Gait Analysis to Monitor Fracture Healing of the Lower Leg
Abstract
:1. Introduction
2. Materials and Methods
3. Changes in Gait throughout the Healing Process after Lower Leg Fractures
3.1. Spatiotemporal Gait Parameters
3.1.1. Pace
3.1.2. Rhythm
3.1.3. Variability
3.1.4. Asymmetry
3.1.5. Spatiotemporal Gait Parameters Are Associated with Gait Speed
3.2. Kinematics
3.3. Kinetics
3.3.1. Ground Reaction Forces
3.3.2. Joint Moments
3.3.3. Generated Power
3.4. Pedography
3.5. Muscle Activity and Mass
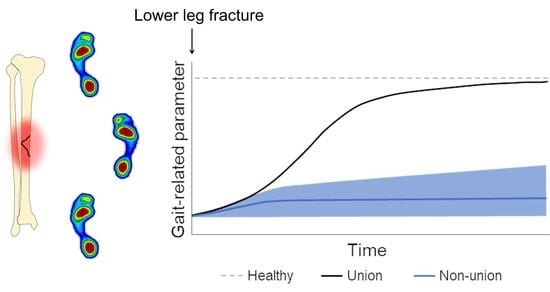
4. Predicting Non-Union Based on Gait
5. Discussion
5.1. Predicting Non-Union Based on Gait
5.2. Suggestions for Future Research Analyzing Gait after Lower Leg Fractures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wennergren, D.; Bergdahl, C.; Ekelund, J.; Juto, H.; Sundfeldt, M.; Möller, M. Epidemiology and Incidence of Tibia Fractures in the Swedish Fracture Register. Injury 2018, 49, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Rydberg, E.M.; Wennergren, D.; Stigevall, C.; Ekelund, J.; Möller, M. Epidemiology of More than 50,000 Ankle Fractures in the Swedish Fracture Register during a Period of 10 Years. J. Orthop. Surg. Res. 2023, 18, 79. [Google Scholar] [CrossRef]
- Sarmiento, A.; Latta, L. The Evolution of Functional Bracing of Fractures. J. Bone Jt. Surg. 2006, 88, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eickhoff, A.M.; Cintean, R.; Fiedler, C.; Gebhard, F.; Schütze, K.; Richter, P. Influence of Weight Bearing on Postoperative Complications after Surgical Treatment of the Lower Extremity. Z. Orthop. Unf. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ganse, B.; Yang, P.F.; Gardlo, J.; Gauger, P.; Kriechbaumer, A.; Pape, H.C.; Koy, T.; Müller, L.P.; Rittweger, J. Partial Weight Bearing of the Tibia. Injury 2016, 47, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed Union and Nonunions: Epidemiology, Clinical Issues, and Financial Aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Warschawski, Y.; Elbaz, A.; Segal, G.; Norman, D.; Haim, A.; Jacov, E.; Grundshtein, A.; Steinberg, E. Gait Characteristics and Quality of Life Perception of Patients Following Tibial Plateau Fracture. Arch. Orthop. Trauma Surg. 2015, 135, 1541–1546. [Google Scholar] [CrossRef]
- Elsoe, R.; Larsen, P. Asymmetry in Gait Pattern Following Bicondylar Tibial Plateau Fractures—A Prospective One-Year Cohort Study. Injury 2017, 48, 1657–1661. [Google Scholar] [CrossRef]
- Tian, R.; Zheng, F.; Zhao, W.; Zhang, Y.; Yuan, J.; Zhang, B.; Li, L. Prevalence and Influencing Factors of Nonunion in Patients with Tibial Fracture: Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 377. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Padilla-Eguiluz, N.G.; Rosset, P. Frontiers in Non-Union Research. EFORT Open Rev. 2020, 5, 574–583. [Google Scholar] [CrossRef]
- Großner, T.; Schmidmaier, G. Konservative Therapieoptionen der Pseudarth. Unfallchirurg 2020, 123, 705–710. [Google Scholar] [CrossRef]
