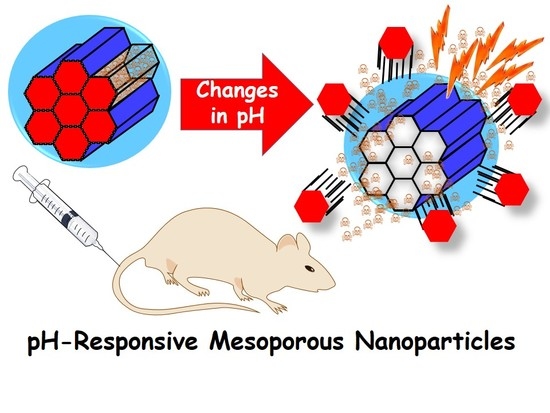
pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery
Abstract
:1. Introduction
1.1. Types of Nanoparticles
1.1.1. Non-Porous
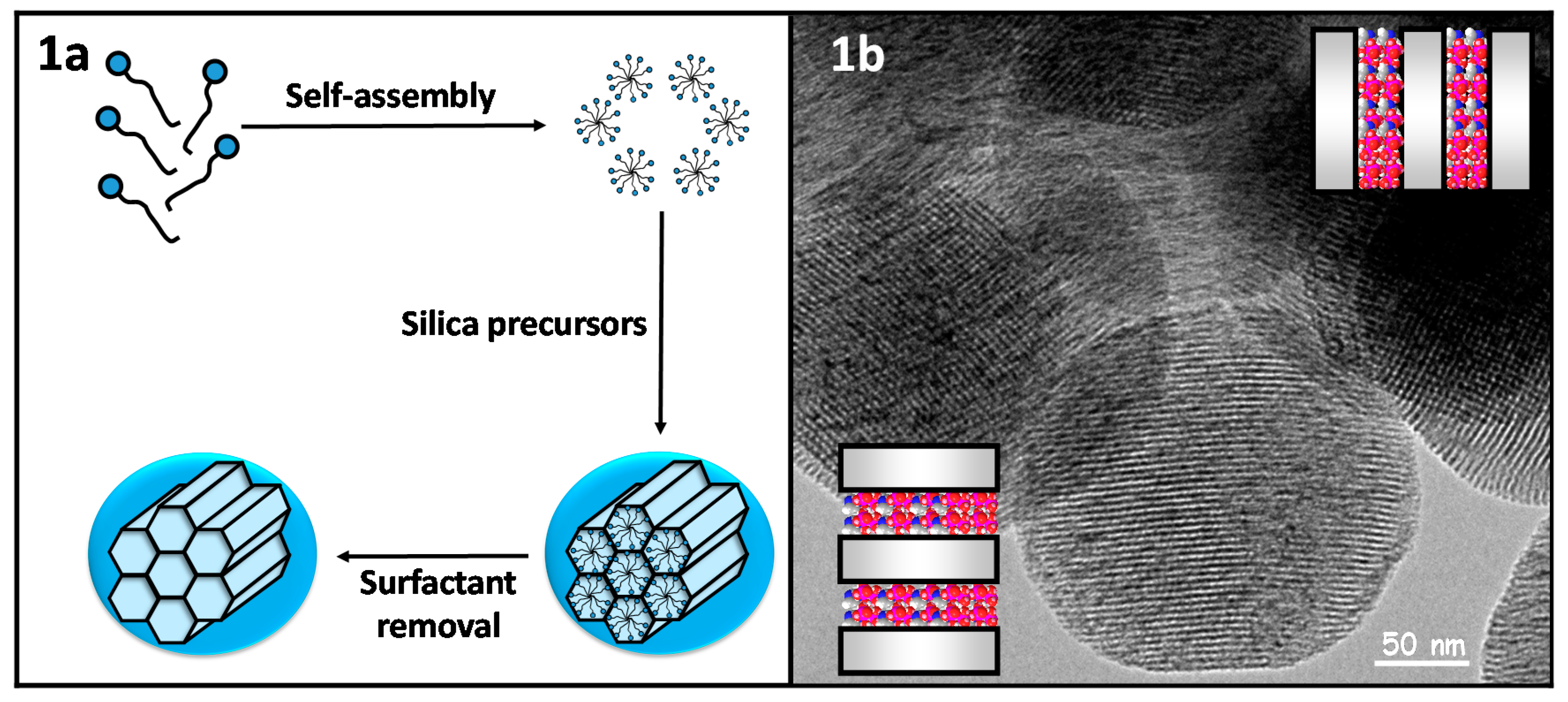
1.1.2. Mesoporous Nanoparticles
Mesoporous Silica Nanoparticles
Mesoporous Carbon Nanoparticles
1.2. Stimuli-Responsive Nanoparticles
1.2.1. External Stimuli-Responsive Nanocarriers
1.2.2. Internal Stimuli-Responsive Nanocarriers
1.2.3. The Role of pH in Drug Delivery
Digestive System
Extracellular Tumor Microenvironment
Intracellular pH
2. Host-Guest Interactions
2.1. DOX Loading and Release Mechanism
2.2. Host-Guest Interaction-Based Nanocarriers
2.2.1. Dual Stimuli-Responsive Nanocarriers
2.2.2. Nanocarriers for Dual Therapy
3. Acid-Labile Bonds
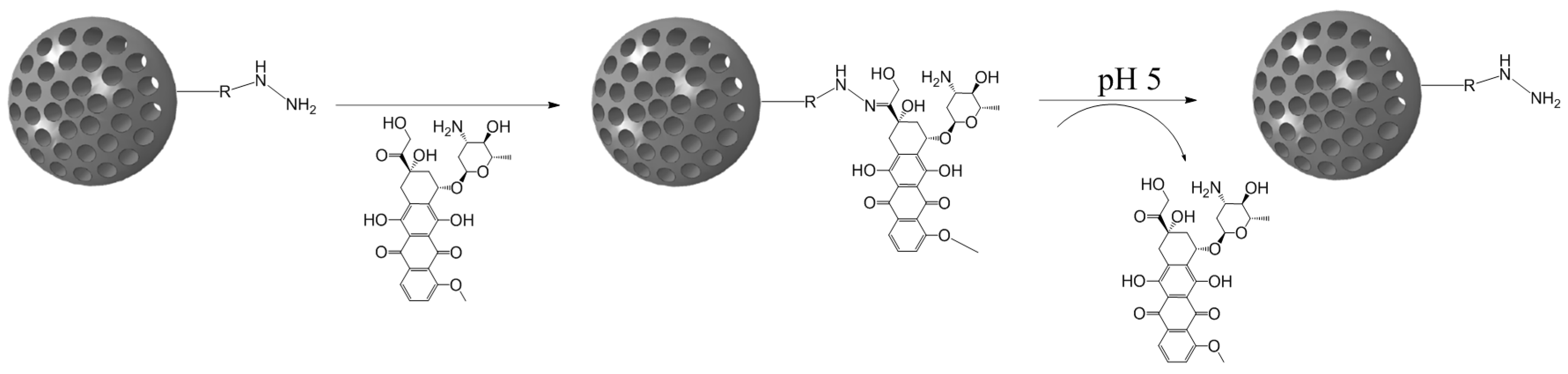
3.1. Hydrazone Bond
3.2. Acetal Bond
3.3. Imine-Based Bond
3.4. Ester-Based Bond
3.5. Coordination Bond
4. Supramolecular Structures as Pore-Capping Agents
4.1. Disassembling Gatekeepers
4.1.1. Self-Immolative Polymers
4.1.2. Small Nanoparticles
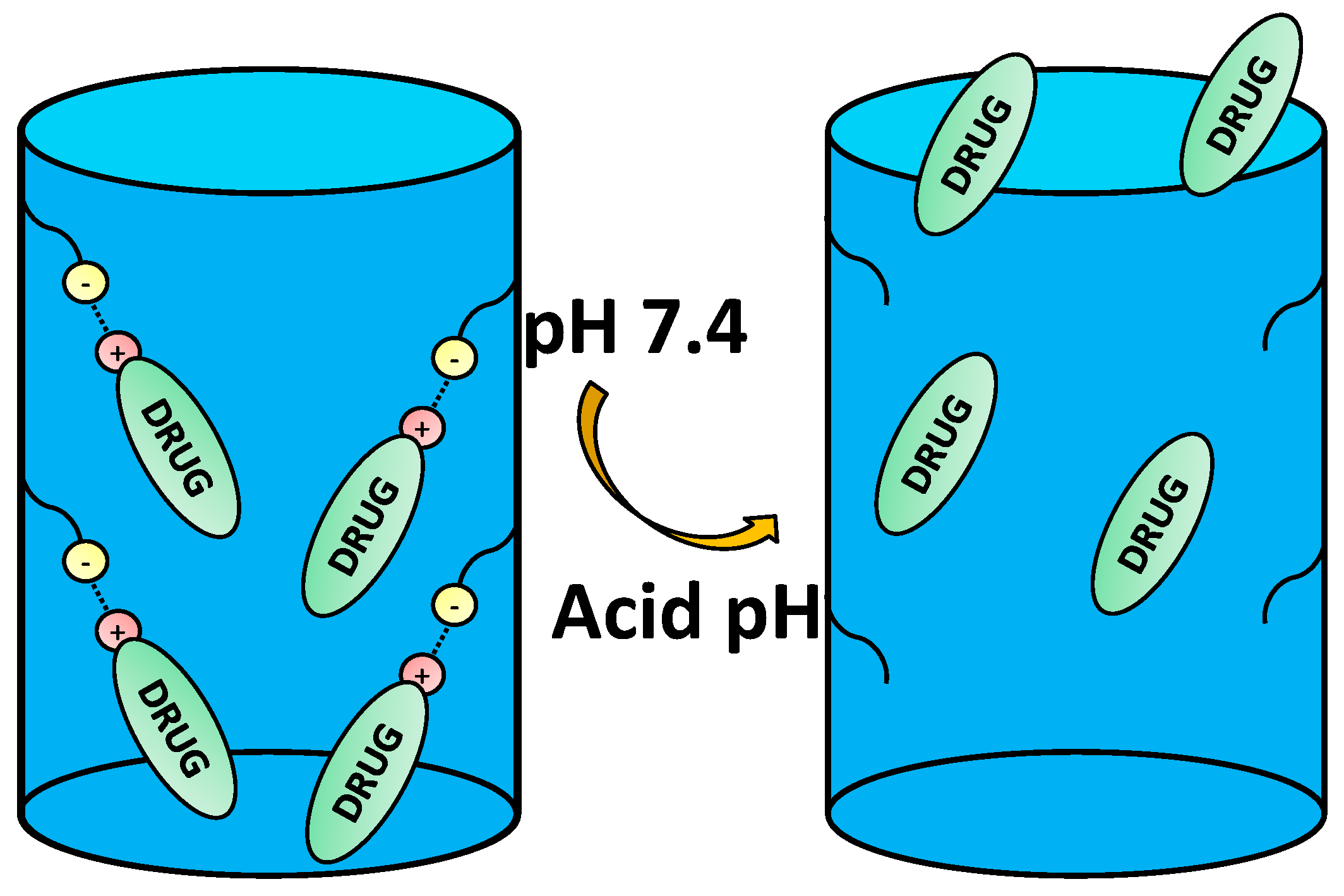
4.2. Pore Capping through Electrostatic Interactions
4.2.1. Polyelectrolyte Multilayers
4.2.2. Electrostatic Interactions between the Surface and the Gatekeeper
4.2.3. Cyclodextrins
4.3. Gatekeepers Suffering Conformational Changes
4.3.1. Anionic Polymers
4.3.2. Cationic Polymers
4.3.3. Peptides and More Complex Biomolecules
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Azarmi, S.; Roa, W.H.; Löbenberg, R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008, 60, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Brede, C.; Labhasetwar, V. Applications of nanoparticles in the detection and treatment of kidney diseases. Adv. Chronic Kidney Dis. 2013, 20, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Gomes, A.C.; Preto, A.; Cavaco-Paulo, A. Folate-targeted nanoparticles for rheumatoid arthritis therapy. Nanomed. Nanotechnol. Biol. Med. 2015, 12, 1113–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zhang, X.; Wu, Z.; Zheng, C.; Li, C. Oral glucose- and pH-sensitive nanocarriers for simulating insulin release in vivo. Polym. Chem. 2014, 5, 1999–2009. [Google Scholar] [CrossRef]
- Spuch, C.; Saida, O.; Navarro, C. Advances in the treatment of neurodegenerative disorders employing nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Schlapschy, M.; Binder, U.; Börger, C.; Theobald, I.; Wachinger, K.; Kisling, S.; Haller, D.; Skerra, A. PASylation: A biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Eng. Des. Sel. 2013, 26, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Estephan, Z.G.; Schlenoff, P.S.; Schlenoff, J.B. Zwitteration as an alternative to PEGylation. Langmuir 2011, 27, 6794–6800. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond.) 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Langer, R.S.; Farokhzad, O. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2011, 63, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J. Mesoporous carbon biomaterials. Sci. China Mater. 2015, 58, 241–257. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A.; Miletto, I.; Brunella, V.; Berlier, G.; Scalarone, D. Controlled post-synthesis grafting of thermoresponsive poly(N-isopropylacrylamide) on mesoporous silica nanoparticles. Polym. Adv. Technol. 2015, 26, 1070–1075. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, X.; Guo, Q.; Zhao, Y.; Wu, Z.; Li, C. Glycopolymer modified magnetic mesoporous silica nanoparticles for MR imaging and targeted drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 98–108. [Google Scholar] [CrossRef]
- Wang, D.; Wu, S. Red-Light-Responsive Supramolecular Valves for Photocontrolled Drug Release from Mesoporous Nanoparticles. Langmuir 2016, 32, 632–636. [Google Scholar] [CrossRef] [PubMed]
- He, D.; He, X.; Wang, K.; Cao, J.; Zhao, Y. A light-responsive reversible molecule-gated system using thymine-modified mesoporous silica nanoparticles. Langmuir 2012, 28, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Baeza, A.; Rodriguez-Milla, M.A.; García-Castro, J.; Vallet-Regí, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Vallet-Regí, M. Design of thermoresponsive polymeric gates with opposite controlled release behaviors. RSC Adv. 2016, 6, 42510–42516. [Google Scholar] [CrossRef]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Moros, M.; De La Fuente, J.M.; Vallet-Regí, M. Magnetic-Responsive Release Controlled by Hot Spot Effect. Langmuir 2015, 31, 12777–12782. [Google Scholar] [CrossRef] [PubMed]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2014, 5, 1519. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Luo, Z.; Ding, X.; Li, J.; Dai, L.; Zhou, J.; Zhao, X.; Ye, J.; Cai, K. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale 2015, 7, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, X.; Li, J.; Luo, Z.; Hu, Y.; Liu, J.; Dai, L.; Zhou, J.; Hou, C.; Cai, K. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology 2015, 26, 145102. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Hu, J.-J.; Xu, Q.; Chen, S.; Jia, H.-Z.; Sun, Y.-X.; Zhuo, R.-X.; Zhang, X.-Z. A redox-responsive drug delivery system based on RGD containing peptide-capped mesoporous silica nanoparticles. J. Mater. Chem. B 2015, 3, 39–44. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, H.; Wang, Y.; Gao, Y.; Huang, J.; Wang, Y.; Zhang, J.; Wang, S. Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20290–20299. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lo, L.-W.; Mou, C.-Y.; Yang, C.-S. Synthesis and characterization of positive-charge functionalized mesoporous silica nanoparticles for oral drug delivery of an anti-inflammatory drug. Adv. Funct. Mater. 2008, 18, 3283–3292. [Google Scholar] [CrossRef]
