Are Activity Wrist-Worn Devices Accurate for Determining Heart Rate during Intense Exercise?
Abstract
:1. Introduction
2. Methods
2.1. Participant Selection
2.2. Study Setting
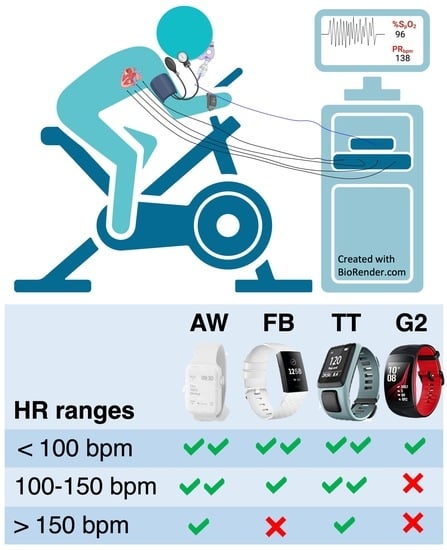
2.3. HR Measuring Devices
2.3.1. Tomtom Runner Cardio (TT)
2.3.2. FitBit (FB)
2.3.3. Apple Watch (AW)
2.3.4. Gear S2 (G2)
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Main Implications and Future Perspectives
4.2. Strengths and Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| bpm | beats per minute |
| ECG | electrocardiogram |
| FB | Fitbit Charge |
| AW | Apple Watch |
| TT | Tomtom Runner Cardio |
| G2 | Samsung G2 |
| LED | light emitting diode |
| PPG | photoplethysmogram |
| accuracy root-mean-square | |
| APE | absolute percent error |
| ICC | interclass correlation coefficient |
| SD | standard deviation |
| R | Spearman’s rank correlation |
References
- Thompson, W. Worldwide Survey of Fitness Trends for 2022. Acsm’s Health Fit. J. 2022, 26, 11–20. [Google Scholar] [CrossRef]
- Scalise, L.; Cosoli, G. Wearables for health and fitness: Measurement characteristics and accuracy. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–6. [Google Scholar]
- Düking, P.; Tafler, M.; Wallmann-Sperlich, B.; Sperlich, B.; Kleih, S. Behavior Change Techniques in Wrist-Worn Wearables to Promote Physical Activity: Content Analysis. JMIR mHealth uHealth 2020, 8, e20820. [Google Scholar] [CrossRef] [PubMed]
- Pedragosa, V.; Angosto, S.; Gonçalves, C. Validity and Reliability of a Wearable Fitness Technology Scale in Portugal. Int. J. Environ. Res. Public Health 2022, 19, 5927. [Google Scholar] [CrossRef] [PubMed]
- Marutani, Y.; Konda, S.; Ogasawara, I.; Yamasaki, K.; Yokoyama, T.; Maeshima, E.; Nakata, K. An Experimental Feasibility Study Evaluating the Adequacy of a Sportswear-Type Wearable for Recording Exercise Intensity. Sensors 2022, 22, 2577. [Google Scholar] [CrossRef] [PubMed]
- Mcdevitt, S.; Hernandez, H.; Hicks, J.; Lowell, R.; Bentahaikt, H.; Burch, R.; Ball, J.; Chander, H.; Freeman, C.; Taylor, C.; et al. Wearables for Biomechanical Performance Optimization and Risk Assessment in Industrial and Sports Applications. Bioeng. Rev. 2022, 9, 33. [Google Scholar] [CrossRef]
- Van Hooren, B.; Goudsmit, J.; Restrepo, J.; Vos, S. Real-time feedback by wearables in running: Current approaches, challenges and suggestions for improvements. J. Sport. Sci. 2020, 38, 214–230. [Google Scholar] [CrossRef]
- Nazarian, S.; Lam, K.; Darzi, A.; Ashrafian, H. Diagnostic accuracy of smartwatches for the detection of cardiac arrhythmia: Systematic review and meta-analysis. J. Med Internet Res. 2021, 23, e28974. [Google Scholar] [CrossRef]
