Applying Acylated Fucose Analogues to Metabolic Glycoengineering
Abstract
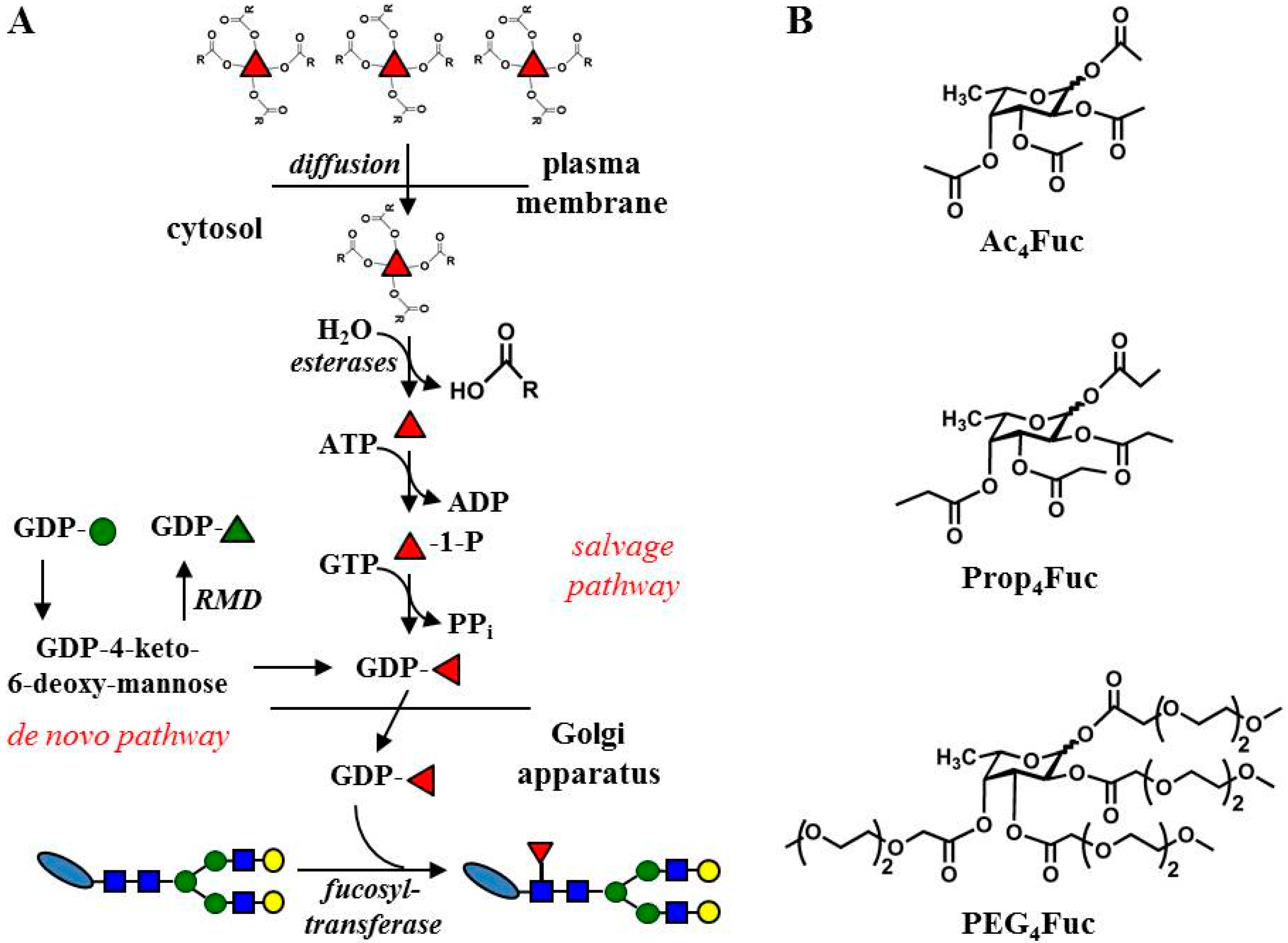
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Syntheses of Fucose Analogues
2.3. Cell Culture
2.4. Cell Treatment with Acylated Fucose Analogues
2.5. Measurement of Cell Viability and Impact on Recombinant Protein Expression
2.6. Isolation of Membrane Proteins
2.7. Release and Separation of N-Linked Glycans
2.8. Permethylation and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry
2.9. Trastuzumab Purification
2.10. Lectin Blotting
2.11. High-pH Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD) Monosaccharide Analysis
2.12. FcγRIIIa Binding Assay
3. Results and Discussion
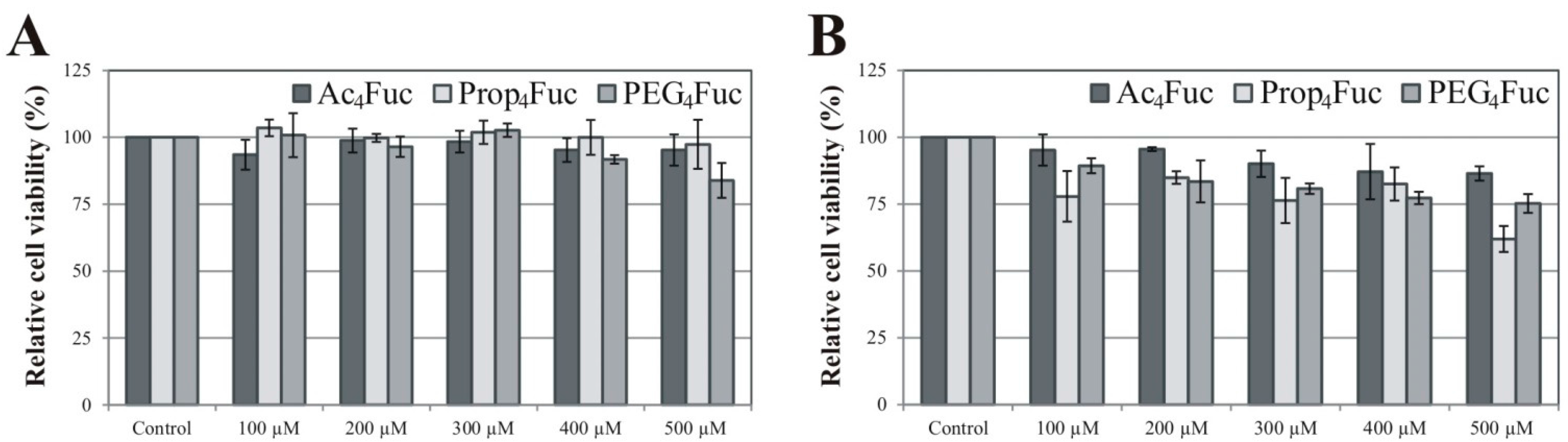
3.1. Effects of Fucose Analogues on HEK-293T Cell Viability and Recombinant A1AT Expression
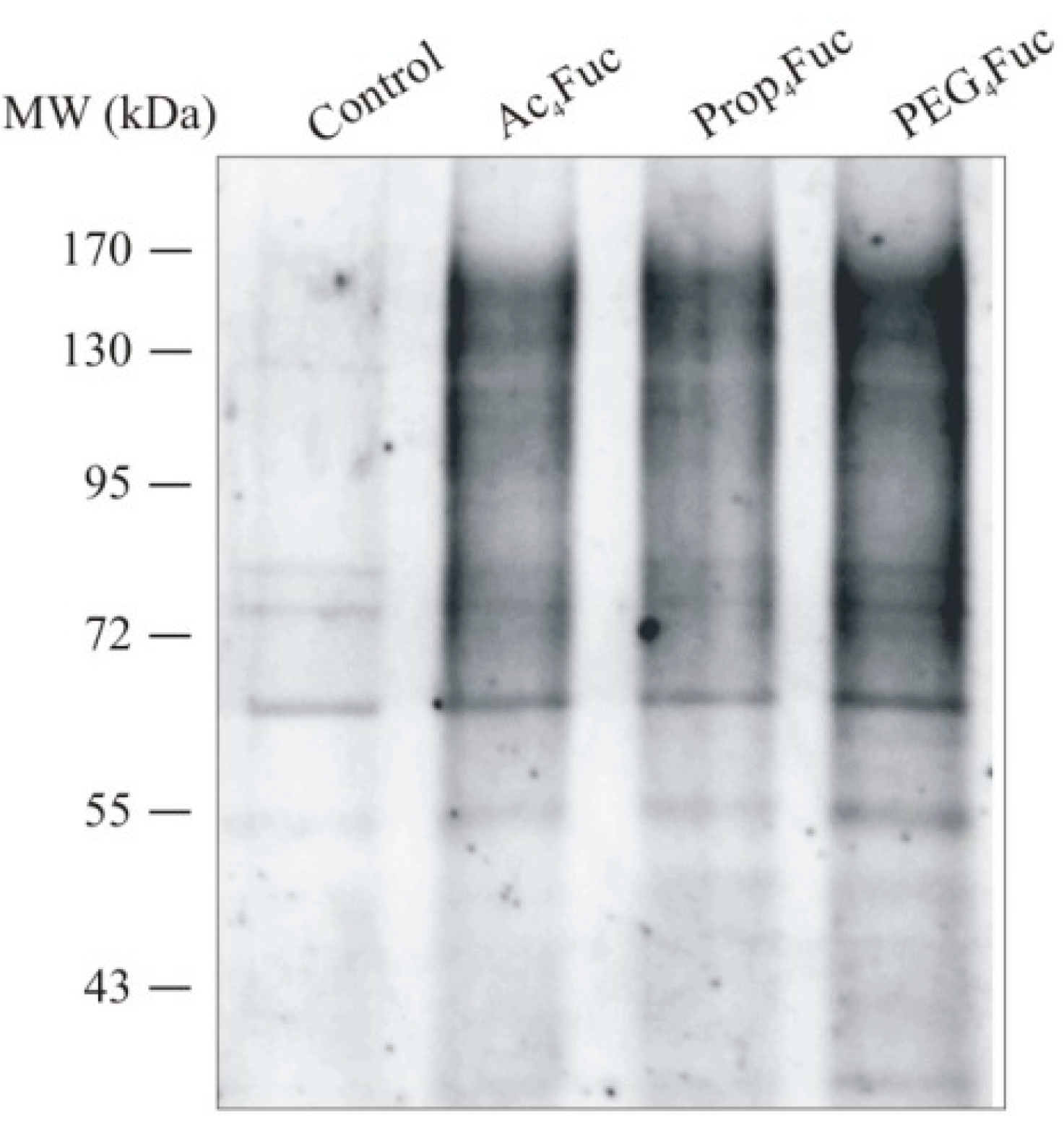
3.2. Incorporation of Fucose Analogues on CHO RMD Cell Membrane

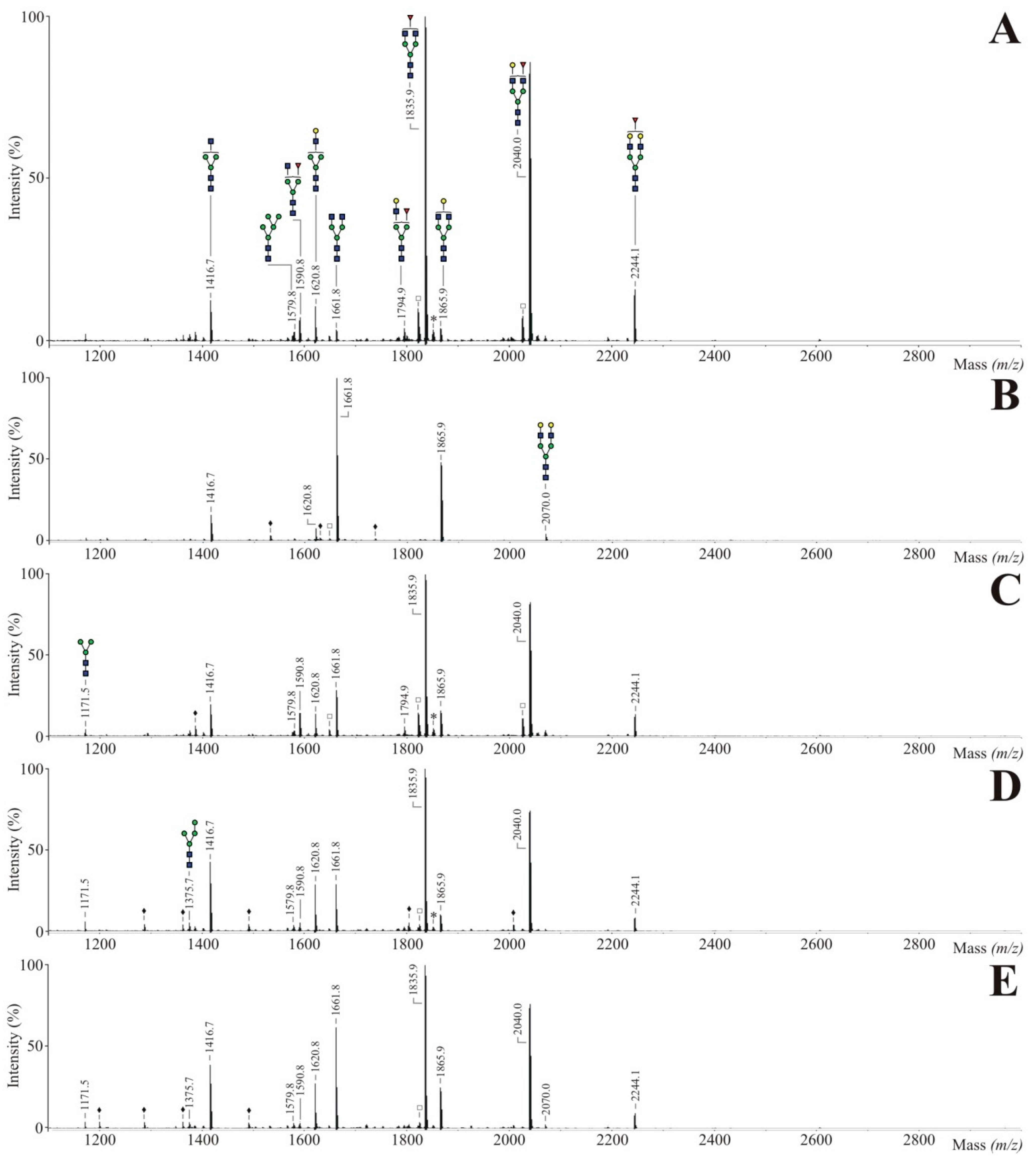
3.3. Incorporation of Fucose Analogues on Recombinant Trastuzumab Expressed in CHO Cells
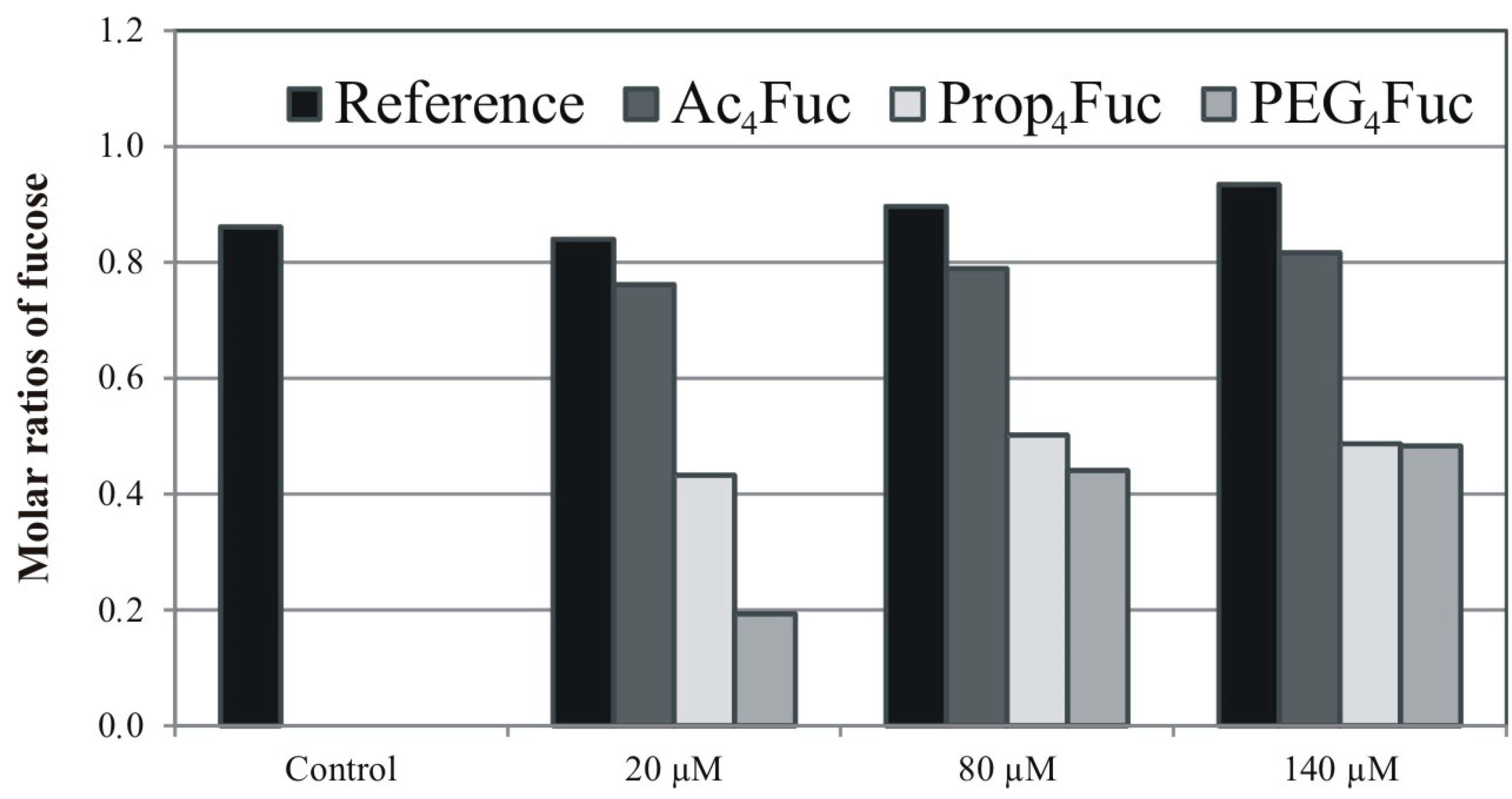
3.4. Effects of Sequential Trastuzumab Fucosylation on FcγRIIIa Binding
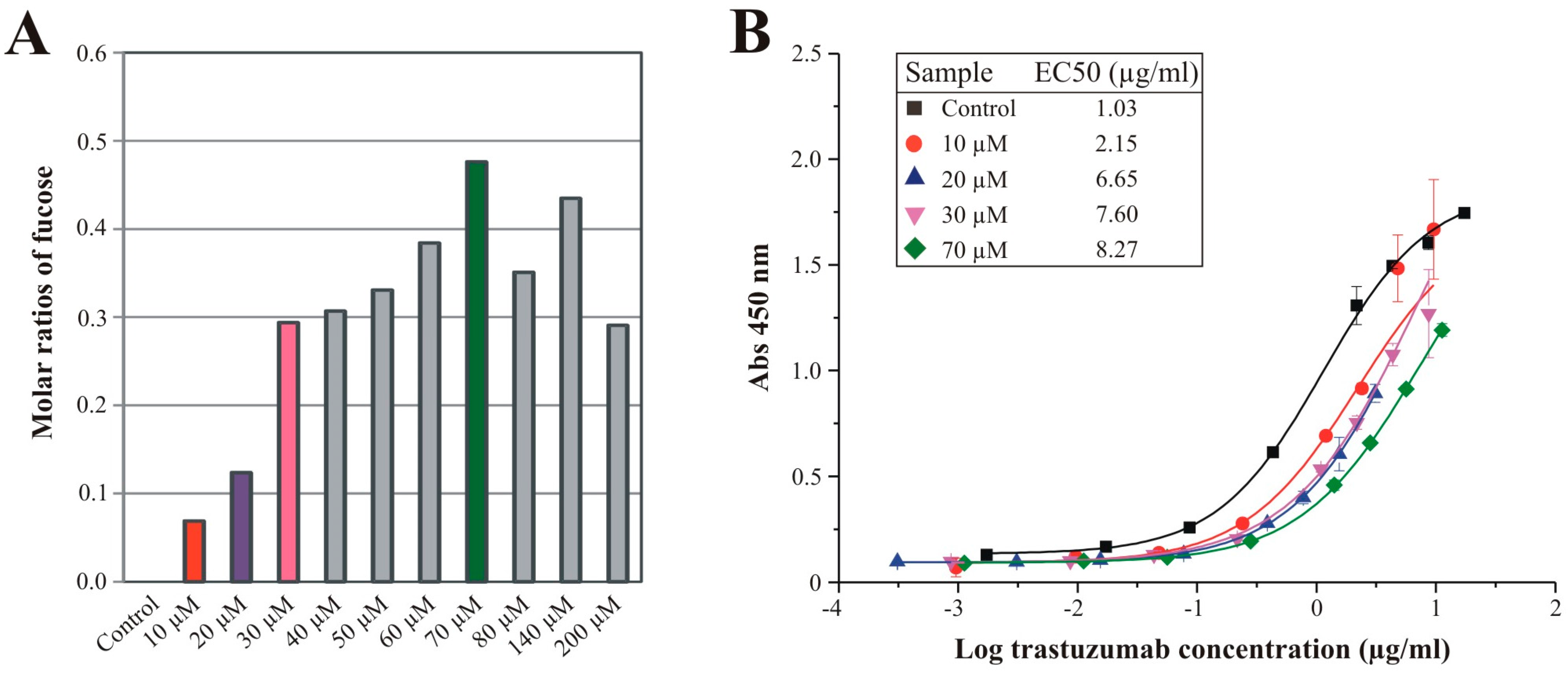
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Kayser, H.; Zeitler, R.; Kannicht, C.; Grunow, D.; Nuck, R.; Reutter, W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J. Biol. Chem. 1992, 267, 16934–16938. [Google Scholar] [PubMed]