- Popat, A.; Jambhrunkar, S.; Zhang, J.; Yang, J.; Zhang, H.; Meka, A.; Yu, C. Programmable drug release using bioresponsive mesoporous silica nanoparticles for site-specific oral drug delivery. Chem. Commun. (Camb.) 2014, 50, 5547–5550. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Onyango, J.O.; Chung, M.S.; Eng, C.H.; Klees, L.M.; Langenbacher, R.; Yao, L.; An, M. Noncanonical amino acids to improve the pH response of pHLIP insertion at tumor acidity. Angew. Chem. Int. Ed. 2015, 54, 3658–3663. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, C.; Liu, X.; Ma, R.; Kong, D.; Shi, L. A Multifunctional Nanocarrier Based on Nanogated Mesoporous Silica for Enhanced Tumor-Specific Uptake and Intracellular Delivery. Macromol. Biosci. 2012, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Van Kirk, E.A.; Zhan, Y.; Murdoch, W.J.; Radosz, M.; Shen, Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew. Chem. Int. Ed. 2007, 46, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, I.M.; Shawesh, A.A.A.; Kodeh, F.S.; Kuhn, S.; Hempelmann, R. Novel pH-responsive swing gate system for adsorption and controlled release of BTB and MG dyes using amine functionalized mesoporous SBA-15 silica. J. Sol-Gel Sci. Technol. 2016, 77, 386–395. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, X.; Liu, W.; Zhang, F.; Zheng, X.; Qiao, P.; Li, J.; Dong, W.; Chen, L. Janus Silver-Mesoporous Silica Nanocarriers for SERS Traceable and pH-Sensitive Drug Delivery in Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, S.; Davaran, S.; Rasouli, F.; Mahkam, M.; Salehi, R. Positively charged functionalized silica nanoparticles as nontoxic carriers for triggered anticancer drug release. Des. Monomers Polym. 2014, 17, 227–237. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Tai, C.-W.; Su, J.; Zou, X.; Gao, F. Ultra-small mesoporous silica nanoparticles as efficient carriers for pH responsive releases of anti-cancer drugs. Dalton Trans. 2015, 44, 20186–20192. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Bae, J.H.; Kim, M.J.; Kim, S.H.; Ha, C.S. Design of a novel mesoporous organosilica hybrid microcarrier: A pH stimuli-responsive dual-drug-delivery vehicle for intracellular delivery of anticancer agents. Part. Part. Syst. Charact. 2013, 30, 1044–1055. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, L.; Zheng, H.; Xing, L.; Li, C.; Cui, J.; Che, S. pH-responsive mitoxantrone (MX) delivery using mesoporous silica nanoparticles (MSN). J. Mater. Chem. 2011, 21, 9483–9486. [Google Scholar] [CrossRef]
- Sanson, C.; Schatz, C.; Le Meins, J.F.; Soum, A.; Thévenot, J.; Garanger, E.; Lecommandoux, S. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J. Control. Release 2010, 147, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Jordan, S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N.; Miller, J.S. Bioresponsive Mesoporous Silica Nanoparticles for Triggered Drug Release Bioresponsive Mesoporous Silica Nanoparticles for Triggered Drug Release. J. Am. Chem. Soc. 2011, 133, 19582–19585. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, H.; Ge, W.; Wu, C.; Chen, D.; Li, Q.; Chen, Y.; Wang, X. Green and facile synthesis of highly biocompatible carbon nanospheres and their pH-responsive delivery of doxorubicin to cancer cells. RSC Adv. 2015, 5, 17532–17540. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Jokar, S. Functionalized mesoporous silica-coated magnetic graphene oxide by polyglycerol-g-polycaprolactone with pH-responsive behavior: Designed for targeted and controlled doxorubicin delivery. J. Ind. Eng. Chem. 2015, 28, 45–53. [Google Scholar] [CrossRef]
- Mohapatra, S.; Rout, S.R.; Das, R.K.; Nayak, S.; Ghosh, S.K. Highly Hydrophilic Luminescent Magnetic Mesoporous Carbon Nanospheres for Controlled Release of Anticancer Drug and Multimodal Imaging. Langmuir 2016, 32, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Wang, Z.; Dong, W.F.; Zhang, X.; Zheng, X.; Xiao, X.A.; Wang, Y.S.; Zhao, X.; Zhang, M.; Li, J.; et al. Facile Synthesis of Core-shell Magnetic Mesoporous Silica Nanoparticles for pH-sensitive Anticancer Drug Delivery. Chem. Biol. Drug Des. 2015, 86, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, L.; Zhang, L.; Xing, S.; Wang, T.; Wang, Y.A.; Wang, C.; Su, Z. Designed fabrication of unique eccentric mesoporous silica nanocluster-based core-shell nanostructures for pH-responsive drug delivery. ACS Appl. Mater. Interfaces 2013, 5, 7282–7290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.; Sun, W.; Xie, Q.R.; Xia, W.; Gu, H. Delivering hydrophilic and hydrophobic chemotherapeutics simultaneously by magnetic mesoporous silica nanoparticles to inhibit cancer cells. Int. J. Nanomed. 2012, 7, 999–1013. [Google Scholar]
- Du, P.; Zhao, X.; Zeng, J.; Guo, J.; Liu, P. Layer-by-layer engineering fluorescent polyelectrolyte coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Appl. Surf. Sci. 2015, 345, 90–98. [Google Scholar] [CrossRef]
- Tang, H.; Guo, J.; Sun, Y.; Chang, B.; Ren, Q.; Yang, W. Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery. Int. J. Pharm. 2011, 421, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hao, X.; Wu, Y.; Zhang, J.; Zhang, X.; Wang, P.C.; Zou, G.; Liang, X.-J. Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and pH dually response. J. Mater. Chem. B. Mater. Biol. Med. 2013, 1, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Zhao, Q.; Wan, L.; Wang, Y.; Gao, Y.; Wang, P.; Wang, Z.; Zhang, J.; Jiang, T.; Wang, S. Hybrid lipid-capped mesoporous silica for stimuli-responsive drug release and overcoming multidrug resistance. ACS Appl. Mater. Interfaces 2015, 7, 3342–3351. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, W.; Liu, X.; Chen, K.; Zhu, S.; Shi, P.; Chen, Y.; Shi, J. Mesoporous manganese silicate coated silica nanoparticles as multi-stimuli-responsive T1-MRI contrast agents and drug delivery carriers. Acta Biomater. 2016, 30, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Monem, A.S.; Elbialy, N.; Mohamed, N. Mesoporous silica coated gold nanorods loaded doxorubicin for combined chemo-photothermal therapy. Int. J. Pharm. 