- Tricás-Vidal, H.J.; Lucha-López, M.O.; Hidalgo-García, C.; Vidal-Peracho, M.C.; Monti-Ballano, S.; Tricás-Moreno, J.M. Health Habits and Wearable Activity Tracker Devices: Analytical Cross-Sectional Study. Sensors 2022, 22, 2960. [Google Scholar] [CrossRef]
- Shei, R.J.; Holder, I.G.; Oumsang, A.S.; Paris, B.A.; Paris, H.L. Wearable activity trackers-advanced technology or advanced marketing? Eur. J. Appl. Physiol. 2022, 122, 1975–1990. [Google Scholar] [CrossRef]
- Picard, R.W.; Boyer, E.W. Smartwatch biomarkers and the path to clinical use. Med 2021, 2, 797–799. [Google Scholar] [CrossRef]
- Cardinale, M.; Varley, M.C. Wearable Training-Monitoring Technology: Applications, Challenges, and Opportunities. Int. J. Sport. Physiol. Perform. 2017, 12, S255–S262. [Google Scholar] [CrossRef] [Green Version]
- Stuart, T.; Hanna, J.; Gutruf, P. Wearable devices for continuous monitoring of biosignals: Challenges and opportunities. APL Bioeng. 2022, 6, 21502. [Google Scholar] [CrossRef]
- Thompson, W. Worldwide Survey of Fitness Trends for 2021. Acsm’s Health Fit. J. 2021, 25, 10–19. [Google Scholar] [CrossRef]
- Bauer, N.; Sperlich, B.; Holmberg, H.C.; Engel, F.A. Effects of High-Intensity Interval Training in School on the Physical Performance and Health of Children and Adolescents: A Systematic Review with Meta-Analysis. Sports Medicine-Open 2022, 8, 1–14. [Google Scholar] [CrossRef]
- Murali, S.; Rincon, F.; Cassina, T.; Cook, S.; Goy, J.J. Heart Rate and Oxygen Saturation Monitoring With a New Wearable Wireless Device in the Intensive Care Unit: Pilot Comparison Trial. JMIR Biomed. Eng. 2020, 5, e18158. [Google Scholar] [CrossRef]
- Salamone, F.; Masullo, M.; Sibilio, S. Wearable Devices for Environmental Monitoring in the Built Environment: A Systematic Review. Sensors 2021, 21, 4727. [Google Scholar] [CrossRef]
- Léger, L.; Thivierge, M. Heart Rate Monitors: Validity, Stability, and Functionality. Physician Sportsmed. 1988, 16, 143–151. [Google Scholar] [CrossRef]
- Adesida, Y.; Papi, E.; McGregor, A.H. Exploring the Role of Wearable Technology in Sport Kinematics and Kinetics: A Systematic Review. Sensors 2019, 19, 1597. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Maeda, Y.; Sekine, M.; Huang, M. The Role of Wearable Monitor for Healthcare. 7th Forum New Mater.-Part D 2016, 100, 159–165. [Google Scholar] [CrossRef]
- Thompson, W. Worldwide survey of fitness trends for 2020. Acsm’s Health Fit. J. 2019, 23, 10–18. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, R.K.; Hickey, A.M.; Freedson, P.S. Advantages and Limitations of Wearable Activity Trackers: Considerations for Patients and Clinicians. Clin. J. Oncol. Nurs. 2016, 20, 606–610. [Google Scholar] [CrossRef] [Green Version]
- Statista. Connected wearable devices worldwide 2016 to 2022. 2021., 2021.