- Padilla-Eguiluz, N.G.; Gómez-Barrena, E. Epidemiology of Long Bone Non-Unions in Spain. Injury 2021, 52, S3–S7. [Google Scholar] [CrossRef]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The Risk of Non-Union per Fracture: Current Myths and Revised Figures from a Population of over 4 Million Adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.; Templeman, D.; Weinlein, J.C. Nonunion of the Femur and Tibia: An Update. Orthop. Clin. N. Am. 2016, 47, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Claes, L. Improvement of Clinical Fracture Healing—What Can Be Learned from Mechano-Biological Research? J. Biomech. 2021, 115, 110148. [Google Scholar] [CrossRef]
- Assiotis, A.; Sachinis, N.P.; Chalidis, B.E. Pulsed Electromagnetic Fields for the Treatment of Tibial Delayed Unions and Nonunions. A Prospective Clinical Study and Review of the Literature. J. Orthop. Surg. Res. 2012, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Blokhuis, J.H.D.; de Bruine, J.A.M.; Bramer den Boer, F.C.; Bakker, P.; Patka, H.J.; Th, M.; Haarman, R.A.; Manoliu, T.F. The Reliability of Plain Radiography in Experimental Fracture Healing. Skelet. Radiol. 2001, 30, 151–156. [Google Scholar] [CrossRef]
- Augat, P.; Faschingbauer, M.; Seide, K.; Tobita, K.; Callary, S.A.; Solomon, L.B.; Holstein, J.H. Biomechanical Methods for the Assessment of Fracture Repair. Injury 2014, 45, S32–S38. [Google Scholar] [CrossRef]
- Ledet, E.H.; Liddle, B.; Kradinova, K.; Harper, S. Smart Implants in Orthopedic Surgery, Improving Patient Outcomes: A Review. Innov. Entrep. Health 2018, 5, 41–51. [Google Scholar] [CrossRef]
- Agar, A.; Sahin, A.; Guclu, S.; Gulabi, D.; Erturk, C. Foot Loading Analysis of Intraarticular Tibia Pilon Fracture. J. Am. Podiatr. Med. Assoc. 2022, 112, 21–107. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.P.; Rosenbaum, D.; Kriese, T.; Gerngroj, H.; Clues, L. Gait Asymmetry Following Successful Surgical Treatment of Ankle Fractures in Young Adults. Clin. Orthopeadics Relat. Res. 1995, 311, 262–269. [Google Scholar]
- Bennett, K.J.; Millar, S.C.; Fraysse, F.; Arnold, J.B.; Atkins, G.J.; Solomon, L.B.; Martelli, S.; Thewlis, D. Postoperative Lower Limb Joint Kinematics Following Tibial Plateau Fracture: A 2-Year Longitudinal Study. Gait Posture 2021, 83, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.J.; Bushuven, E.; Hell, R.; Veith, N.T.; Buschbaum, J.; Holstein, J.H.; Pohlemann, T. A Novel Tool for Continuous Fracture Aftercare—Clinical Feasibility and First Results of a New Telemetric Gait Analysis Insole. Injury 2016, 47, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Deleanu, B.; Prejbeanu, R.; Crisan, D.; Predescu, V.; Popa, I.; Poenaru, D.V. Gait Characteristics before Hardware Removal in Patients Operated upon for Tibial Plateau Fractures. Int. Orthop. 2015, 39, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Birisik, F.; Ersin, M.; Sahinkaya, T.; Öztürk, I. A Prospective Evaluation of Strength and Endurance of Ankle Dorsiflexors-Plantar Flexors after Conservative Management of Lateral Malleolar Fractures. Turk. J. Phys. Med. Rehabil. 2021, 67, 300–307. [Google Scholar] [CrossRef]
- Elbaz, A.; Mor, A.; Segal, G.; Bar, D.; Monda, M.K.; Kish, B.; Nyska, M.; Palmanovich, E. Lower Extremity Kinematic Profile of Gait of Patients After Ankle Fracture: A Case-Control Study. J. Foot Ankle Surg. 2016, 55, 918–921. [Google Scholar] [CrossRef]
- Falzarano, G.; Pica, G.; Medici, A.; Rollo, G.; Bisaccia, M.; Cioffi, R.; Pavone, M.; Meccariello, L. Foot Loading and Gait Analysis Evaluation of Nonarticular Tibial Pilon Fracture: A Comparison of Three Surgical Techniques. J. Foot Ankle Surg. 2018, 57, 894–898. [Google Scholar] [CrossRef]