2014, 470, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lv, R.; He, F.; Qu, F.; Gai, S.; Du, S.; Wei, Z.; Yang, P. A core/shell/satellite anticancer platform for 808 NIR light-driven multimodal imaging and combined chemo-/photothermal therapy. Nanoscale 2015, 7, 13747–13758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Jin, Z.; Chan, K.M.; Yu, J.C. Mesoporous carbon/CuS nanocomposites for pH-dependent drug delivery and near-infrared chemo-photothermal therapy. RSC Adv. 2015, 5, 93226–93233. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhang, R.; Liu, X.; Yan, X.; Wang, J.; Wagner, E.; Huang, R. Synthesis of Core-Shell Graphitic Carbon @ Silica Nanospheres with Photothermochemotherapy. ACS Nano 2014, 7870–7879. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yao, H.; Meng, Y.; Wang, Y.; Yan, X.; Huang, R. Specific aptamer-conjugated mesoporous silica-carbon nanoparticles for HER2-targeted chemo-photothermal combined therapy. Acta Biomater. 2015, 16, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, R.; Liang, G.; Zhang, Z.; Zhang, P.; Yu, S.; Kong, J. MRI-visualized, dual-targeting, combined tumor therapy using magnetic graphene-based mesoporous silica. Small 2014, 10, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Xie, Z.; Liu, M.; Sun, H.; Zhu, H.; Guo, H. Research on redox-responsive mesoporous silica nanoparticles functionalized with PEG via a disulfide bond linker as drug carrier materials. Colloid Polym. Sci. 2015, 7, 2121–2128. [Google Scholar] [CrossRef]
- Lin, C.H.; Cheng, S.H.; Liao, W.N.; Wei, P.R.; Sung, P.J.; Weng, C.F.; Lee, C.H. Mesoporous silica nanoparticles for the improved anticancer efficacy of cis-platin. Int. J. Pharm. 2012, 429, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kong, J. Doxorubicin-tethered fluorescent silica nanoparticles for pH-responsive anticancer drug delivery. Talanta 2015, 134, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Cheng, S.H.; Huang, I.P.; Souris, J.S.; Yang, C.S.; Mou, C.Y.; Lo, L.W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed. 2010, 49, 8214–8219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jeon, J.B.; Chang, J.Y. Selectively functionalized mesoporous silica particles with the PEGylated outer surface and the doxorubicin-grafted inner surface: Improvement of loading content and solubility. Microporous Mesoporous Mater. 2013, 172, 118–124. [Google Scholar] [CrossRef]
- Cui, L.; Lin, H.; Yang, C.; Han, X.; Zhang, T.; Qu, F. Synthesis of multifunctional Fe3O4@mSiO2@Au core-shell nanocomposites for pH-responsive drug delivery. Eur. J. Inorg. Chem. 2014, 2014, 6156–6164. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Li, H.; Zhou, X.; Liu, M. pH-Triggered Au-fluorescent mesoporous silica nanoparticles for 19F MR/fluorescent multimodal cancer cellular imaging. Chem. Commun. 2014, 50, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Aioub, M.S.; El-Sayed, M.A. A Real-Time Surface Enhanced Raman Spectroscopy Study of Plasmonic Photothermal Cell Death Using Targeted Gold Nanoparticles. J. Am. Chem. Soc. 2016, 138, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, Q.; Shen, X.; Sun, Q.; Mu, C.; Gu, H.; Cai, K. pH-responsive nanocontainer based on hydrazone-bearing hollow silica nanoparticles for targeting tumor therapy. J. Mater. Chem. B 2016, 4, 4594–4604. [Google Scholar] [CrossRef]
- Schlossbauer, A.; Dohmen, C.; Schaffert, D.; Wagner, E.; Bein, T. PH-responsive release of acetal-linked melittin from SBA-15 mesoporous silica. Angew. Chem. Int. Ed. 2011, 50, 6828–6830. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Zhao, X.; Agarwal, A.; Mueller, L.J.; Feng, P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J. Am. Chem. Soc. 2010, 132, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, H.; Yang, N.; Wang, M.; Ding, C.; Fu, J. Graphene quantum dot-capped mesoporous silica nanoparticles through an acid-cleavable acetal bond for intracellular drug delivery and imaging. J. Mater. Chem. B 2014, 2, 4979–4982. [Google Scholar] [CrossRef]
- Chen, M.; He, X.; Wang, K.; He, D.; Yang, S.; Qiu, P.; Chen, S. A pH-responsive polymer/mesoporous silica nano-container linked through an acid cleavable linker for intracellular controlled release and tumor therapy in vivo. J. Mater. Chem. B 2014, 2, 428–436. [Google Scholar] [CrossRef]
- Yang, K.; Luo, H.; Zeng, M.; Jiang, Y.; Li, J.; Fu, X. Intracellular pH-Triggered, Targeted Drug Delivery to Cancer Cells by Multifunctional Envelope-Type Mesoporous Silica Nanocontainers. ACS Appl. Mater. Interfaces 2015, 7, 17399–17407. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Yao, X.; Chen, X.; Chen, X. Intracellular pH-operated mechanized mesoporous silica nanoparticles as potential drug carries. Microporous Mesoporous Mater. 2015, 201, 169–175. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Z.; Zhang, J.; Luo, T.; Zhou, J.; Zhao, X.; Cai, K. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials 2016, 83, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Jia, H.Z.; Zhang, J.; Liu, C.W.; Zhuo, R.X.; Zhang, X.Z. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug delivery. Small 2014, 10, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Leng, F.; Zheng, L.; Yang, J.; Wang, W.; Huang, C.Z. Autofluorescent and pH-responsive mesoporous silica for cancer-targeted and controlled drug release. Microporous Mesoporous Mater. 2014, 186, 187–193. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, D.; Liu, J.; Kuang, Y.; Li, Q.; Zhang, M.; Ye, H.; Qin, H.; Xu, Y.; Li, C.; Jiang, B. pH-Sensitive drug delivery system based on modified dextrin coated mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2016, 85, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Lan, J.; Zhang, Y.; Wu, Z.L.; Li, C.M.; Wang, J.; Huang, C.Z. Reduced graphene oxide gated mesoporous silica nanoparticles as a versatile chemo-photothermal therapy system through pH controllable release. J. Mater. Chem. B 2015, 3, 6377–6384. [Google Scholar] [CrossRef]