- Aich, U.; Yarema, K.J. Non-Natural Sugar Analogues: Chemical Probes for Metabolic Oligosaccharide Engineering Metabolic Engineering Non-Natural Sugar Glycosylation Pathways Mucins O-GlcNAc Protein Modification Chemoselective Ligation; Fraser-Reid, B., Tatsuta, K., Thiem, J., Eds.; Glycoscience, Springer: Berlin, Germany; Heidelberg, Germany, 2008; pp. 2133–2190. [Google Scholar]
- Du, J.; Meledeo, M.A.; Wang, Z.; Khanna, H.S.; Paruchuri, V.D.; Yarema, K.J. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 2009, 19, 1382–1401. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Rabuka, D.; Hubbard, S.C.; Laughlin, S.T.; Argade, S.P.; Bertozzi, C.R. A chemical reporter strategy to probe glycoprotein fucosylation. J. Am. Chem. Soc. 2006, 128, 12078–12079. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Hsu, T.L.; Itoh, T.; Sugiyama, M.; Hanson, S.R.; Vogt, P.K.; Wong, C.H. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 12371–12376. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.L.; Hanson, S.R.; Kishikawa, K.; Wang, S.K.; Sawa, M.; Wong, C.H. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc. Natl. Acad. Sci. USA 2007, 104, 2614–2619. [Google Scholar] [CrossRef] [PubMed]
- Hinderlich, S.; Stasche, R.; Zeitler, R.; Reutter, W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Purification and characterization of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. J. Biol. Chem. 1997, 272, 24313–24318. [Google Scholar] [CrossRef] [PubMed]