- Lee, J.m.; Kim, Y.; Welk, G.J. Validity of Consumer-Based Physical Activity Monitors. MedicineScience Sports Exerc. 2014, 14, 1840–1848. [Google Scholar] [CrossRef] [Green Version]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [Green Version]
- Alian, A.A.; Shelley, K.H. Photoplethysmography. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 395–406. [Google Scholar] [CrossRef]
- Lee, I.; Park, N.; Lee, H.; Hwang, C.; Kim, J.H.; Park, S. Systematic Review on Human Skin-Compatible Wearable Photoplethysmography Sensors. Appl. Sci. 2021, 11, 2313. [Google Scholar] [CrossRef]
- Zakeri, I.; Adolph, A.L.; Puyau, M.R.; Vohra, F.A.; Butte, N.F. Application of cross-sectional time series modeling for the prediction of energy expenditure from heart rate and accelerometry. J. Appl. Physiol. 2008, 104, 1665–1673. [Google Scholar] [CrossRef]
- Chowdhury, E.A.; Western, M.J.; Nightingale, T.E.; Peacock, O.J.; Thompson, D. Assessment of laboratory and daily energy expenditure estimates from consumer multi-sensor physical activity monitors. PLoS ONE 2017, 12, e0171720. [Google Scholar] [CrossRef] [Green Version]
- Rankovic, G.; Mutavdzic, V.; Toskic, D.; Preljevic, A.; Kocic, M.; Nedin Rankovic, G.; Damjanovic, N. Aerobic capacity as an indicator in different kinds of sports. Bosn. J. Basic Med Sci. 2010, 10, 44–48. [Google Scholar] [CrossRef]
- Montero, D.; Cathomen, A.; Jacobs, R.A.; Flück, D.; de Leur, J.; Keiser, S.; Bonne, T.; Kirk, N.; Lundby, A.K.; Lundby, C. Haematological rather than skeletal muscle adaptations contribute to the increase in peak oxygen uptake induced by moderate endurance training. J. Physiol. 2015, 593, 4677–4688. [Google Scholar] [CrossRef] [Green Version]
- Min, J. Maximal Oxygen Uptake in Breathing Exercises and Heart Rate Exercises Based on In-Depth Regression Equations. Adv. Multimed. 2022, 2022, 9664346. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. Age-Predicted Maximal Heart Rate in Recreational Marathon Runners: A Cross-Sectional Study on Fox’s and Tanaka’s Equations. Front. Physiol. 2018, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Ismail, S.; Siddiqi, I.; Akram, U. Heart rate estimation in PPG signals using Convolutional-Recurrent Regressor. Comput. Biol. Med. 2022, 145, 105470. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.; Simoes-Capela, N.; Van Hoof, C.; Van Helleputte, N. Heart Rate Estimation from Wrist-Worn Photoplethysmography: A Review. IEEE Sens. J. 2019, 19, 6560–6570. [Google Scholar] [CrossRef]
- Parak, J.; Uuskoski, M.; Machek, J.; Korhonen, I. Estimating heart rate, energy expenditure, and physical performance with a wrist photoplethysmographic device during running. JMIR mHealth uHealth 2017, 5, 1–14. [Google Scholar] [CrossRef]
- Madhan Mohan, P.; Nagarajan, V.; Vignesh, J.C. Spot measurement of heart rate based on morphology of PhotoPlethysmoGraphic (PPG) signals. J. Med Eng. Technol. 2017, 41, 87–96. [Google Scholar] [CrossRef]
- Berggren, G.; Christensen, E.H. Heart rate and body temperature as indices of metabolic rate during work. Arbeitsphysiologie 1950, 14, 255–260. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Dooley, E.E.; Golaszewski, N.M.; Bartholomew, J.B. Estimating accuracy at exercise intensities: A comparative study of self-monitoring heart rate and physical activity wearable devices. JMIR mHealth uHealth 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, R.M.; Virtanen, P.K. Heart rate monitors: State of the art. J. Sport. Sci. 1998, 16 (Suppl. 1), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Vagedes, J. How accurate is pulse rate variability as an estimate of heart rate variability? A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Heart rate monitoring: Applications and limitations. Sport. Med. 2003, 33, 517–538. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Hinde, K.; White, G.; Armstrong, N. Wearable Devices Suitable for Monitoring Twenty Four Hour Heart Rate Variability in Military Populations. Sensors 2021, 21, 1061. [Google Scholar] [CrossRef]
