Metabolic Engineering Strategies for Co-Utilization of Carbon Sources in Microbes
Abstract
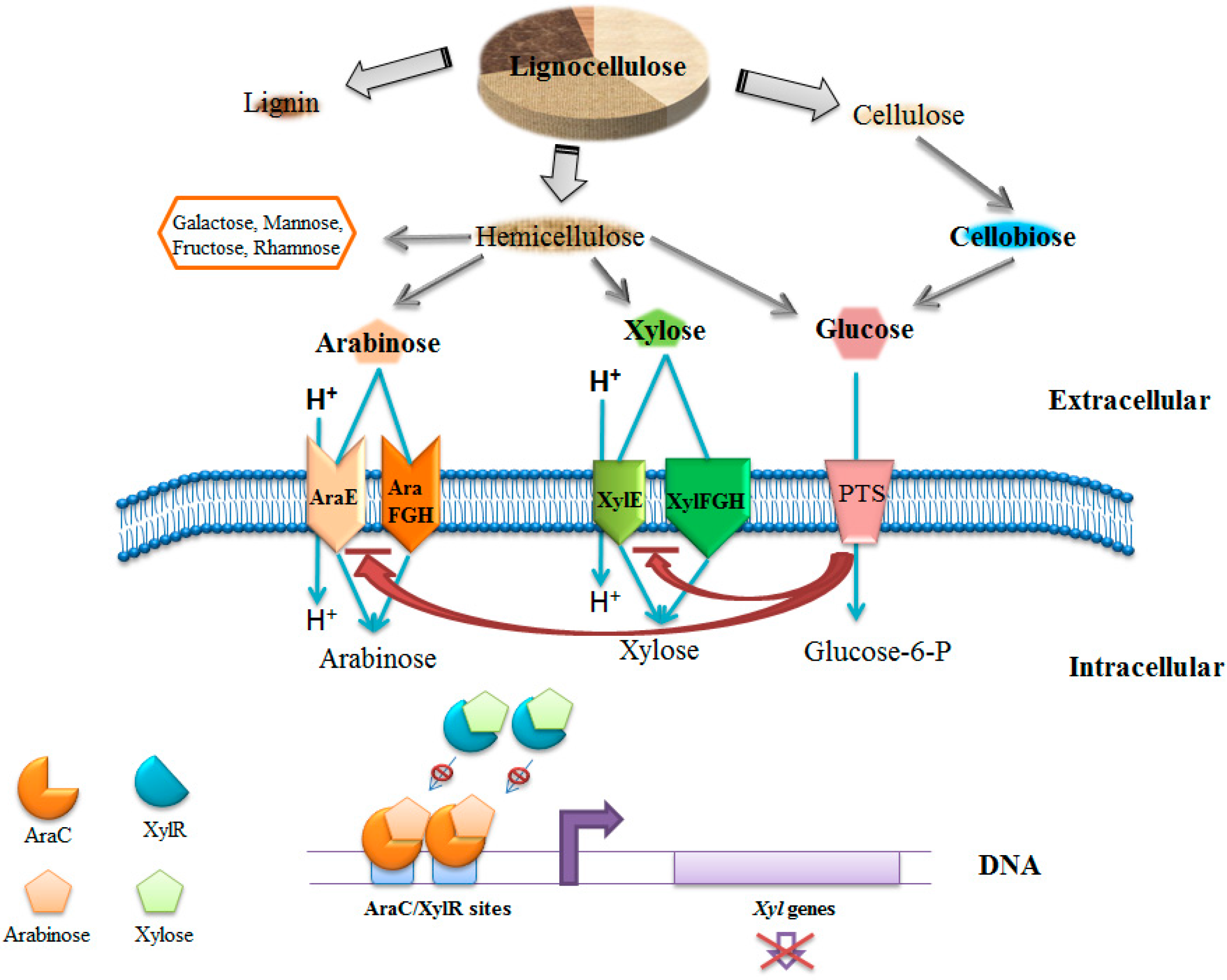
:1. Introduction
2. Glucose and Pentoses from Lignocellulosic Biomass
| Microbe | Strategy | Carbohydrates | Product | References |
|---|---|---|---|---|
| Escherichia coli | Inactivation of ptsG gene | Glucose and xylose | Ethanol | [13] |
| Deletion of ptsG gene | Glucose and xylose | Polyhydroxyalkanoates | [10] | |
| Replacement of native cyclic AMP receptor protein with a cyclic AMP-independent mutant | Glucose and xylose | Xylitol | [14] | |
| Engineering of chb and asc operons and adaptive evolution | Cellobiose and xylose | - | [37] | |
| Expression of xylR at the appropriate level | Xylose and arabinose | Ethanol | [16] | |
| Deletion of araC, constitutive expression of genes required for pentose metabolism and adaptive evolution | Glucose and xylose | Xylitol | [15] | |
| Inactivation of ptsHIcrr gene; overexpression of galP | Glucose, xylose, and arabinose | Cinnamic and p-hydroxycinnamicacid | [11] | |
| Saccharomyces cerevisiae | Expression of the xylose isomerase; Overexpression of XKS1, RPE1, RKI1, TAL1, and TKL1; Deletion of GRE3 and COX4 genes; Adaptive evolution | Glucose and xylose | Ethanol | [38] |
| Construction of a growth-based screening system for mutant hexose transporters | Glucose and xylose | - | [19] | |
| Deletion of d-ribulose-5-phosphate 3-epimerase | Glucose and xylose | Ethanol | [20] | |
| Maintaining glucose in the useful concentration range in fed-batch reaction | Glucose and xylose | Ethanol | [39] | |
| Expression of xylose reductase, xylitol dehydrogenase and xylulokinase; Engineering of hexose transporters | Glucose and xylose | Ethanol | [18] | |
| Evolutionary engineering strategy based on repeated batch cultivation with repeated cycles of consecutive growth | Glucose, xylose, and arabinose | Ethanol | [24] | |
| Evolutionary engineering via continuous culture using xylose and arabinose as limiting carbon sources | Xylose and arabinose | Ethanol | [23] | |
| Expression of a cellodextrin transporter, intracellular β-glucosidase and xylose reductase and optimization of the expression | Cellobiose and xylose | Xylitol Ethanol | [22] | |
| Integration of the fermentation pathways of cellobiose and xylose and an acetic acid reduction pathway | Cellobiose, xylose, and acetic acid | Ethanol | [40] | |
| Saccharomyces pastorianus | Co-expression of all three classes of cellulase genes with the xyl1/xdh1/XKS genes | Xylose and cellulose | Alcohol | [41] |
| Clostridium acetobutylicum | CcpA mutagenesis | Glucose and xylose | Acetone, Butanol, Ethanol | [25,26] |
| Inactivation of glcG and overexpression of the rate-limiting steps in xylose pathway | Glucose, xylose, and arabinose | Acetone, Butanol, Ethanol | [27] | |
| Clostridium sp. Strain BOH3 | Expression of xylose isomerase and xylulokinase | Glucose and xylose | Butanol Riboflavin | [28] |
| Clostridium tyrobutyricum | Overexpression of xylT, xylA, and xylB | Glucose and xylose | n-Butanol | [42] |
| Corynebacterium glutamicum | Expression of araBAD operon and/or the xylA gene from Escherichia coli | Glucose, xylose, and arabinose | Amino Acid | [43] |
| Enterococcus mundtii QU 25 | Maintaining the glucose concentration lower than 25 g/L | Glucose and xylose | L-Lactic acid | [44] |
| Cryptococcus curvatus | Decreasing glucose concentration | Glucose, xylose, and cellobiose | Microbial lipid | [45] |

3. Glucose and Galactose from Marine Plant Biomass
4. Glucose and Non-Carbohydrates