- Keppler, O.T.; Hinderlich, S.; Langner, J.; Schwartz-Albiez, R.; Reutter, W.; Pawlita, M. UDP-GlcNAc 2-epimerase: A regulator of cell surface sialylation. Science 1999, 284, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Niethamer, T.K.; Yardeni, T.; Leoyklang, P.; Ciccone, C.; Astiz-Martinez, A.; Jacobs, K.; Dorward, H.M.; Zerfas, P.M.; Gahl, W.A.; Huizing, M. Oral monosaccharide therapies to reverse renal and muscle hyposialylation in a mouse model of GNE myopathy. Mol. Genet. Metab. 2012, 107, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Nishino, I.; Noguchi, S. Sialic acid supplementation therapy for distal myopathy with rimmed vacuoles (GNE myopathy). Rinsho Shinkeigaku 2012, 52, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- De Lonlay, P.; Seta, N. The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochim. Biophys. Acta 2009, 1792, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, T.; Luhn, K.; Srikrishna, G.; Freeze, H.H.; Harms, E.; Vestweber, D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 1999, 94, 3976–3985. [Google Scholar] [PubMed]
- Gu, X.; Wang, D.I. Improvement of interferon-gamma sialylation in Chinese hamster ovary cell culture by feeding of N-acetylmannosamine. Biotechnol. Bioeng. 1998, 58, 642–648. [Google Scholar] [CrossRef]