- Hargens, T.A.; Chambers, S.; Luden, N.D.; Womack, C.J. Reliability of the heart rate variability threshold during treadmill exercise. Clin. Physiol. Funct. Imaging 2022, 42, 292–299. [Google Scholar] [CrossRef]
- Rodrigues, E.; Lima, D.; Barbosa, P.; Gonzaga, K.; Guerra, R.O.; Pimentel, M.; Barbosa, H.; Maciel, Á. HRV Monitoring Using Commercial Wearable Devices as a Health Indicator for Older Persons during the Pandemic. Sensors 2022, 22, 2001. [Google Scholar] [CrossRef]
- Tamura, T.; Chen, W. Seamless Healthcare Monitoring: Advancements in Wearable, Attachable, and Invisible Devices; Springer International Publishing: Berlin, Germany, 2017. [Google Scholar]
- Zhang, Z. Photoplethysmography-Based Heart Rate Monitoring in Physical Activities via Joint Sparse Spectrum Reconstruction. IEEE Trans. bio-Med. Eng. 2015, 62, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Kaewkannate, K.; Kim, S. A comparison of wearable fitness devices. BMC Public Health 2016, 16, 433. [Google Scholar] [CrossRef]
- Reddy, R.K.; Pooni, R.; Zaharieva, D.P.; Senf, B.; El Youssef, J.; Dassau, E.; Doyle Iii, F.J.; Clements, M.A.; Rickels, M.R.; Patton, S.R.; et al. Accuracy of Wrist-Worn Activity Monitors During Common Daily Physical Activities and Types of Structured Exercise: Evaluation Study. JMIR mHealth uHealth 2018, 6, e10338. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Zhao, J.; Zhang, X.; Chen, X. An effective photoplethysmography heart rate estimation framework integrating two-level denoising method and heart rate tracking algorithm guided by finite state machine. IEEE J. Biomed. Health Inform. 2022, 26, 3731–3742. [Google Scholar] [CrossRef]
- Bellenger, C.R.; Miller, D.J.; Halson, S.L.; Roach, G.D.; Sargent, C. Wrist-Based Photoplethysmography Assessment of Heart Rate and Heart Rate Variability: Validation of WHOOP. Sensors 2021, 21, 3571. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Xie, Y.; Gao, F.; Xu, S.; Wu, X.; Ye, Z. Wearable Health Devices in Health Care: Narrative Systematic Review. JMIR mHealth uHealth 2020, 8, e18907. [Google Scholar] [CrossRef]
- Nantume, A.; Kiwanuka, N.; Muyinda, A.; Cauvel, T.; Shah, S. Accuracy and reliability of a wireless vital signs monitor for hospitalized patients in a low-resource setting. Digit. Health 2022, 8, 20552076221102262. [Google Scholar] [CrossRef]
- Reis, V.M.; Vianna, J.M.; Barbosa, T.M.; Garrido, N.; Vilaça Alves, J.; Carneiro, A.L.; Aidar, F.J.; Novaes, J. Are wearable heart rate measurements accurate to estimate aerobic energy cost during low-intensity resistance exercise? PLoS ONE 2019, 14, e0221284. [Google Scholar] [CrossRef]
- Normand-Gravier, T.; Britto, F.; Launay, T.; Renfree, A.; Toussaint, J.F.; Desgorces, F.D. Exercise Dose Equalization in High-Intensity Interval Training: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 4980. [Google Scholar] [CrossRef]
- Sperlich, B.; Holmberg, H.C. Wearable, yes, but able…? It is time for evidence-based marketing claims! Br. J. Sport. Med. 2017, 51, 1240. [Google Scholar] [CrossRef]
- Stahl, S.E.; An, H.S.; Dinkel, D.M.; Noble, J.M.; Lee, J.M. How accurate are the wrist-based heart rate monitors during walking and running activities? Are they accurate enough? BMJ Open Sport Exerc. Med. 2016, 2, e000106. [Google Scholar] [CrossRef] [Green Version]