- Fändriks, A.; Tranberg, R.; Karlsson, J.; Möller, M.; Zügner, R. Gait Biomechanics in Patients with Intra-Articular Tibial Plateau Fractures—Gait Analysis at Three Months Compared with Age- and Gender-Matched Healthy Subjects. BMC Musculoskelet. Disord. 2021, 22, 702. [Google Scholar] [CrossRef]
- van Hoeve, S.; Houben, M.; Verbruggen, J.P.A.M.; Willems, P.; Meijer, K.; Poeze, M. Gait Analysis Related to Functional Outcome in Patients Operated for Ankle Fractures. J. Orthop. Res. 2019, 37, 1658–1666. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Tsai, Y.S.; Yau, C.S.; Shie, H.H.; Wu, C.M. Differences in Gait and Trunk Movement between Patients after Ankle Fracture and Healthy Subjects. Biomed. Eng. Online 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, E.; Agarwal, S.; Khaleel, A. Walking Impairments after Severe Tibia Plateau Fractures. A Gait Pattern Analysis. J. Orthop. Sci. 2020, 25, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.; Fenwick, A.; Doht, S.; Frey, S.; Meffert, R. Clinical Outcome and Changes in Gait Pattern after Pilon Fractures. Int. Orthop. 2013, 37, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joslin, C.C.; Eastaugh-Waring, S.J.; Hardy, J.R.W.; Cunningham, J.L. Weight Bearing after Tibial Fracture as a Guide to Healing. Clin. Biomech. 2008, 23, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Kröger, I.; Müßig, J.; Brand, A.; Pätzold, R.; Wackerle, H.; Klöpfer-Krämer, I.; Augat, P. Recovery of Gait and Function during the First Six Months after Tibial Shaft Fractures. Gait Posture 2022, 91, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lajevardi-Khosh, A.; Bamberg, S.; Rothberg, D.; Kubiak, E.; Petelenz, T.; Hitchcock, R. Center of Pressure in a Walking Boot Shifts Posteriorly in Patients Following Lower Leg Fracture. Gait Posture 2019, 70, 218–221. [Google Scholar] [CrossRef]
- Larsen, P.; Laessoe, U.; Rasmussen, S.; Graven-Nielsen, T.; Berre Eriksen, C.; Elsoe, R. Asymmetry in Gait Pattern Following Tibial Shaft Fractures—A Prospective One-Year Follow-up Study of 49 Patients. Gait Posture 2017, 51, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Larsen, P.; Eriksen, C.B.; Stokholm, R.; Elsoe, R. Results Following Prolonged Recovery Show Satisfactory Functional and Patient-Reported Outcome after Intramedullary Nailing of a Tibial Shaft Fracture: A Prospective 5-Year Follow-up Cohort Study. Arch. Orthop. Trauma Surg. 2021, 141, 1303–1310. [Google Scholar] [CrossRef]
- Losch, A.; Meybohm, P.; Schmalz, T.; Fuchs, M.; Vamvukakis, F.; Dresing, K.; Blumentritt, S.; Stürmer, K.M. Funktionelle Ergebnisse Bei Freizeitsportlern in Der Dynamischen Ganganalyse 1 Jahr Nach Operativ Versorgten Sprunggelenkfrakturen. Sportverletz. Sportschaden 2002, 16, 101–107. [Google Scholar] [CrossRef]
- Perttunen, J.R.; Nieminen, H.; Tukiainen, E.; Kuokkanen, H.; Asko-Seljavaara, S.; Komi, P.V. Asymmetry of Gait after Free Flap Reconstruction of Severe Tibial Fractures with Extensive Soft-Tissue Damage. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2000, 34, 237–243. [Google Scholar] [CrossRef]
