) | 199.25 c | (3.64) |
| Fluka | 1892 | 2-Phenylethanol | 15,901.89 c | (4.93) | 12,891.41 b | (6.44) | 10,081.29 a | (6.41) |
| Alcohols | 417,955.34 | 382,357.00 | 360,007.40 | |||||
| Sigma-Aldrich | 834 | Ethyl acetate | 29,080.00 c | (3.02) | 21,165.00 b | (2.24) | 12,385.00 a | (1.77) |
| Fluka | 1080 | Ethyl butyrate | 780.20 c | (3.41) | 253.55 b | (6.82) | 173.05 a | (8.05) |
| Sigma-Aldrich | 1145 | Isoamyl acetate | 2369.15 c | (8.01) | 1424.89 b | (6.01) | 300.75 a | (4.47) |
| Fluka | 1185 | Ethyl hexanoate | 266.35 b | (2.23) | 394.38 c | (6.64) | 48.14 a | (6.18) |
| Sigma-Aldrich | 1294 | Hexyl acetate | 72.09 b | (2.82) | 249.67 c | (1.12) | 17.29 a | (1.09) |
| Sigma-Aldrich | 1326 | Ethyl lactate | 1870.36 b | (6.65) | 652.73 a | (1.74) | 794.73 a | (1.95) |
| Sigma-Aldrich | 1436 | Ethyl octanoate | 612.75 b | (4.10) | 594.03 b | (2.12) | 20.36 a | (8.32) |
| Tentatively identified | 1499 | 3-hidroxy-ethyl butyrate | 141.29 b | (3.87) | 22.12 a | (6.90) | 210.31 c | (7.38) |
| Sigma-Aldrich | 1605 | Diethyl malonate | 4.22 a | (0.84) | n.d. | n.d. | ||
| Fluka | 1655 | Ethyl decanoate | 207.26 b | (2.81) | 137.42 a | (5.56) | n.d. | |
| Fluka | 1702 | Diethyl succinate | 2986.58 b | (5.02) | 130.56 a | (2.68) | 42.50 a | (2.82) |
| Tentatively identified | 1827 | 4-hidroxy-ethyl butyrate | n.d. | n.d. | 8.02 a | (0.76) | ||
| Fluka | 1936 | 2-phenylethyl acetate | 446.72 c | (7.47) | 185.84 b | (6.98) | 21.26 a | (6.23) |
| Sigma-Aldrich | 2070 | Diethyl malate | 1.37 a | (0.62) | 176.20 c | (1.65) | 63.17 b | (5.64) |
| Tentatively identified | 2331 | Ethyl monosuccinate | 114.04 a | (9.14) | n.d. | n.d. | ||
| Esters | 38,952.38 | 25,386.39 | 14,084.57 | |||||
| Sigma-Aldrich | 1426 | Acetic acid | 24.72 c | (5.63) | 16.16 b | (5.63) | 9.36 a | (3.84) |
| Sigma-Aldrich | 1546 | Propanoic acid | 6.14 b | (3.96) | 10.06 c | (2.55) | 1.92 a | (1.72) |
| Fluka | 1583 | Isobutyric acid | 115.66 b | (5.83) | 53.84 a | (4.21) | 273.40 c | (5.26) |
| Fluka | 1600 | Butyric acid | 122.78 a | (3.53) | 223.30 b | (5.00) | 546.70 c | (3.55) |
| Sigma-Aldrich | 1642 | Isovaleric acid | 394.94 c | (1.25) | 288.85 b | (0.68) | 265.96 a | (2.03) |
| Fluka | 1703 | Pentanoic acid | 12.33 a | (6.27) | 13.78 a | (2.82) | 16.55 b | (2.01) |
| Fluka | 1816 | Hexanoic acid | 1300.20 c | (3.91) | 1195.53 b | (2.08) | 1007.67 a | (2.13) |