- Bork, K.; Reutter, W.; Weidemann, W.; Horstkorte, R. Enhanced sialylation of EPO by overexpression of UDP-GlcNAc 2-epimerase/ManAc kinase containing a sialuria mutation in CHO cells. FEBS Lett. 2007, 581, 4195–4198. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Lawrence, S.; Hinderlich, S.; Yarema, K.J.; Lee, Y.C.; Betenbaugh, J.M. Engineering sialic acid synthetic ability into insect cells: Identifying metabolic bottlenecks and devising strategies to overcome them. Biochemistry 2003, 42, 15215–15225. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Fritz, T.A.; Taylor, W.H.; Esko, J.D. Disaccharide uptake and priming in animal cells: Inhibition of sialyl Lewis X by acetylated Galβ1—>4GlcNAcβ-O-naphthalenemethanol. Proc. Natl. Acad. Sci. USA 1995, 92, 3323–3327. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.B.; Teng, H.; Rhee, J.K.; Lahar, N.; Baskaran, G.; Yarema, K.J. Characterization of the cellular uptake and metabolic conversion of acetylated N-acetylmannosamine (ManNAc) analogues to sialic acids. Biotechnol. Bioeng. 2004, 85, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Sampathkumar, S.G.; Jones, M.B.; Rhee, J.K.; Baskaran, G.; Goon, S.; Yarema, K.J. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acyl-modified N-acetylmannosamine analogs in Jurkat cells. J. Biol. Chem. 2004, 279, 18342–18352. [Google Scholar] [CrossRef] [PubMed]