- Psatha, M.; Wu, Z.; Gammie, F.M.; Ratkevicius, A.; Wackerhage, H.; Lee, J.H.; Redpath, T.W.; Gilbert, F.J.; Ashcroft, G.P.; Meakin, J.R.; et al. A Longitudinal MRI Study of Muscle Atrophy during Lower Leg Immobilization Following Ankle Fracture. J. Magn. Reson. Imaging 2012, 35, 686–695. [Google Scholar] [CrossRef]
- Quacinella, M.; Bernstein, E.; Mazzone, B.; Wyatt, M.; Kuhn, K.M. Do Spatiotemporal Gait Parameters Improve after Pilon Fracture in Patients Who Use the Intrepid Dynamic Exoskeletal Orthosis? Clin. Orthop. Relat. Res. 2019, 477, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Schoenmakers, S.; Houben, M.; van Hoeve, S.; Willems, P.; Meijer, K.; Poeze, M. The Influence of Size and Comminution of the Posterior Malleolus Fragment on Gait in Trimalleolar Ankle Fractures. Clin. Biomech. 2022, 91, 105550. [Google Scholar] [CrossRef]
- Segal, G.; Elbaz, A.; Parsi, A.; Heller, Z.; Palmanovich, E.; Nyska, M.; Feldbrin, Z.; Kish, B. Clinical Outcomes Following Ankle Fracture: A Cross-Sectional Observational Study. J. Foot Ankle Res. 2014, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Suciu, O.; Onofrei, R.R.; Totorean, A.D.; Suciu, S.C.; Amaricai, E.C. Gait Analysis and Functional Outcomes after Twelve-Week Rehabilitation in Patients with Surgically Treated Ankle Fractures. Gait Posture 2016, 49, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Thewlis, D.; Callary, S.A.; Fraysse, F.; Solomon, L.B. Peak Loading during Walking Is Not Associated with Fracture Migration Following Tibial Plateau Fracture: A Preliminary Case Series. J. Orthop. Res. 2015, 33, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Thur, C.K.; Gutierrez-Farewik, E.M.; Wretenberg, P.; Broström, E. One Year Follow-up after Operative Ankle Fractures: A Prospective Gait Analysis Study with a Multi-Segment Foot Model. Gait Posture 2010, 31, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Warschawski, Y.; Drexler, M.; Batko, B.; Elias, S.; Goldstein, Y.; Frenkel Rutenberg, T.; Schermann, H.; Steinberg, E.L. Correlation between Preoperative Imaging Parameters and Postoperative Basic Kinematics-Based Functional Outcome in Patients with Tibial Plateau Fractures. Clin. Biomech. 2019, 65, 87–91. [Google Scholar] [CrossRef]
- Thingstad, P.; Taraldsen, K.; Saltvedt, I.; Sletvold, O.; Vereijken, B.; Lamb, S.E.; Helbostad, J.L. The Long-Term Effect of Comprehensive Geriatric Care on Gait after Hip Fracture: The Trondheim Hip Fracture Trial—a Randomised Controlled Trial. Osteoporosis International 2016, 27, 933–942. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.J.; Godinho, C.; Santos, A.T.; Domingos, J.; Abreu, D.; Lobo, R.; Gonçalves, N.; Barra, M.; Larsen, F.; Fagerbakke, Ø.; et al. Quantitative Home-Based Assessment of Parkinson’s Symptoms: The SENSE-PARK Feasibility and Usability Study. BMC Neurol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, C.A.; Fukuchi, R.K.; Duarte, M. Effects of Walking Speed on Gait Biomechanics in Healthy Participants: A Systematic Review and Meta-Analysis. Syst. Rev. 2019, 8, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejek, Z.; Paróczai, R.; Illyés, Á.; Kiss, R.M. The Influence of Walking Speed on Gait Parameters in Healthy People and in Patients with Osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2006, 14, 612–622. [Google Scholar] [CrossRef]