- Shcherbina, A.; Mattsson, C.M.; Waggott, D.; Salisbury, H.; Christle, J.W.; Hastie, T.; Wheeler, M.T.; Ashley, E.A. Accuracy in Wrist-Worn, Sensor-Based Measurements of Heart Rate and Energy Expenditure in a Diverse Cohort. JPM 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weaver, R.G.; Armstrong, B.; Burkart, S.; Zhang, S.; Beets, M.W. Validity of Wrist-Worn photoplethysmography devices to measure heart rate: A systematic review and meta-analysis. J. Sport. Sci. 2020, 38, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Nuuttila, O.P.; Korhonen, E.; Laukkanen, J.; Kyröläinen, H. Validity of the Wrist-Worn Polar Vantage V2 to Measure Heart Rate and Heart Rate Variability at Rest. Sensors 2022, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Jachymek, M.; Jachymek, M.T.; Kiedrowicz, R.M.; Kaźmierczak, J.; Płońska-Gościniak, E.; Peregud-Pogorzelska, M. Wristbands in Home-Based Rehabilitation—Validation of Heart Rate Measurement. Sensors 2022, 22, 60. [Google Scholar] [CrossRef]
- Patel, M.S.; Polsky, D.; Kennedy, E.H.; Small, D.S.; Evans, C.N.; Rareshide, C.A.L.; Volpp, K.G. Smartphones vs Wearable Devices for Remotely Monitoring Physical Activity After Hospital Discharge: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e1920677. [Google Scholar] [CrossRef] [Green Version]
- Düking, P.; Fuss, F.K.; Holmberg, H.C.; Sperlich, B. Recommendations for Assessment of the Reliability, Sensitivity, and Validity of Data Provided by Wearable Sensors Designed for Monitoring Physical Activity. JMIR mHealth uHealth 2018, 6, e102. [Google Scholar] [CrossRef]
- Wang, R.; Blackburn, G.; Desai, M.; Phelan, D.; Gillinov, L.; Houghtaling, P.; Gillinov, M. Accuracy of Wrist-Worn Heart Rate Monitors. JAMA Cardiol. 2017, 2, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Hannan, A.L.; Harders, M.P.; Hing, W.; Climstein, M.; Coombes, J.S.; Furness, J. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: Systematic review and meta-analysis. BMC Sport. Sci. Med. Rehabil. 2019, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Abt, G.; Bray, J.; Benson, A.C. Measuring Moderate-Intensity Exercise with the Apple Watch: Validation Study. JMIR Cardio 2018, 2, e6. [Google Scholar] [CrossRef] [Green Version]
- Van Gastel, M.; Stuijk, S.; De Haan, G. Motion robust remote-PPG in infrared. IEEE Trans. Biomed. Eng. 2015, 62, 1425–1433. [Google Scholar] [CrossRef]
- van Gastel, M.; Stuijk, S.; de Haan, G. New principle for measuring arterial blood oxygenation, enabling motion-robust remote monitoring. Sci. Rep. 2016, 6, 38609. [Google Scholar] [CrossRef]
- Iyriboz, Y.; Powers, S.; Morrow, J.; Ayers, D.; Landry, G. Accuracy of pulse oximeters in estimating heart rate at rest and during exercise. Br. J. Sport. Med. 1991, 25, 162–164. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T. Current progress of photoplethysmography and SPO(2) for health monitoring. Biomed. Eng. Lett. 2019, 9, 21–36. [Google Scholar] [CrossRef]
- Gillinov, S.; Etiwy, M.; Wang, R.; Blackburn, G.; Phelan, D.; Gillinov, A.M.; Houghtaling, P.; Javadikasgari, H.; Desai, M.Y. Variable Accuracy of Wearable Heart Rate Monitors during Aerobic Exercise. Med. Sci. Sport. Exerc. 2017, 49, 1697–1703. [Google Scholar] [CrossRef]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-ananta, T.; Ramella-Roman, J.C.; McShane, M.J.; Coté, G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef]
- Bassett, D.R.J.; Rowlands, A.; Trost, S.G. Calibration and validation of wearable monitors. Med. Sci. Sport. Exerc. 2012, 44, S32–S38. [Google Scholar] [CrossRef] [Green Version]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Sperlich, B.; Aminian, K.; Düking, P.; Holmberg, H.C. Editorial: Wearable Sensor Technology for Monitoring Training Load and Health in the Athletic Population. Front. Physiol. 2019, 10, 1520. [Google Scholar] [CrossRef]
- ISO 80601-2-61:2017; Medical Electrical Equipment—Part 2-61: Particular Requirements for Basic Safety and Essential Performance of Pulse Oximeter Equipment. Technical Report. 2017.