- Von Horsten, H.H.; Ogorek, C.; Blanchard, V.; Demmler, C.; Giese, C.; Winkler, K.; Kaup, M.; Berger, M.; Jordan, I.; Sandig, V. Production of non-fucosylated antibodies by co-expression of heterologous GDP-6-deoxy-D-lyxo-4-hexulose reductase. Glycobiology 2010, 20, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Rosenlöcher, J.; Sandig, G.; Kannicht, C.; Blanchard, V.; Reinke, S.O.; Hinderlich, S. Recombinant glycoproteins: The impact of cell lines and culture conditions on the generation of protein species. J. Proteom. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reinke, S.O.; Bayer, M.; Berger, M.; Hinderlich, S.; Blanchard, V. The analysis of N-glycans of cell membrane proteins from human hematopoietic cell lines reveals distinctions in their pattern. Biol. Chem. 2012, 393, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.O.; Bayer, M.; Berger, M.; Blanchard, V.; Hinderlich, S. Analysis of cell surface N-glycosylation of the human embryonic kidney 293t cell line. J. Carbohydr. Chem. 2011, 30, 218–232. [Google Scholar] [CrossRef]
- Wada, Y.; Azadi, P.; Costello, C.E.; Dell, A.; Dwek, R.A.; Geyer, H.; Geyer, R.; Kakehi, K.; Karlsson, N.G.; Kato, K.; et al. Comparison of the methods for profiling glycoprotein glycans—HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study. Glycobiology 2007, 17, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Dell, A.; Khoo, K.-.; Panico, M.; McDowell, R.A.; Etienne, A.T.; Reason, A.J.; Morris, H.R. Glycobiology: A Practical Approach; Fukuda, M., Kobata, A., Eds.; IRL Press at Oxford University Press: Oxford, UK, 1993; pp. 187–222. [Google Scholar]