- Lewek, M.D. The Influence of Body Weight Support on Ankle Mechanics during Treadmill Walking. J. Biomech. 2011, 44, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kirtley, C.; Whittle, M.W.; Jefferson, R.J. Influence of Walking Speed on Gait Parameters. J. Biomed. Eng. 1985, 7, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Challis, J.H.; Newell, K.M. Walking Speed Influences on Gait Cycle Variability. Gait Posture 2007, 26, 128–134. [Google Scholar] [CrossRef] [PubMed]
- James, E.G.; Conatser, P.; Karabulut, M.; Leveille, S.G.; Hausdorff, J.M.; Travison, T.; Bean, J.F. Walking Speed Affects Gait Coordination and Variability Among Older Adults With and Without Mobility Limitations. Arch. Phys. Med. Rehabil. 2020, 101, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Bartsch, R.P.; Zeev, A.; Giladi, N.; Hausdorff, J.M. Effects of Walking Speed on Asymmetry and Bilateral Coordination of Gait. Gait Posture 2013, 38, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Kanko, R.M.; Laende, E.; Selbie, W.S.; Deluzio, K.J. Inter-Session Repeatability of Markerless Motion Capture Gait Kinematics. J. Biomech. 2021, 121, 110422. [Google Scholar] [CrossRef]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; van Rompaey, V.; Saeys, W. Do Spatiotemporal Parameters and Gait Variability Differ across the Lifespan of Healthy Adults? A Systematic Review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef]
- Zadik, S.; Benady, A.; Gutwillig, S.; Florentine, M.M.; Solymani, R.E.; Plotnik, M. Age Related Changes in Gait Variability, Asymmetry, and Bilateral Coordination—When Does Deterioration Starts? Gait Posture 2022, 96, 87–92. [Google Scholar] [CrossRef]
- Pau, M.; Capodaglio, P.; Leban, B.; Porta, M.; Galli, M.; Cimolin, V. Kinematics Adaptation and Inter-Limb Symmetry during Gait in Obese Adults. Sensors 2021, 21, 5980. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lim, J.; Lee, S. Body Fat-Related Differences in Gait Parameters and Physical Fitness Level in Weight-Matched Male Adults. Clin. Biomech. 2021, 81, 105243. [Google Scholar] [CrossRef]
- Phillips, A.; McClinton, S. Gait Deviations Associated with Plantar Heel Pain: A Systematic Review. Clin. Biomech. 2017, 42, 55–64. [Google Scholar] [CrossRef]
- Ogawa, E.F.; Shi, L.; Bean, J.F.; Hausdorff, J.M.; Dong, Z.; Manor, B.; McLean, R.R.; Leveille, S.G. Chronic Pain Characteristics and Gait in Older Adults: The MOBILIZE Boston Study II. Arch. Phys. Med. Rehabil. 2020, 101, 418–425. [Google Scholar] [CrossRef]
- Rittweger, J.; Felsenberg, D. Recovery of Muscle Atrophy and Bone Loss from 90 Days Bed Rest: Results from a One-Year Follow-Up. Bone 2009, 44, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P. Impact of Bone Fracture on Muscle Strength and Physical Performance—Narrative Review. Curr. Osteoporos. Rep. 2020, 18, 633–645. [Google Scholar] [CrossRef]
- Manjra, M.A.; Naude, J.; Birkholtz, F.; Glatt, V.; Tetsworth, K.; Hohmann, E. The Relationship between Gait and Functional Outcomes in Patients Treated with Circular External Fixation for Malunited Tibial Fractures. Gait Posture 2019, 68, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Interactions among Obesity and Age-Related Effects on the Gait Pattern and Muscle Activity across the Ankle Joint. Exp. Gerontol. 2020, 140, 111054. [Google Scholar] [CrossRef]
- Cohen, J.A.; Verghese, J.; Zwerling, J.L. Cognition and Gait in Older People. Maturitas 2016, 93, 73–77. [Google Scholar] [CrossRef]