- Batchelder, P.B.; Raley, D.M. Maximizing the Laboratory Setting for Testing Devices and Understanding Statistical Output in Pulse Oximetry. Anesth. Analg. 2007, 105. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. bio-Med. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Martín-Escudero, P.; Cabanas, A.M.; Fuentes-Ferrer, M.; Galindo-Canales, M. Oxygen Saturation Behavior by Pulse Oximetry in Female Athletes: Breaking Myths. Biosensors 2021, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Wallen, M.P.; Gomersall, S.R.; Keating, S.E.; Wisløff, U.; Coombes, J.S. Accuracy of Heart Rate Watches: Implications for Weight Management. PLoS ONE 2016, 11, e0154420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunn, J.A.; Navalta, J.W.; Fountaine, C.J.; Reece, J.D. Current State of Commercial Wearable Technology in Physical Activity Monitoring 2015–2017. Int. J. Exerc. Sci. 2018, 11, 503. [Google Scholar] [PubMed]
- Zong, C.; Jafari, R. Robust heart rate estimation using wrist-based PPG signals in the presence of intense physical activities. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; Volume 2015, pp. 8078–8082. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Jamnik, V.; Bredin, S.S.D.; Shephard, R.J.; Gledhill, N. The 2018 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+): 2018 PAR-Q+. Health Fit. J. Can. 2018, 11, 31–34. [Google Scholar] [CrossRef]
- Seok, D.; Lee, S.; Kim, M.; Cho, J.; Kim, C. Motion Artifact Removal Techniques for Wearable EEG and PPG Sensor Systems. Front. Electron. 2021, 2, 685513. [Google Scholar] [CrossRef]
- Garcia-Lopez, I.; Rodriguez-Villegas, E. Characterization of Artifact Signals in Neck Photoplethysmography. IEEE Trans. Biomed. Eng. 2020, 67, 2849–2861. [Google Scholar] [CrossRef] [Green Version]
- López-Silva, S.; Giannetti, R.; Dotor, M.L.; Silveira, J.P.; Golmayo, D.; Miguel-Tobal, F.; Bilbao, A.; Galindo Canales, M.; Martin-Escudero, P. Heuristic algorithm for photoplethysmographic heart rate tracking during maximal exercise test. J. Med. Biol. Eng. 2012, 32, 181–188. [Google Scholar] [CrossRef]
- Such, O. Motion tolerance in wearable sensors–the challenge of motion artifact. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Volume 2007, pp. 1542–1545. [Google Scholar] [CrossRef]
- Falter, M.; Budts, W.; Goetschalckx, K.; Cornelissen, V.; Buys, R. Accuracy of Apple Watch Measurements for Heart Rate and Energy Expenditure in Patients With Cardiovascular Disease: Cross-Sectional Study. JMIR mHealth uHealth 2019, 7, e11889. [Google Scholar] [CrossRef]
- Rafolt, D.; Gallasch, E. Influence of contact forces on wrist photoplethysmography–prestudy for a wearable patient monitor. Biomed. Technik. Biomed. Eng. 2004, 49, 22–26. [Google Scholar] [CrossRef]
- Wang, W.; den Brinker, A.C.; Stuijk, S.; de Haan, G. Algorithmic Principles of Remote PPG. IEEE Trans. bio-Med. Eng. 2017, 64, 1479–1491. [Google Scholar] [CrossRef] [Green Version]
- Parak, J.; Korhonen, I. Evaluation of wearable consumer heart rate monitors based on photopletysmography. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 3670–3673. [Google Scholar] [CrossRef]