- Frisch, E.; Kaup, M.; Egerer, K.; Weimann, A.; Tauber, R.; Berger, M.; Blanchard, V. Profiling of endo H-released serum N-glycans using CE-LIF and MALDI-TOF-MS--application to rheumatoid arthritis. Electrophoresis 2011, 32, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Marth, J.D.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. Symbol nomenclature for glycan representation. Proteomics 2009, 9, 5398–5399. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jones, M.B.; Rhee, J.K.; Sampathkumar, S.G.; Yarema, K.J. Establishment of N-acetylmannosamine (ManNAc) analogue-resistant cell lines as improved hosts for sialic acid engineering applications. Biotechnol. Prog. 2004, 20, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, S.G.; Jones, M.B.; Meledeo, M.A.; Campbell, C.T.; Choi, S.S.; Hida, K.; Gomutputra, P.; Sheh, A.; Gilmartin, T.; Head, S.R.; et al. Targeting glycosylation pathways and the cell cycle: Sugar-dependent activity of butyrate-carbohydrate cancer prodrugs. Chem. Biol. 2006, 13, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D.; Atkinson, P.H. Equilibration of fucosyl glycoprotein pools in hela-cells. Biochemistry 1977, 16, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Stanley, P. A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc: Glycopeptide beta-4-N-acetylglucosaminyltransferase III activity. J. Biol. Chem. 1984, 259, 13370–13378. [Google Scholar] [PubMed]