| Sigma-Aldrich | 1929 | (E)-2-hexenoic acid | 24.59 b | (3.32) | 12.20 a | (8.70) | 23.61 b | (3.83) |
| Sigma-Aldrich | 1957 | (E)-3-hexenoic acid | 25.05 a | (8.49) | 41.45 c | (2.96) | 37.09 b | (3.30) |
| Fluka | 2024 | Octanoic acid | 4241.85 b | (6.43) | 4320.21 a | (2.40) | 1690.47 b | (1.05) |
| Sigma-Aldrich | 2108 | Nonanoic acid | n.d. | n.d. | 40.33 a | (1.26) | ||
| Sigma-Aldrich | 2289 | Decanoic acid | 1340.91 | (5.23) | 1287.92 | (5.46) | 163.46 | (6.12) |
| Sigma-Aldrich | 2439 | Dodecanoic acid | n.d. | 25.32 a | (5.26) | n.d. | ||
| Acids | 7609.17 | 7488.62 | 4076.51 | |||||
| Sigma-Aldrich | 1650 | γ-butyrolactone | 15.31 a | (5.04) | 2.84 a | (0.99) | 11.82 a | (9.69) |
| Sigma-Aldrich | 1902 | Pantoic lactone | 29.00 a | (8.06) | 31.40 a | (3.69) | 25.96 b | (6.49) |
| Lactones | 44.30 | 34.24 | 37.78 | |||||
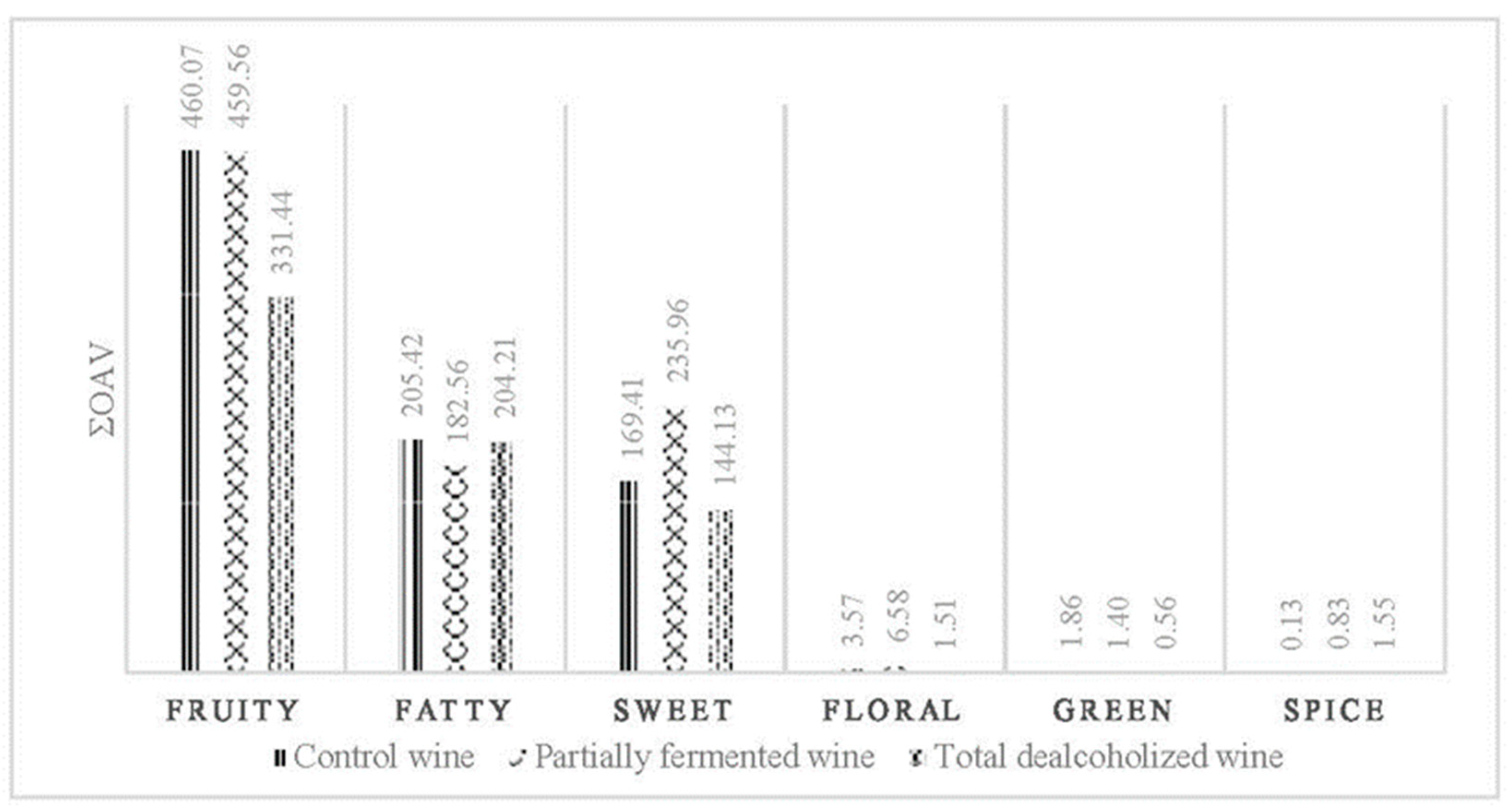
| Compound | Odor Descriptor | Odor Threshold (ug/L) | Aromatic Series | OAV Control Wine | OAV Partially Fermented Wine | OAV Total Dealcoholized Wine |
|---|---|---|---|---|---|---|
| Acetaldehyde | Rough, ripe apple | 500 | 1, 6 | 168.40 | 148.30 | 178.00 |
| Ethyl octanoate | Caramel, fruity | 5 | 1, 4 | 122.55 | 118.81 | 4.07 |
| Isoamyl acetate | Banana | 30 | 1 | 78.97 | 47.50 | 10.03 |
| Ethyl butyrate | Fruity | 20 | 1 | 39.01 | 12.68 | 8.65 |
| β-Damascenone | Sweet, fruity | 0.05 | 1, 4 | 27.20 | 100.60 | 125.60 |
| Ethyl hexanoate | Green apple | 14 | 1 | 19.02 | 28.17 | 3.44 |
| Isovaleric acid | Acid, rancid | 33 | 4, 6 | 11.97 | 8.75 | 8.06 |
| Octanoic acid | Sweet, cheese | 500 | 6 | 8.48 | 8.64 | 3.36 |
| 3-methyl-1-butanol | Burnt, alcohol | 30,000 | 4, 6 | 6.63 | 6.42 | 6.28 |
| Ethyl acetate | Fruity, solvent | 7500 | 1, 6 | 3.88 | 2.82 | 1.65 |
| Hexanoic acid | Sweet | 420 | 6 | 2.86 | 3.44 | 2.44 |
| 2-phenylethyl acetate | Floral | 250 | 2 | 1.79 | 0.74 | 0.09 |
| 2-phenylethyl alcohol | Floral, rose | 10,000 | 2 | 1.59 | 4.91 | 1.15 |
| Decanoic acid | Rancid fat | 1000 | 6 | 1.34 | 1.29 | 0.17 |
| Ethyl decanoate | Caramel, fruity | 200 | 1, 4 | 1.04 | 0.69 | 0.00 |
| Isobutanol | Bitter, green | 40,000 | 3, 6 | 0.87 | 0.59 | 0.52 |
| (Z)-3-hexen-1-ol | Green, cut grass | 400 | 3 | 0.82 | 0.46 | 0.01 |
| Butyric acid | Rancid, cheese, sweet | 173 | 6 | 0.71 | 1.29 | 3.16 |
| 3-(methylthio)-1-propanol | Cooked vegetable | 1000 | 6 | 0.13 | 0.16 | 0.20 |