- Zhang, Z.; Pi, Z.; Liu, B. TROIKA: A general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE Trans. bio-Med. Eng. 2015, 62, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Liu, I.; Ni, S.; Peng, K. Enhancing the Robustness of Smartphone Photoplethysmography: A Signal Quality Index Approach. Sensors 2020, 20, 1923. [Google Scholar] [CrossRef] [Green Version]
- Majeed, I.A.; Jos, S.; Arora, R.; Choi, K.; Bae, S. Motion Artifact Removal of Photoplethysmogram (PPG) Signal. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 5576–5580. [Google Scholar] [CrossRef]
- Zhu, S.; Tan, K.; Zhang, X.; Liu, Z.; Liu, B. MICROST: A mixed approach for heart rate monitoring during intensive physical exercise using wrist-type PPG Signals. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; Volume 2015, pp. 2347–2350. [Google Scholar] [CrossRef]
- Asada, H.H.; Shaltis, P.; Reisner, A.; Rhee, S.; Hutchinson, R.C. Mobile monitoring with wearable photoplethysmographic biosensors. IEEE Eng. Med. Biol. Mag. 2003, 22, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Cennini, G.; Arguel, J.; Aksit, K.; van Leest, A. Heart rate monitoring via remote photoplethysmography with motion artifacts reduction. Opt. Express 2010, 18, 4867–4875. [Google Scholar] [CrossRef]
- Han, H.; Kim, M.J.; Kim, J. Development of real-time motion artifact reduction algorithm for a wearable photoplethysmography. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Volume 2007, pp. 1538–1541. [Google Scholar] [CrossRef]
- Poh, M.Z.; Swenson, N.C.; Picard, R.W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef]
- Wilkosz, M.; Szczȩsna, A. Multi-Headed Conv-LSTM Network for Heart Rate Estimation during Daily Living Activities. Sensors 2021, 21, 5212. [Google Scholar] [CrossRef]
- Sushames, A.; Edwards, A.; Thompson, F.; McDermott, R.; Gebel, K. Validity and Reliability of Fitbit Flex for Step Count, Moderate to Vigorous Physical Activity and Activity Energy Expenditure. PLoS ONE 2016, 11, e0161224. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wen, D.; Liang, L.; Jia, Y.; Gao, L.; Lei, J. Evaluating the Validity of Current Mainstream Wearable Devices in Fitness Tracking Under Various Physical Activities: Comparative Study. JMIR mHealth uHealth 2018, 6, e94. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Gonzalo, R.; Parak, J.; Tarniceriu, A.; Renevey, P.; Bertschi, M.; Korhonen, I. Evaluation of accuracy and reliability of PulseOn optical heart rate monitoring device. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; Volume 2015, pp. 430–433. [Google Scholar] [CrossRef]
- Nelson, B.W.; Allen, N.B. Accuracy of Consumer Wearable Heart Rate Measurement During an Ecologically Valid 24-Hour Period: Intraindividual Validation Study. JMIR mHealth uHealth 2019, 7, e10828. [Google Scholar] [CrossRef]
- Fuller, D.; Colwell, E.; Low, J.; Orychock, K.; Tobin, M.A.; Simango, B.; Buote, R.; Van Heerden, D.; Luan, H.; Cullen, K.; et al. Reliability and Validity of Commercially Available Wearable Devices for Measuring Steps, Energy Expenditure, and Heart Rate: Systematic Review. JMIR mHealth uHealth 2020, 8, e18694. [Google Scholar] [CrossRef]