- Sauerzapfe, B.; Krenek, K.; Schmiedel, J.; Wakarchuk, W.W.; Pelantova, H.; Kren, V.; Elling, L. Chemo-enzymatic synthesis of poly-N-acetyllactosamine (poly-LacNAc) structures and their characterization for CGL2-galectin-mediated binding of ECM glycoproteins to biomaterial surfaces. Glycoconj. J. 2009, 26, 141–159. [Google Scholar] [CrossRef] [PubMed]
- North, S.J.; Huang, H.H.; Sundaram, S.; Jang-Lee, J.; Etienne, A.T.; Trollope, A.; Chalabi, S.; Dell, A.; Stanley, P.; Haslam, S.M. Glycomics profiling of Chinese hamster ovary cell glycosylation mutants reveals N-glycans of a novel size and complexity. J. Biol. Chem. 2010, 285, 5759–5775. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A. Trastuzumab—Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarenko, P.V.; Flynn, G.C. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Horstkorte, R.; Weidemann, W. Increasing the sialylation of therapeutic glycoproteins: The potential of the sialic acid biosynthetic pathway. J. Pharm. Sci. 2009, 98, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, D.; Zhang, M.; Hurtado-Ziola, N.; Varki, A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol. Genet. Eng. Rev. 2012, 28, 147–175. [Google Scholar] [CrossRef] [PubMed]