- Yang, Y.; Achazi, K.; Jia, Y.; Wei, Q.; Haag, R.; Li, J. Complex Assembly of Polymer Conjugated Mesoporous Silica Nanoparticles for Intracellular pH-Responsive Drug Delivery. Langmuir 2016, 32, 12453–12460. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.R.; Ferris, D.P.; Frasconi, M.; Malin, D.; Strekalova, E.; Yilmaz, M.D.; Ambrogio, M.W.; Algaradah, M.M.; Hong, M.P.; Chen, X.; et al. Esterase- and pH-responsive poly(β-amino ester)-capped mesoporous silica nanoparticles for drug delivery. Nanoscale 2015, 7, 7178–7183. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yang, M.Y.; Wu, H.X.; Tang, Z.W.; Xiao, J.Y.; Liu, C.J.; Zhuo, R.X. Glucose- and pH-responsive nanogated ensemble based on polymeric network capped mesoporous silica. ACS Appl. Mater. Interfaces 2015, 7, 6310–6316. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P.; Guillem, C. pH- and photo-switched release of guest molecules from mesoporous silica supports. J. Am. Chem. Soc. 2009, 131, 6833–6843. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Lu, X.; Yuan, Y.; Qian, J.; Zhou, H.; Lu, X.; Shi, J.; Liu, C. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials 2011, 32, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Cai, K.; Hu, Y.; Zhang, B.; Xu, D. Cell-specific intracellular anticancer drug delivery from mesoporous silica nanoparticles with pH sensitivity. Adv. Healthc. Mater. 2012, 1, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, X.; Wu, Y.; Li, J.; Cheng, W.; He, J.; Tian, H.; Huang, Y. Preparation of pH-responsive mesoporous hydroxyapatite nanoparticles for intracellular controlled release of an anticancer drug. Biomater. Sci. 2015, 5, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.G.; An, J.X.; Su, C.; Guo, Q.Q.; Li, C.X. Boronate ester bond-based core-shell nanocarriers with pH response for anticancer drug delivery. Rsc Adv. 2014, 4, 20208–20215. [Google Scholar] [CrossRef]
- Chen, X.; Yao, X.; Wang, C.; Chen, L.; Chen, X. Mesoporous silica nanoparticles capped with fluorescence-conjugated cyclodextrin for pH-activated controlled drug delivery and imaging. Microporous Mesoporous Mater. 2015, 217, 46–53. [Google Scholar] [CrossRef]
- Yilmaz, M.D.; Xue, M.; Ambrogio, M.W.; Buyukcakir, O.; Wu, Y.; Frasconi, M.; Chen, X.; Nassar, M.S.; Stoddart, J.F.; Zink, J.I. Sugar and pH dual-responsive mesoporous silica nanocontainers based on competitive binding mechanisms. Nanoscale 2015, 7, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-L.; Li, Q.-L.; Zhou, Y.; Jin, X.-Y.; Qi, A.-D.; Yang, Y.-W. Sugar and pH dual-responsive snap-top nanocarriers based on mesoporous silica-coated Fe3O4 magnetic nanoparticles for cargo delivery. Chem. Commun. 2015, 51, 4237–4240. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Fu, J.; Liu, X.; Wang, T.; Lu, X. Acid-responsive intracellular doxorubicin release from click chemistry functionalized mesoporous silica nanoparticles. RSC Adv. 2015, 5, 30640–30646. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Li, M.F.; Feng, J. Multifunctional mesoporous silica nanoparticles based on charge-reversal plug-gate nanovalves and acid-decomposable ZnO quantum dots for intracellular drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 26666–26673. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.J.; Anderson, D.J.; Grainger, D.W. Comparison of hydrazone heterobifunctional cross-linking agents for reversible conjugation of thiol-containing chemistry. Bioconjug. Chem. 2010, 21, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Raunio, H.; Rautio, J. Prodrugs—From serendipity to rational design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, K.; Liu, J.; Sun, G.; Yin, Q.; Lu, L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Tavares, A.J.; Krull, U.J. Beyond labels: A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal. Chim. Acta 2010, 673, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, J.X. Upconversion nanomaterials: Synthesis, mechanism, and applications in sensing. Sensors 2012, 12, 2414–2435. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Simplício, A.L.; Clancy, J.M.; Gilmer, J.F. Prodrugs for amines. Molecules 2008, 13, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.D.; Davidson, M.G.; Van Den Elsen, J.M.H.; Fossey, J.S.; Jenkins, A.T.A.; Jiang, Y.B.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; et al. Exploiting the reversible covalent bonding of boronic acids: Recognition, sensing, and assembly. Acc. Chem. Res. 2013, 46, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zheng, H.; Xing, L.; Shu, M.; Che, S. Designable coordination bonding in mesopores as a pH-responsive release system. Chem. Mater. 2010, 22, 5437–5444. [Google Scholar] [CrossRef]
- Wu, M.; Meng, Q.; Chen, Y.; Xu, P.; Zhang, S.; Li, Y.; Zhang, L.; Wang, M.; Yao, H.; Shi, J. Ultrasmall confined Iron oxide nanoparticle MSNs as a pH-responsive theranostic platform. Adv. Funct. Mater. 2014, 24, 4273–4283. [Google Scholar] [CrossRef]
- Xing, L.; Zheng, H.; Cao, Y.; Che, S. Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release. Adv. Mater. 2012, 24, 6433–6437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Huang, Z.; Che, S. Mesostructured chitosan–silica hybrid as a biodegradable carrier for a pH-responsive drug delivery system. Dalton Trans. 2012, 41, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Che, S. Amino/quaternary ammonium groups bifunctionalized large pore mesoporous silica for pH-responsive large drug delivery. RSC Adv. 2012, 2, 4421–4429. [Google Scholar] [CrossRef]