| 1-hexanol | Resinous, floral, green | 8000 | 2, 3 | 0.13 | 0.18 | 0.01 |
| Methanol | Chemical, medicine | 668,000 | 6 | 0.13 | 0.14 | 0.14 |
| 4-vinyl-guaiacol | Spicy, curry | 40 | 5 | 0.13 | 0.83 | 1.55 |
| Hexyl acetate | Green, floral | 1500 | 2, 3 | 0.05 | 0.17 | 0.01 |
| Guaicol | Medicine, caramel, smoke | 10 | 4, 6 | 0.03 | 0.69 | 0.13 |
| Linalool | Floral | 15 | 2 | 0.01 | 0.58 | 0.25 |
| Isobutyric acid | Rancid, butter, cheese | 2300 | 6 | 0.01 | 0.02 | 0.12 |
| Compound | OAV Max. | OAV Min. | OAV Max./OAV Min. |
|---|---|---|---|
| (Z)-3-hexen-1-ol | 0.82 | 0.01 | 82.00 |
| Linalool | 0.58 | 0.01 | 58.00 |
| Ethyl octanoate | 122.55 | 4.07 | 30.09 |
| Guaicol | 0.69 | 0.03 | 23.00 |
| 2-phenylethyl aceteate | 1.79 | 0.09 | 19.89 |
| 1-hexanol | 0.18 | 0.01 | 18.00 |
| Hexyl acetate | 0.17 | 0.01 | 17.00 |
| Isobutyric acid | 0.12 | 0.01 | 12.00 |
| 4-vinyl-guaiacol | 1.55 | 0.13 | 11.92 |
| Ethyl hexanoate | 28.17 | 3.44 | 8.19 |
| Decanoic acid | 1.34 | 0.17 | 7.88 |
| Isoamyl acetate | 78.97 | 10.03 | 7.88 |
| β-Damascenone | 125.60 | 27.20 | 4.62 |
| Ethyl butyrate | 39.01 | 8.65 | 4.51 |
| Butyric acid | 3.16 | 0.71 | 4.45 |
| 2-phenylethyl alcohol | 4.91 | 1.15 | 4.27 |
| Octanoic acid | 8.64 | 3.36 | 2.57 |
| Ethyl acetate | 3.88 | 1.65 | 2.35 |
| Isobutanol | 0.87 | 0.52 | 1.67 |
| 3-(methylthio)-1-propanol | 0.20 | 0.13 | 1.54 |
| Isovaleric acid | 11.97 | 8.06 | 1.49 |
| Hexanoic acid | 3.44 | 2.44 | 1.41 |
| Acetaldehyde | 178.00 | 148.30 | 1.20 |
| Methanol | 0.14 | 0.13 | 1.08 |
| 3-methyl-1-butanol | 6.63 | 6.28 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio Alises, M.; Sánchez-Palomo, E.; González-Viñas, M.A. Influence of Different Alcohol Reduction Technologies on the Volatile Composition of La Mancha Tempranillo Rosé Wines. Beverages 2023, 9, 63. https://doi.org/10.3390/beverages9030063
Osorio Alises M, Sánchez-Palomo E, González-Viñas MA. Influence of Different Alcohol Reduction Technologies on the Volatile Composition of La Mancha Tempranillo Rosé Wines. Beverages. 2023; 9(3):63. https://doi.org/10.3390/beverages9030063
Chicago/Turabian StyleOsorio Alises, Maria, Eva Sánchez-Palomo, and Miguel A. González-Viñas. 2023. "Influence of Different Alcohol Reduction Technologies on the Volatile Composition of La Mancha Tempranillo Rosé Wines" Beverages 9, no. 3: 63. https://doi.org/10.3390/beverages9030063