- Vergara, I.; Vrotsou, K.; Orive, M.; Gonzalez, N.; Garcia, S.; Quintana, J.M. Factors Related to Functional Prognosis in Elderly Patients after Accidental Hip Fractures: A Prospective Cohort Study. BMC Geriatr. 2014, 14, 124. [Google Scholar] [CrossRef]
- Gao, F.; Lv, T.R.; Zhou, J.C.; Qin, X.D. Effects of Obesity on the Healing of Bone Fracture in Mice. J. Orthop. Surg. Res. 2018, 13, 145. [Google Scholar] [CrossRef] [Green Version]
- Braun, B.J.; Veith, N.T.; Rollmann, M.; Orth, M.; Fritz, T.; Herath, S.C.; Holstein, J.H.; Pohlemann, T. Weight-Bearing Recommendations after Operative Fracture Treatment—Fact or Fiction? Gait Results with and Feasibility of a Dynamic, Continuous Pedobarography Insole. Int. Orthop. 2017, 41, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Nadkarni, N.K.; Black, S.E.; McIlroy, W.E. Gait Symmetry and Velocity Differ in Their Relationship to Age. Gait Posture 2012, 35, 590–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, D.; Martin, X.; Lamah, L.; Delhumeau, C.; Farpour-Lambert, N.; de Coulon, G.; Ferrière, V.D. Recovery of Physical Activity Levels in Adolescents after Lower Limb Fractures: A Longitudinal, Accelerometry-Based Activity Monitor Study. BMC Musculoskelet. Disord. 2012, 13, 131. [Google Scholar] [CrossRef] [PubMed]






| Authors | Fracture Type(s) Included | n | Age (Years) | Longitudinal Measurements | Time After Fracture/Surgery That Measurement Occurred | Measurement Device(s) | Type of Parameters Calculated |
|---|---|---|---|---|---|---|---|
| Agar et al., 2022 [21] | Intraarticular distal tibial fractures | 62 | 43 | No | 24–58 months | Pressure plate | Pedographic measures |
| Becker et al., 1995 [22] | Malleolar fractures | 40 | 24 | No | 18.5 months | Pressure plate | Pedographic measures |
| Bennet et al., 2021 [23] | Proximal tibial fractures | 18 | 52 | Yes | 2 weeks, 6 weeks, 3 months, 6 months, 1 year, 2 years | Optical motion capture, force plate | Kinematics |
| Braun et al., 2016 [24] | Malleolar fractures | 10 | 53 | Yes | Continuously for 6 weeks | Pressure insoles | Pedographic measures |
| Deleanu et al., 2015 [25] | Proximal tibial fractures | 25 | 39 | No | Before hardware removal | Pressure plate, ultrasound-based motion capture | Spatiotemporal gait parameters |
| Ekinci et al., 2021 [26] | Malleolar fractures | 24 | 41 | Yes | At cast removal, 3 and 6 months after rehabilitation | Isokinetic dynamometer | Muscle strength |
| Elbaz et al., 2016 [27] | Malleolar fractures | 24 | 49 | No | <6 weeks from weight-bearing approval | Inertial measurement units | Kinematics |
| Elsoe et al., 2017 [8] | Proximal tibial fractures | 23 | 54 | No | 12 months after ring fixator removal | Electronic walkway | Spatiotemporal gait parameters |
| Falzarano et al., 2018 [28] | Nonarticular distal tibial fractures | 34 | 32 | No | 12 months | Pressure plate | Kinetics |
| Fändriks et al., 2021 [29] | Proximal tibial fractures | 20 | 44 | No | 85 days | Optical motion capture | Spatiotemporal gait parameters, kinematics |
| Hoeve et al., 2019 [30] | Malleolar fractures | 33 | 57 | No | 18 months | Optical motion capture, force plate | Kinematics |
| Hsu et al., 2019 [31] | Malleolar fractures | 10 | 38 | No | 4 months | accelerometer | Spatiotemporal gait parameters |