- Möller, H.; Böhrsch, V.; Lucka, L.; Hackenberger, C.P.R.; Hinderlich, S. Efficient metabolic oligosaccharide engineering of glycoproteins by UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) knock-down. Mol. BioSyst. 2011, 7, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenlöcher, J.; Böhrsch, V.; Sacharjat, M.; Blanchard, V.; Giese, C.; Sandig, V.; Hackenberger, C.P.R.; Hinderlich, S. Applying Acylated Fucose Analogues to Metabolic Glycoengineering. Bioengineering 2015, 2, 213-234. https://doi.org/10.3390/bioengineering2040213
Rosenlöcher J, Böhrsch V, Sacharjat M, Blanchard V, Giese C, Sandig V, Hackenberger CPR, Hinderlich S. Applying Acylated Fucose Analogues to Metabolic Glycoengineering. Bioengineering. 2015; 2(4):213-234. https://doi.org/10.3390/bioengineering2040213
Chicago/Turabian StyleRosenlöcher, Julia, Verena Böhrsch, Michael Sacharjat, Véronique Blanchard, Christoph Giese, Volker Sandig, Christian P. R. Hackenberger, and Stephan Hinderlich. 2015. "Applying Acylated Fucose Analogues to Metabolic Glycoengineering" Bioengineering 2, no. 4: 213-234. https://doi.org/10.3390/bioengineering2040213
APA StyleRosenlöcher, J., Böhrsch, V., Sacharjat, M., Blanchard, V., Giese, C., Sandig, V., Hackenberger, C. P. R., & Hinderlich, S. (2015). Applying Acylated Fucose Analogues to Metabolic Glycoengineering. Bioengineering, 2(4), 213-234. https://doi.org/10.3390/bioengineering2040213