- Tarn, D.; Xue, M.; Zink, J.I. PH-responsive dual cargo delivery from mesoporous silica nanoparticles with a metal-latched nanogate. Inorg. Chem. 2013, 52, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, Q.; Li, J.; Shen, X.; Mu, C.; Cai, K. Dendrimerlike mesoporous silica nanoparticles as pH-responsive nanocontainers for targeted drug delivery and bioimaging. ACS Appl. Mater. Interfaces 2015, 7, 7357–7372. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cheng, X.; Soeriyadi, A.H.; Sagnella, S.M.; Lu, X.; Scott, J.A.; Lowe, S.B.; Kavallaris, M.; Gooding, J.J. Stimuli-responsive functionalized mesoporous silica nanoparticles for drug release in response to various biological stimuli. Biomater. Sci. 2014, 2, 121. [Google Scholar] [CrossRef]
- Kankala, R.K.; Kuthati, Y.; Liu, C.-L.; Mou, C.-Y.; Lee, C.-H. Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ROS. RSC Adv. 2015, 5, 86072–86081. [Google Scholar] [CrossRef]
- Mourino, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gao, C.; Peng, B.; Shu, M.; Che, S. pH-Responsive Drug Delivery System Based on Coordination Bonding in a Mesostructured Surfactant/Silica Hybrid. J. Phys. Chem. C 2011, 115, 7230–7237. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Che, S. Coordination bonding-based mesoporous silica for pH-responsive anticancer drug doxorubicin delivery. J. Phys. Chem. C 2011, 115, 16803–16813. [Google Scholar] [CrossRef]
- Kim, S.; Philippot, S.; Fontanay, S.; Duval, R.E.; Lamouroux, E.; Canilho, N.; Pasc, A. pH- and glutathione-responsive release of curcumin from mesoporous silica nanoparticles coated using tannic acid–Fe(iii) complex. RSC Adv. 2015, 5, 90550–90558. [Google Scholar] [CrossRef]
- Fang, W.; Yang, J.; Gong, J.; Zheng, N. Photo- and pH-triggered release of anticancer drugs from mesoporous silica-coated Pd@Ag nanoparticles. Adv. Funct. Mater. 2012, 22, 842–848. [Google Scholar] [CrossRef]
- Jiao, Y.; Shen, S.; Sun, Y.; Jiang, X.; Yang, W. A functionalized hollow mesoporous silica nanoparticles-based controlled dual-drug delivery system for improved tumor cell cytotoxicity. Part. Part. Syst. Charact. 2015, 32, 222–233. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Lozano, D.; Vallet-Regí, M.; Manzano, M. Self-Immolative Polymers as novel pH-responsive gate keepers for drug delivery. RSC Adv. 2017, 7, 132–136. [Google Scholar] [CrossRef]
- Gnaim, S.; Shabat, D. Quinone-methide species, a gateway to functional molecular systems: From self-immolative dendrimers to long-wavelength fluorescent dyes. Acc. Chem. Res. 2014, 47, 2970–2984. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-Triggered Controlled Drug Release from Mesoporous Silica. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar]
- Huang, X.; Wu, S.; Du, X. Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon N. Y. 2016, 101, 135–142. [Google Scholar] [CrossRef]
- Wu, S.; Huang, X.; Du, X. pH- and redox-triggered synergistic controlled release of a ZnO-gated hollow mesoporous silica drug delivery system. J. Mater. Chem. B 2015, 3, 1426–1432. [Google Scholar] [CrossRef]
- Muhammad, F.; Wang, A.; Guo, M.; Zhao, J.; Qi, W.; Yingjie, G.; Gu, J.; Zhu, G. PH dictates the release of hydrophobic drug cocktail from mesoporous nanoarchitecture. ACS Appl. Mater. Interfaces 2013, 5, 11828–11835. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Liu, L.; You, X.; Zhang, C.; Wang, Y. A pH-sensitive nanocarrier for co-delivery of doxorubicin and camptothecin to enhance chemotherapeutic efficacy and overcome multidrug resistance in vitro. RSC Adv. 2015, 5, 77097–77105. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Liu, C.-H.; Liang, Y.-H.; Lin, F.-H.; Wu, K.C.-W. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. B 2013, 1, 2447–2450. [Google Scholar] [CrossRef]
- Lin, K.L.; Chen, L.; Liu, P.Y.; Zou, Z.Y.; Zhang, M.L.; Shen, Y.H.; Qiao, Y.Q.; Liu, X.Y.; Chang, J. Hollow magnetic hydroxyapatite microspheres with hierarchically mesoporous microstructure for pH-responsive drug delivery. Crystengcomm 2013, 15, 2999–3008. [Google Scholar] [CrossRef]
- Cui, L.; Lin, H.; Guo, W.; Xiang, D.; Zhou, D.; Zhang, T.; Qu, F. A novel pH-responsive controlled release system based on mesoporous silica coated with hydroxyapatite. J. Sol-Gel Sci. Technol. 2014, 72, 106–113. [Google Scholar] [CrossRef]
- Rim, H.P.; Min, K.H.; Lee, H.J.; Jeong, S.Y.; Lee, S.C. PH-tunable calcium phosphate covered mesoporous silica nanocontainers for intracellular controlled release of guest drugs. Angew. Chem. Int. Ed. 2011, 50, 8853–8857. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Gaspar, V.M.; Costa, E.C.; De Melo-Diogo, D.; Machado, P.; Paquete, C.M.; Correia, I.J. Preparation of end-capped pH-sensitive mesoporous silica nanocarriers for on-demand drug delivery. Eur. J. Pharm. Biopharm. 2014, 88, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Biomineralization inspired surface engineering of nanocarriers for pH-responsive, targeted drug delivery. Biomaterials 2013, 34, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yin, Q.; Ji, X.; Zhang, S.; Chen, H.; Zheng, Y.; Sun, Y.; Qu, H.; Wang, Z.; Li, Y.; et al. Manganese oxide-based multifunctionalized mesoporous silica nanoparticles for pH-responsive MRI, ultrasonography and circumvention of MDR in cancer cells. Biomaterials 2012, 33, 7126–7137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qian, X.; Zhang, L.; Peng, W.; Chen, Y. Composition-property relationships in multifunctional hollow mesoporous carbon nanosystems for PH-responsive magnetic resonance imaging and on-demand drug release. Nanoscale 2015, 7, 7632–7643. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Mazaheri Tehrani, Z.; Jokar, S. Chitosan based supramolecular polypseudorotaxane as a pH-responsive polymer and their hybridization with mesoporous silica-coated magnetic graphene oxide for triggered anticancer drug delivery. Polymer 2015, 76, 52–61. [Google Scholar] [CrossRef]