| Iliopoulos et al., 2020 [32] | Proximal tibial fractures | 16 | 49 | No | 3–6 months after frame removal | Force plate | Kinetics, spatiotemporal gait parameters |
| Jansen et al., 2013 [33] | Distal tibial fractures | 41 | 48 | No | 50 months | Pressure plate | Pedographic measures |
| Joslin et al., 2008 [34] | Tibial shaft fractures | 12 | 32 | No | 20 weeks | Force plate | Kinetics |
| Kröger et al., 2022 [35] | Tibial shaft fractures | 23 | 39 | Yes | 2, 3, and 6 months | Optical motion capture, force plate | Kinematics, kinetics, spatiotemporal gait parameters |
| Lajevardi-Khosh et al., 2019 [36] | Tibial shaft fractures and malleolar fractures | 7 | Yes | Continuously for 2–12 weeks | Pressure insoles | Pedographic measures | |
| Larsen et al., 2017 [37] | Tibial shaft fractures | 49 | 43 | Yes | 6 months, 12 months | Electronic walkway | Spatiotemporal gait parameters |
| Larsen et al., 2021 [38] | Tibial shaft fractures | 29 | 46 | No | 5 years | Electronic walkway | Spatiotemporal gait parameters |
| Losch et al., 2002 [39] | Malleolar fractures | 20 | 43 | No | 1 year | Optical motion capture, force plate | Kinematics, kinetics, spatiotemporal gait parameters |
| Perttunen et al., 2000 [40] | Tibial fractures | 17 | 51 | No | 9 months–14 years | Pressure insoles, electromyography | Pedographic measures, muscle activity |
| Psatha et al., 2012 [41] | Malleolar fractures | 18 | 43 | Yes | 5, 8, 15, 29 and 43 days | Magnetic resonance imaging | Muscle volume |
| Quacinella et al., 2019 [42] | Distal tibial fractures | 7 | 25 | No | 12 months | Optical motion capture, force plate | Spatiotemporal gait parameters |
| Schoenmakers et al., 2022 [43] | Malleolar fractures | 26 | 58 | No | 24 months | Optical motion capture | Kinematics |
| Segal et al., 2014 [44] | Malleolar fractures | 41 | 48 | No | 67 days | Electronic walkway | Spatiotemporal gait parameters |
| Suciu et al., 2016 [45] | Malleolar fractures | 30 | 53 | Yes | 7 weeks, 12 weeks | Pressure plate | Spatiotemporal gait parameters |
| Thewlis et al., 2015 [46] | Proximal tibial fractures | 9 | 69 | Yes | 2 weeks, 3 months, 6 months, 1 year | Optical motion capture, force plate | Kinetics |
| Wang et al., 2010 [47] | Malleolar fractures | 18 | 39 | No | 1 year | Optical motion capture | Kinematics, spatiotemporal gait parameters |
| Warschawski et al., 2015 [7] | Proximal tibial fractures | 22 | 46 | No | 3 years | Floor-based photocell system | Spatiotemporal gait parameters |
| Warschawski et al., 2019 [48] | Proximal tibial fractures | 21 | 44 | No | 3 years | Floor-based photocell system | Spatiotemporal gait parameters |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warmerdam, E.; Orth, M.; Pohlemann, T.; Ganse, B. Gait Analysis to Monitor Fracture Healing of the Lower Leg. Bioengineering 2023, 10, 255. https://doi.org/10.3390/bioengineering10020255
Warmerdam E, Orth M, Pohlemann T, Ganse B. Gait Analysis to Monitor Fracture Healing of the Lower Leg. Bioengineering. 2023; 10(2):255. https://doi.org/10.3390/bioengineering10020255
Chicago/Turabian StyleWarmerdam, Elke, Marcel Orth, Tim Pohlemann, and Bergita Ganse. 2023. "Gait Analysis to Monitor Fracture Healing of the Lower Leg" Bioengineering 10, no. 2: 255. https://doi.org/10.3390/bioengineering10020255