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhatia, L.; Johri, S.; Ahmad, R. An economic and ecological perspective of ethanol production from renewable agro waste. AMB Express 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Desai, S.H.; Atsumi, S. Two-dimensional isobutyl acetate production pathways to improve carbon yield. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Quarterman, J.; Kim, S.R.; Cate, J.H.D.; Jin, Y.S. Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Vinuselvi, P.; Kim, M.K.; Lee, S.K.; Ghim, C.M. Rewiring carbon catabolite repression for microbial cell factory. BMB Rep. 2012, 45, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Da Costa Sousa, L.; Chundawat, S.P.; Balan, V.; Dale, B.E. ‘Cradle-to-grave’ assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 2009, 20, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Karimova, G.; Ladant, D.; Ullmann, A. Relief of catabolite repression in a cAMP-independent catabolite gene activator mutant of Escherichia coli. Res. Microbiol. 2004, 155, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, J. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2008, 11, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, Q.; Wang, P.G.; Qi, Q.S. A novel-designed Escherichia coli for the production of various polyhydroxyalkanoates from inexpensive substrate mixture. Appl. Microbiol. Biotechnol. 2007, 75, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Tah, A.; Martínez, L.M.; Hernández-Chávez, G.; Rocha, M.; Martínez, A.; Bolívar, F.; Gosset, G. Production of cinnamic and p-hydroxycinnamic acid from sugar mixtures with engineered Escherichia coli. Microb. Cell Fact. 2015, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montalvo, V.; Martínez, A.; Hernández- Chávez, G.; Bolivar, F.; Valle, F.; Gosset, G. Expression of galp and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol. Bioeng. 2003, 83, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; Lee, H.M.; Guo, H.J.; Wang, Z.W.; Lin, L.J.; Chao, Y.P. Systematic approach to engineer Escherichia coli pathways for co-utilization of a glucose-xylose mixture. J. Agric. Food Chem. 2013, 61, 7583–7590. [Google Scholar] [CrossRef] [PubMed]
- Cirino, P.C.; Chin, J.W.; Ingram, L.O. Engineering Escherichia coli for xylitol production from glucose-xylose mixtures. Biotechnol. Bioeng. 2006, 95, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Choi, B.Y.; Ryu, Y.S.; Jung, S.H.; Park, J.M.; Kim, G.H.; Lee, S.K. Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli. Metab. Eng. 2015, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Benke, P.I.; Batth, T.S.; Bokinsky, G.; Petzold, C.J.; Adams, P.D.; Keasling, J.D. Supplementation of intracellular XylR leads to coutilization of hemicellulose sugars. Appl. Environ. Microbiol. 2012, 78, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.Z.; Chao, R.; Zhao, H.M. Metabolic engineering of a Saccharomyces cerevisiae strain capable of simultaneously utilizing glucose and galactose to produce enantiopure (2R,3R)-butanediol. Metab. Eng. 2014, 23, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.L.; Matsushika, A.; de Sales, B.B.; Goshima, T.; Bon, E.P.S.; Stambuk, B.U. Xylose and xylose/glucose co-fermentation by recombinant Saccharomyces cerevisiae strains expressing individual hexose transporters. Enzym. Microb. Technol. 2014, 63, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Farwick, A.; Bruder, S.; Schadeweg, V.; Oreb, M.; Boles, E. Engineering of yeast hexose transporters to transport D-xylose without inhibition by D-glucose. Proc. Natl. Acad. Sci. USA 2014, 111, 5159–5164. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.H.; Song, H.; Li, B.Z.; Yuan, Y.J. Deletion of d-ribulose-5-phosphate 3-epimerase (RPE1) induces simultaneous utilization of xylose and glucose in xylose-utilizing Saccharomyces cerevisiae. Biotechnol. Lett. 2015, 37, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Ha, S.J.; Kim, S.R.; Lee, W.H.; Galazka, J.M.; Cate, J.H.D.; Jin, Y.S. Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyces cerevisiae. Metab. Eng. 2013, 15, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Li, B.Z.; Shen, M.H.; Hu, M.L.; Song, H.; Yuan, Y.J. Optimization of CDT-1 and XYL1 expression for balanced co-production of ethanol and xylitol from cellobiose and xylose by engineered Saccharomyces cerevisiae. PLoS ONE 2013, 8, e68317. [Google Scholar]
- Sanchez, R.G.; Karhumaa, K.; Fonseca, C.; Nogué, V.S.; Almeida, J.R.M.; Larsson, C.U.; Bengtsson, O.; Bettiga, M.; Hahn-Hägerdal, B.; Gorwa-Grauslund, M.F. Research improved xylose and arabinose utilization by an industrial recombinant Saccharomyces cerevisiae strain using evolutionary engineering. Biotechnol. Biofuels 2010, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisselink, H.W.; Toirkens, M.J.; Wu, Q.; Pronk, J.T.; van Maris, A.J.A. Novel evolutionary engineering approach for accelerated utilization of glucose, xylose, and arabinose mixtures by engineered Saccharomyces cerevisiae strains. Appl. Environ. Microbiol. 2009, 75, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Gu, Y.; Hu, S.Y.; Wu, Y.; Wang, P.; Yang, Y.L.; Yang, C.; Yang, S.; Jiang, W.H. Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum. Metab. Eng. 2010, 12, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.P.; Ren, C.; Yang, C.; Yang, S.; Gu, Y.; Jiang, W.H. Molecular modulation of pleiotropic regulator CcpA for glucose and xylose coutilization by solvent-producing Clostridium acetobutylicum. Metab. Eng. 2015, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Gu, Y.; Ning, Y.Y.; Yang, Y.L.; Mitchell, W.J.; Jiang, W.H.; Yang, S. Confirmation and elimination of xylose metabolism bottlenecks in glucose phosphoenolpyruvate-dependent phosphotransferase system-deficient Clostridium acetobutylicum for simultaneous utilization of glucose, xylose, and arabinose. Appl. Environ. Microbiol. 2011, 77, 7886–7895. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.X.; Wu, Y.R.; He, J.Z. Simultaneous fermentation of glucose and xylose to butanol by Clostridium sp. strain BOH3. Appl. Environ. Microbiol. 2014, 80, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Quarterman, J.; Jin, Y.S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.J.; Wei, Q.S.; Kim, S.R.; Galazka, J.M.; Cate, J.; Jin, Y.S. Cofermentation of cellobiose and galactose by an engineered Saccharomyces cerevisiae strain. Appl. Environ. Microbiol. 2011, 77, 5822–5825. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.J.; Bae, H.J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour. Technol. 2009, 100, 6658–6660. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.G.; Seo, S.W.; Jung, G.Y. Engineered Escherichia coli for simultaneous utilization of galactose and glucose. Bioresour. Technol. 2013, 135, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, K.; de Anda, R.; Hernández, G.; Escalante, A.; Gosset, G.; Ramírez, O.T.; Bolívar, F.G. Coutilization of glucose and glycerol enhances the production of aromatic compounds in an Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system. Microb. Cell Fact. 2008, 7. [Google Scholar] [CrossRef] [PubMed]