- Liu, W.T.; Yang, Y.; Shen, P.H.; Gao, X.J.; He, S.Q.; Liu, H.; Zhu, C.S. Facile and simple preparation of pH-sensitive chitosan-mesoporous silica nanoparticles for future breast cancer treatment. Express Polym. Lett. 2015, 9, 1068–1075. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M. Mesoporous Silica Nanoparticles (MCM-41) Coated PEGylated Chitosan as a pH-Responsive Nanocarrier for Triggered Release of Erythromycin. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 692–697. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y. Chitosan enclosed mesoporous silica nanoparticles as drug nano-carriers: Sensitive response to the narrow pH range. Microporous Mesoporous Mater. 2012, 150, 83–89. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.L.; Wang, L.; Ma, J.; Yang, Y.W.; Gao, H. Nanoassembles constructed from mesoporous silica nanoparticles and surface-coated multilayer polyelectrolytes for controlled drug delivery. Microporous Mesoporous Mater. 2014, 185, 245–253. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, G.; Liu, S. PH-disintegrable polyelectrolyte multilayer-coated mesoporous silica nanoparticles exhibiting triggered co-release of cisplatin and model drug molecules. Macromol. Rapid Commun. 2011, 32, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Liu, X.; Yang, X.; Liu, Z.; Ren, J.; Wang, S.; Qu, X. Versatile fluorescent conjugated polyelectrolyte-capped mesoporous silica nanoparticles for controlled drug delivery and imaging. Chempluschem 2013, 78, 656–662. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y.; Ng, K.W.; Zhao, Y. Integrated hollow mesoporous silica nanoparticles for target drug/siRNA co-delivery. Chem. A Eur. J. 2013, 19, 15593–15603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gao, J.; Qian, J.; Zhang, L.; Zheng, K.; Zhong, K.; Cai, D.; Zhang, X.; Wu, Z. Hydroxylated Mesoporous Nanosilica Coated by Polyethylenimine Coupled with Gadolinium and Folic Acid: A Tumor-Targeted T 1 Magnetic Resonance Contrast Agent and Drug Delivery System. ACS Appl. Mater. Interfaces 2015, 7, 14192–14200. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Tehrani, Z.M.; Bennett, C. PEG-co-Polyvinyl Pyridine Coated Magnetic Mesoporous Silica Nanoparticles for pH-Responsive Controlled Release of Doxorubicin. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 570–577. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M. Mesoporous silica nanoparticles with bilayer coating of poly(acrylic acid-co-itaconic acid) and human serum albumin (HSA): A pH-sensitive carrier for gemcitabine delivery. Mater. Sci. Eng. C 2016, 61, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Nie, W.; He, C.; Zhou, X.; Chen, L.; Qiu, K.; Wang, W.; Yin, Z. Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake and biodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl. Mater. Interfaces 2014, 6, 8447–8460. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Sun, G.; Li, Q.; Wang, E.; Gu, J. A dual-responsive superparamagnetic Fe3O4/Silica/PAH/PSS material used for controlled release of chemotherapeutic agent, keggin polyoxotungstate, PM-19. Solid State Sci. 2010, 12, 1720–1725. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Z.; Liu, Z.; Ren, J.; Qu, X. Luminescent carbon dot-gated nanovehicles for pH-triggered intracellular controlled release and imaging. Langmuir 2013, 29, 6396–6403. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, S.; Fan, P.; Wang, L.; Di, Y.; Lin, K.; Xiao, F.S. pH-responsive carrier system based on carboxylic acid modified mesoporous silica and polyelectrolyte for drug delivery. Chem. Mater. 2005, 17, 5999–6003. [Google Scholar] [CrossRef]
- Fu, J.K.; Zhu, Y.C.; Zhao, Y. Controlled free radical generation against tumor cells by pH-responsive mesoporous silica nanocomposite. J. Mater. Chem. B 2014, 2, 3538–3548. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Peng, B. Chitosan-capped mesoporous silica nanoparticles as pH-responsive nanocarriers for controlled drug release. Chem. Asian J. 2014, 9, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Gulfam, M.; Chung, B.G. Development of pH-responsive chitosan-coated mesoporous silica nanoparticles. Macromol. Res. 2014, 22, 412–417. [Google Scholar] [CrossRef]
- Popat, A.; Liu, J.; Lu, G.Q.; Qiao, S.Z. A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 11173–11178. [Google Scholar] [CrossRef]
- Mishra, A.K.; Pandey, H.; Agarwal, V.; Ramteke, P.W.; Pandey, A.C. Nanoengineered mesoporous silica nanoparticles for smart delivery of doxorubicin. J. Nanopart. Res. 2014, 16, 2515. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Selective topotecan delivery to cancer cells by targeted pH-sensitive mesoporous silica nanoparticles. RSC Adv. 2016, 6, 50923–50932. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Li, Z.; Kabehie, S.; Botros, Y.Y.; Stoddart, J.F.; Zink, J.I. pH-operated nanopistons on the surfaces of mesoporous silica nanoparticles. J. Am. Chem. Soc. 2010, 132, 13016–13025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Neoh, K.G.; Xu, L.; Lu, S.; Kang, E.T.; Mahendran, R.; Chiong, E. Functionalized mesoporous silica nanoparticles with mucoadhesive and sustained drug release properties for potential bladder cancer therapy. Langmuir 2014, 30, 6151–6161. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Clemens, D.L.; Lee, B.Y.; Dillon, B.J.; Horwitz, M.A.; Zink, J.I. Mesoporous Silica Nanoparticles with pH-Sensitive Nanovalves for Delivery of Moxifloxacin Provide Improved Treatment of Lethal Pneumonic Tularemia. ACS Nano 2015, 9, 10778–10789. [Google Scholar] [CrossRef] [PubMed]
- Théron, C.; Gallud, A.; Carcel, C.; Gary-Bobo, M.; Maynadier, M.; Garcia, M.; Lu, J.; Tamanoi, F.; Zink, J.I.; Wong Chi Man, M. Hybrid mesoporous silica nanoparticles with pH-operated and complementary H-bonding caps as an autonomous drug-delivery system. Chemistry 2014, 20, 9372–9380. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Dong, R.; Wang, S.; Zhang, Z.; Luo, M.; Bai, C.; Zhao, Q.; Li, J.; Chen, L.; Xiong, H. A pH-responsive nano-carrier with mesoporous silica nanoparticles cores and poly(acrylic acid) shell-layers: Fabrication, characterization and properties for controlled release of salidroside. Int. J. Pharm. 2013, 446, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Samart, C.; Prawingwong, P.; Amnuaypanich, S.; Zhang, H.; Kajiyoshi, K.; Reubroycharoen, P. Preparation of poly acrylic acid grafted-mesoporous silica as pH responsive releasing material. J. Ind. Eng. Chem. 2014, 20, 2153–2158. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Li, X.; Pan, C.-Y. Fabrication of smart nanocontainers with a mesoporous core and a pH-responsive shell for controlled uptake and release. J. Mater. Chem. 2009, 19, 5155. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, Q.; Yang, D.; Zhang, J.Z.; Zhang, F.; Hu, J. Preparation of pH-Responsive Mesoporous Silica Nanoparticles and Their Application in Controlled Drug Delivery. J. Phys. Chem. C 2011, 115, 9926–9932. [Google Scholar] [CrossRef]
- Wu, H.; Tang, L.; An, L.; Wang, X.; Zhang, H.; Shi, J.; Yang, S. PH-responsive magnetic mesoporous silica nanospheres for magnetic resonance imaging and drug delivery. React. Funct. Polym. 2012, 72, 329–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Hu, L.-L.; Chang, Y.-Q.; He, R.-H.; Chen, M.-L.; Shu, Y.; Wang, J.-H. Mesoporous carbon nanoparticles capped with polyacrylic acid as drug carrier for bi-trigger continuous drug release. J. Mater. Chem. B 2016, 4, 5178–5184. [Google Scholar] [CrossRef]
- Han, J.; Sun, J.; Bai, S.; Panezai, H.; Jin, X.; Wu, X. “Graft to” Synthesis and Ibuprofen-Loading Performance of pH-Sensitive PMAA-Silica Hybrid Nanoparticles with Controlled Bimodal Mesopores. J. Pharm. Sci. 2015, 104, 4299–4306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, S.; Sun, J.; Han, J.; Guo, Y. PH-responsive ibuprofen delivery in silane-modified poly(methylacrylic acid) coated bimodal mesoporous silicas. Mater. Res. Bull. 2014, 53, 266–271. [Google Scholar] [CrossRef]
- Wen, H.; Guo, J.; Chang, B.; Yang, W. PH-responsive composite microspheres based on magnetic mesoporous silica nanoparticle for drug delivery. Eur. J. Pharm. Biopharm. 2013, 84, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Lin, H.; Jiang, P.; Qu, F. Biofunctional mesoporous silica nanoparticles for magnetically oriented target and pH-responsive controlled release of ibuprofen. Colloids Surf. A Physicochem. Eng. Asp. 2012, 403, 7–14. [Google Scholar] [CrossRef]
- Ma, J.; Lin, H.; Xing, R.; Li, X.; Bian, C.; Xiang, D.; Guo, W.; Qu, F. Synthesis of pH-responsive mesoporous silica nanotubes for controlled release. J. Sol-Gel Sci. Technol. 2014, 69, 364–369. [Google Scholar] [CrossRef]
- Chang, B.; Chen, D.; Wang, Y.; Chen, Y.; Jiao, Y.; Sha, X.; Yang, W. Bioresponsive controlled drug release based on mesoporous silica nanoparticles coated with reductively sheddable polymer shell. Chem. Mater. 2013, 25, 574–585. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Dai, Y.; Ma, P.; Li, X.; Cheng, Z.; Hou, Z.; Kang, X.; Li, C.; Lin, J. Multifunctional up-converting nanocomposites with smart polymer brushes gated mesopores for cell imaging and thermo/pH dual-responsive drug controlled release. Adv. Funct. Mater. 2013, 23, 4067–4078. [Google Scholar] [CrossRef]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Yang, G.; Dai, Y.; Hou, Z.; Lin, J. An imaging-guided platform for synergistic photodynamic/photothermal/chemo-therapy with pH/temperature-responsive drug release. Biomaterials 2015, 63, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xu, Y.; Wu, D.; Shen, W.; Deng, F. Synthesis, characterization, and in vitro pH-controllable drug release from mesoporous silica spheres with switchable gates. Langmuir 2010, 26, 17133–17138. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lü, S.; Gao, C.; Wang, X.; Bai, X.; Gao, N.; Liu, M. Facile preparation of pH-sensitive and self-fluorescent mesoporous silica nanoparticles modified with PAMAM dendrimers for label-free imaging and drug delivery. Chem. Eng. J. 2015, 266, 171–178. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Moghanaki, A.A. Folate-Conjugated pH-Responsive Nanocarrier Designed for Active Tumor Targeting and Controlled Release of Gemcitabine. Pharm. Res. 2015, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Glogowska, A.; Zhong, W.; Klonisch, T.; Xing, M. Polymeric mesoporous silica nanoparticles as a pH-responsive switch to control doxorubicin intracellular delivery. J. Mater. Chem. B 2013, 1, 5264. [Google Scholar] [CrossRef]
- Tian, Y.; Kong, Y.; Li, X.; Wu, J.; Ko, A.C.-T.; Xing, M. Light- and pH-activated intracellular drug release from polymeric mesoporous silica nanoparticles. Colloids Surf. B. Biointerfaces 2015, 134, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, W.; Xiao, H.; Chen, Y.; Chen, M.; Li, J. Intelligent Drug Delivery System Based on Mesoporous Silica Nanoparticles Coated with an Ultra-pH-Sensitive Gatekeeper and Poly(ethylene glycol). ACS Macro Lett. 2016, 5, 55–58. [Google Scholar] [CrossRef]
- Sun, J.T.; Hong, C.Y.; Pan, C.Y. Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release. J. Phys. Chem. C 2010, 114, 12481–12486. [Google Scholar] [CrossRef]
- Yu, F.; Tang, X.; Pei, M. Facile synthesis of PDMAEMA-coated hollow mesoporous silica nanoparticles and their pH-responsive controlled release. Microporous Mesoporous Mater. 2013, 173, 64–69. [Google Scholar] [CrossRef]
- Yang, M.Y.; Tan, L.; Wu, H.X.; Liu, C.J.; Zhuo, R.X. Dual-stimuli-responsive polymer-coated mesoporous silica nanoparticles used for controlled drug delivery. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Ang, C.Y.; Li, M.; Tan, S.Y.; Qu, Q.; Luo, Z. Polymer Coated Hollow Mesoporous Nanoparticles for Triple-Responsive Delivery Silica Drug. ACS Appl. Mater. Interfaces 2015, 7, 18179–18187. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Qin, J.; Fan, L. Chondroitin sulfate functionalized mesostructured silica nanoparticles as biocompatible carriers for drug delivery. Int. J. Nanomed. 2012, 7, 5235–5247. [Google Scholar]