- Shiue, E.; Brockman, I.M.; Prather, K.L.J. Improving product yields on D-glucose in Escherichia coli via knockout of pgi and zwf and feeding of supplemental carbon sources. Biotechnol. Bioeng. 2015, 112, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Sabido, A.; Sigala, J.C.; Hernández-Chávez, G.; Flores, N.; Gosset, G.; Bolívar, F. Physiological and transcriptional characterization of Escherichia coli strains lacking interconversion of phosphoenolpyruvate and pyruvate when glucose and acetate are coutilized. Biotechnol. Bioeng. 2014, 111, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Vinuselvi, P.; Lee, S.K. Engineered Escherichia coli capable of co-utilization of cellobiose and xylose. Enzym. Microb. Technol. 2012, 50, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, X.; Peng, B.Y.; Chen, L.Y.; Hou, J.; Bao, X.M. An efficient xylose-fermenting recombinant Saccharomyces cerevisiae strain obtained through adaptive evolution and its global transcription profile. Appl. Microbiol. Biotechnol. 2012, 96, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Krahulec, S.; Petschacher, B.; Wallner, M.; Longus, K.; Klimacek, M.; Nidetzky, B. Fermentation of mixed glucose-xylose substrates by engineered strains of Saccharomyces cerevisiae: role of the coenzyme specificity of xylose reductase, and effect of glucose on xylose utilization. Microb. Cell Fact. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Oh, E.J.; Million, G.; Cate, J.H.D.; Jin, Y.S. Simultaneous utilization of cellobiose, xylose, and acetic acid from lignocellulosic biomass for biofuel production by an engineered yeast platform. ACS Synth. Biol. 2015, 4, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Kricka, W.; James, T.C.; Fitzpatrick, J.; Bond, U. Engineering Saccharomyces pastorianus for the co-utilisation of xylose and cellulose from biomass. Microb. Cell Fact. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, M.; Tang, I.C.; Yang, S.T. Metabolic engineering of Clostridium tyrobutyricum for n-butanol production through co-utilization of glucose and xylose. Biotechnol. Bioeng. 2015, 112, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Meiswinkel, T.M.; Wendisch, V.F.; Nampoothiri, K.M. Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2011, 92, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Xiao, Y.; Tashiro, Y.; Wang, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Fed-batch fermentation for enhanced lactic acid production from glucose/xylose mixture without carbon catabolite repression. J. Biosci. Bioeng. 2015, 119, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.C.; Zheng, Y.B.; Xiong, X.C.; Chen, S.L. Co-utilization of glucose, xylose and cellobiose by the oleaginous yeast Cryptococcus curvatus. Biomass Bioenerg. 2014, 71, 340–349. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Shen, X.; Yuan, Q.; Yan, Y. Metabolic Engineering Strategies for Co-Utilization of Carbon Sources in Microbes. Bioengineering 2016, 3, 10. https://doi.org/10.3390/bioengineering3010010
Wu Y, Shen X, Yuan Q, Yan Y. Metabolic Engineering Strategies for Co-Utilization of Carbon Sources in Microbes. Bioengineering. 2016; 3(1):10. https://doi.org/10.3390/bioengineering3010010
Chicago/Turabian StyleWu, Yifei, Xiaolin Shen, Qipeng Yuan, and Yajun Yan. 2016. "Metabolic Engineering Strategies for Co-Utilization of Carbon Sources in Microbes" Bioengineering 3, no. 1: 10. https://doi.org/10.3390/bioengineering3010010