- Chang, B.; Sha, X.; Guo, J.; Jiao, Y.; Wang, C.; Yang, W. Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release. J. Mater. Chem. 2011, 21, 9239. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Chang, B.; Hu, H.; Fang, X.; Sha, X. In vivo distribution and antitumor activity of doxorubicin-loaded N-isopropylacrylamide-co-methacrylic acid coated mesoporous silica nanoparticles and safety evaluation. Eur. J. Pharm. Biopharm. 2013, 85, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, D.; Jin, C.; Song, X.; Cheng, J.; Zhao, X.; Qi, X.; Zhang, G. A dual responsive targeted drug delivery system based on smart polymer coated mesoporous silica for laryngeal carcinoma treatment. New J. Chem. 2014, 38, 4830–4836. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Zhu, D.; Zong, S.; Yang, L.; Zhong, Y.; Cui, Y. PH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer-lipid bilayer. ACS Appl. Mater. Interfaces 2013, 5, 10895–10903. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, P.; Tziveleka, L.-A.; Varlas, S.; Iatrou, H. pH-Sensitive nanogates based on poly(l-histidine) for controlled drug release from mesoporous silica nanoparticles. Polym. Chem. 2016, 7, 1475–1485. [Google Scholar] [CrossRef]
- Liu, R.; Liao, P.; Liu, J.; Feng, P. Responsive polymer-coated mesoporous silica as a pH-sensitive nanocarrier for controlled release. Langmuir 2011, 27, 3095–3099. [Google Scholar] [CrossRef] [PubMed]
- Rafi, A.A.; Mahkam, M.; Davaran, S.; Hamishehkar, H. A Smart pH-responsive Nano-Carrier as a Drug Delivery System: A hybrid system comprised of mesoporous nanosilica MCM-41 (as a nano-container) & a pH-sensitive polymer (as smart reversible gatekeepers): Preparation, characterization and in vitro releas. Eur. J. Pharm. Sci. 2016, 93, 64–73. [Google Scholar]
- Niedermayer, S.; Weiss, V.; Herrmann, A.; Schmidt, A.; Datz, S.; Müller, K.; Wagner, E.; Bein, T.; Bräuchle, C. Multifunctional polymer-capped mesoporous silica nanoparticles for pH-responsive targeted drug delivery. Nanoscale 2015, 7, 7953–7964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, T.; Kong, J.L. In situ monitoring of intracellular controlled drug release from mesoporous silica nanoparticles coated with pH-responsive charge-reversal polymer. ACS Appl. Mater. Interfaces 2014, 6, 17446–17453. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tang, C.; Yin, C. pH-Responsive Core-Shell Structured Nanoparticles for Triple-Stage Targeted Delivery of Doxorubicin to Tumors. ACS Appl. Mater. Interfaces 2016, 8, 23498–23508. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pu, F.; Huang, Z.; Liu, Z.; Ren, J.; Qu, X. Stimuli-responsive controlled-release system using quadruplex DNA-capped silica nanocontainers. Nucleic Acids Res. 2011, 39, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Findenegg, G.H. Lysozyme as a pH-responsive valve for the controlled release of guest molecules from mesoporous silica. Langmuir 2012, 28, 17578–17584. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Di, J.; Cao, C.; Zhao, Y.; Ma, Y.; Luo, J.; Wen, Y.; Song, W.; Song, Y.; Jiang, L. A pH-driven DNA nanoswitch for responsive controlled release. Chem. Commun. 2011, 47, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, S.; He, X.; Wang, K.; Qiu, P.; He, D. Co-loading of coralyne and indocyanine green into adenine DNA-functionalized mesoporous silica nanoparticles for pH- and near-infrared-responsive chemothermal treatment of cancer cells. J. Mater. Chem. B 2014, 2, 6064–6071. [Google Scholar] [CrossRef]
- He, D.G.; He, X.X.; Wang, K.M.; Chen, M.A.; Zhao, Y.X.; Zou, Z. Intracellular acid-triggered drug delivery system using mesoporous silica nanoparticles capped with T-Hg2+-T base pairs mediated duplex DNA. J. Mater. Chem. B 2013, 1, 1552–1560. [Google Scholar] [CrossRef]
- Murai, K.; Higuchi, M.; Kinoshita, T.; Nagata, K.; Kato, K. Design of a nanocarrier with regulated drug release ability utilizing a reversible conformational transition of a peptide, responsive to slight changes in pH. Phys. Chem. Chem. Phys. 2013, 15, 11454–11460. [Google Scholar] [CrossRef] [PubMed]


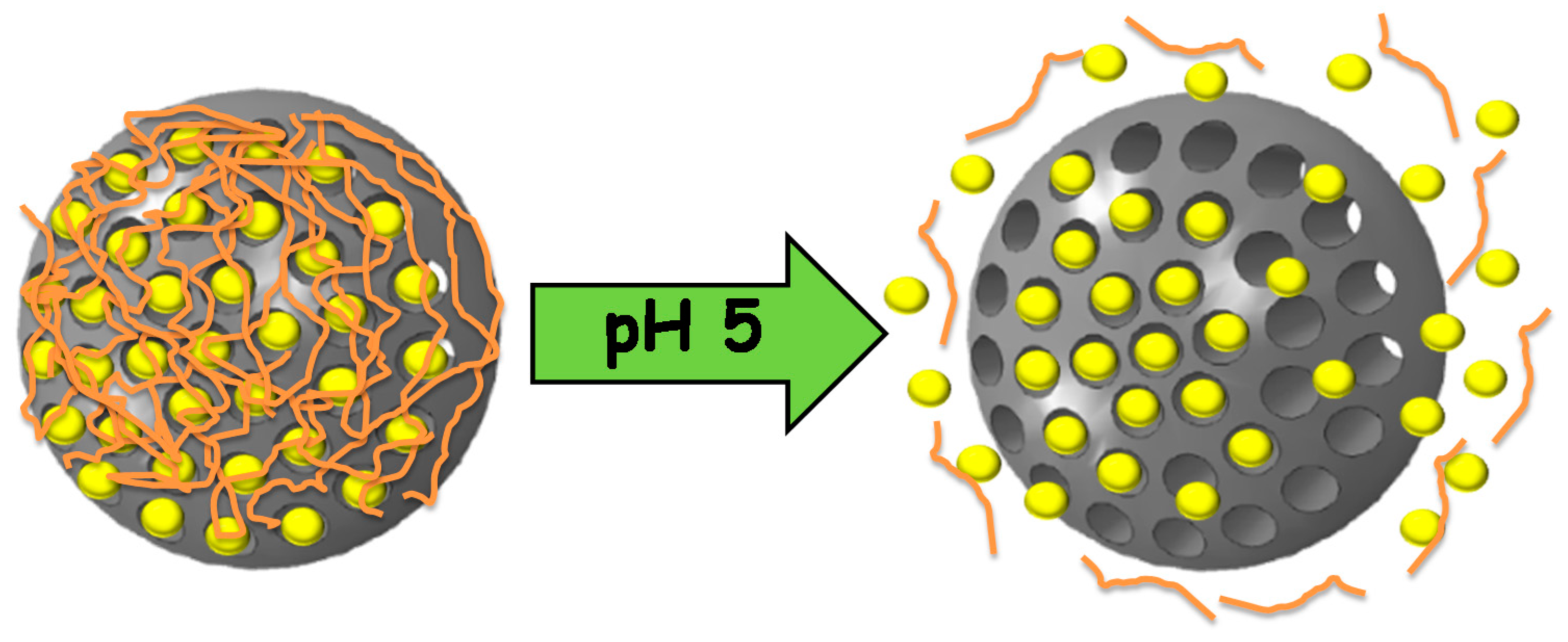
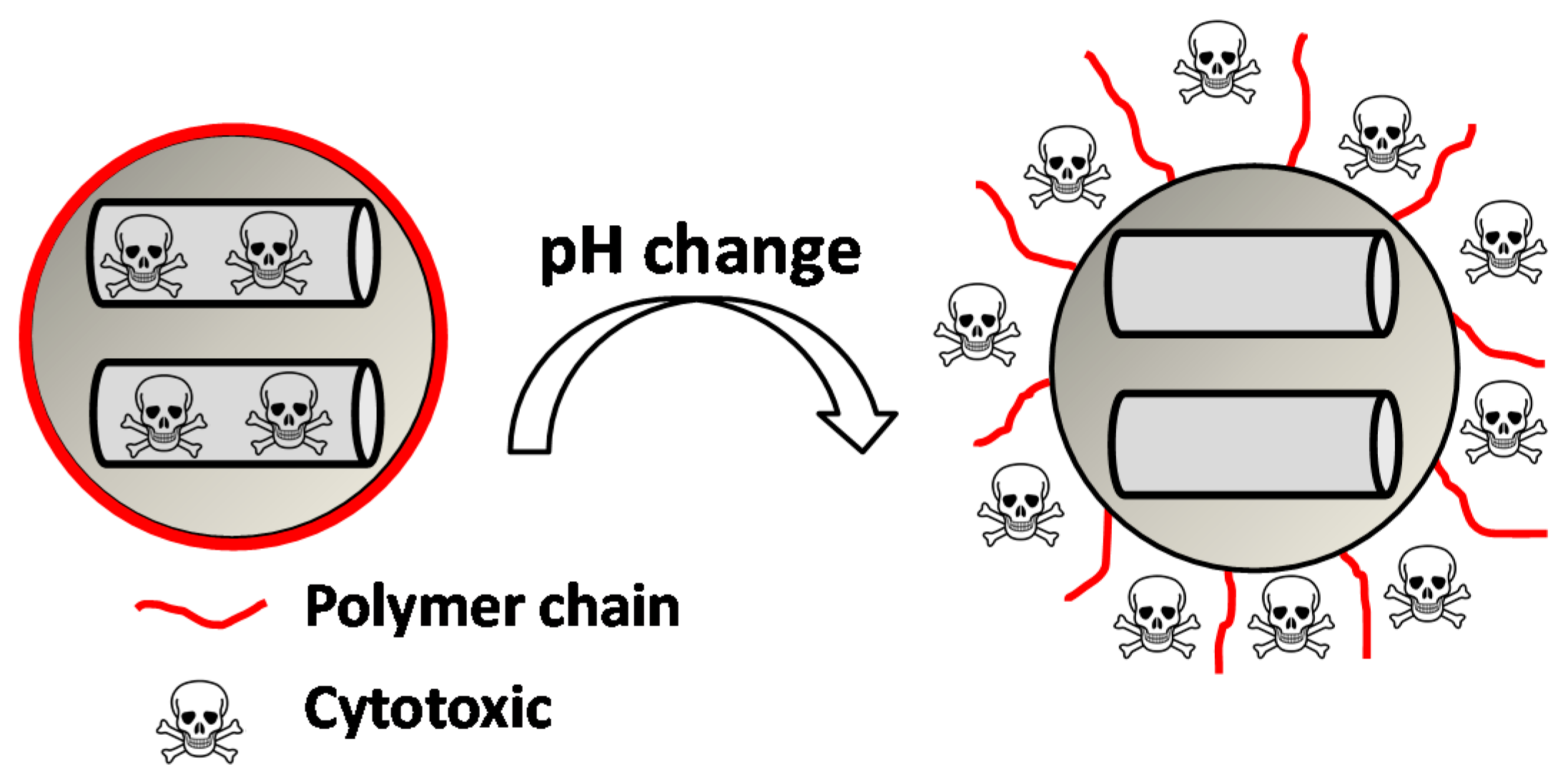
| pH-Responsive Bond | pH-Responsive Mechanism | References |
|---|---|---|
| Hydrazone |  | [70,71,72,73,74,75,76,77] |
| Acetal |  | [78,79,80,81,82] |
| Imine |  | [83,84,85,86,87,88,89] |
| Ester-based |  | [90,91,92,93,94,95,96,97,98,99] |
| Citraconic |  | [100,101] |
| Polycation | Structure | References |
|---|---|---|
| Chitosan |  | [140,141,142,143] |
| Poly(allylamine hydrochloride) |  | [144,145,146] |
| Polyethyleneimine |  | [147,148] |
| Polyvinyl pyridine |  | [149] |
| Polyanion | Structure | References |
| Poly(styrene sulfonate) |  | [146] |
| Poly(acrylic acid-co-itaconic acid) |  | [150] |
| Anionic Polymers | Structure | References |
|---|---|---|
| PAA |  | [167,168,169,170,171,172] |
| PMAA |  | [173,174,175,176,177,178,179,180] |
| Cationic Polymers | Structure | References |
| Polyamine-based | -- | [181,182,183] |
| PDMAEA |  | [184,185] |
| PPEMA |  | [186] |
| PDEAEMA |  | [187,188,189,190] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery. Bioengineering 2017, 4, 3. https://doi.org/10.3390/bioengineering4010003
Gisbert-Garzarán M, Manzano M, Vallet-Regí M. pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery. Bioengineering. 2017; 4(1):3. https://doi.org/10.3390/bioengineering4010003
Chicago/Turabian StyleGisbert-Garzarán, Miguel, Miguel Manzano, and María Vallet-Regí. 2017. "pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery" Bioengineering 4, no. 1: 3. https://doi.org/10.3390/bioengineering4010003
APA StyleGisbert-Garzarán, M., Manzano, M., & Vallet-Regí, M. (2017). pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery. Bioengineering, 4(1), 3. https://doi.org/10.3390/bioengineering4010003