- Boudreaux, B.D.; Hebert, E.P.; Hollander, D.B.; Williams, B.M.; Cormier, C.L.; Naquin, M.R.; Gillan, W.W.; Gusew, E.E.; Kraemer, R.R. Validity of Wearable Activity Monitors during Cycling and Resistance Exercise. Med. Sci. Sport. Exerc. 2018, 50, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.A.; Nuss, K.; Comstock, A.; Reinwald, S.; Blake, S.; Pimentel, R.E.; Tracy, B.L.; Li, K. Heart rate measures from the Apple Watch, Fitbit Charge HR 2, and electrocardiogram across different exercise intensities. J. Sport. Sci. 2019, 37, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.F.; Stergiou, P.R.O.; Fung, T.A.K.S.; Katz, L. Comparison of Polar M600 Optical Heart Rate and ECG Heart Rate during Exercise. Med. Sci. Sport. Exerc. 2017, 49, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Chevance, G.; Golaszewski, N.M.; Tipton, E.; Hekler, E.B.; Buman, M.; Welk, G.J.; Patrick, K.; Godino, J.G. Accuracy and Precision of Energy Expenditure, Heart Rate, and Steps Measured by Combined-Sensing Fitbits Against Reference Measures: Systematic Review and Meta-analysis. JMIR mHealth uHealth 2022, 10, e35626. [Google Scholar] [CrossRef]
- Jo, E.; Lewis, K.; Directo, D.; Kim, M.J.; Dolezal, B.A. Validation of Biofeedback Wearables for Photoplethysmographic Heart Rate Tracking. J. Sport. Sci. Med. 2016, 15, 540–547. [Google Scholar]
- Takacs, J.; Pollock, C.L.; Guenther, J.R.; Bahar, M.; Napier, C.; Hunt, M.A. Validation of the Fitbit One activity monitor device during treadmill walking. J. Sci. Med. Sport 2014, 17, 496–500. [Google Scholar] [CrossRef]
- Cassirame, J.; Vanhaesebrouck, R.; Chevrolat, S.; Mourot, L. Accuracy of the Garmin 920 XT HRM to perform HRV analysis. Australas. Phys. Eng. Sci. Med. 2017, 40, 831–839. [Google Scholar] [CrossRef]
- Giles, D.; Draper, N.; Neil, W. Validity of the Polar V800 heart rate monitor to measure RR intervals at rest. Eur. J. Appl. Physiol. 2016, 116, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Gamelin, F.X.; Berthoin, S.; Bosquet, L. Validity of the polar S810 heart rate monitor to measure R-R intervals at rest. Med. Sci. Sport. Exerc. 2006, 38, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Spierer, D.K.; Rosen, Z.; Litman, L.L.; Fujii, K. Validation of photoplethysmography as a method to detect heart rate during rest and exercise. J. Med. Eng. Technol. 2015, 39, 264–271. [Google Scholar] [CrossRef]
- Porto, L.G.G.; Junqueira, L.F.J. Comparison of time-domain short-term heart interval variability analysis using a wrist-worn heart rate monitor and the conventional electrocardiogram. Pacing Clin. Electrophysiol. PACE 2009, 32, 43–51. [Google Scholar] [CrossRef]
- Claes, J.; Buys, R.; Avila, A.; Finlay, D.; Kennedy, A.; Guldenring, D.; Budts, W.; Cornelissen, V. Validity of heart rate measurements by the Garmin Forerunner 225 at different walking intensities. J. Med Eng. Technol. 2017, 41, 480–485. [Google Scholar] [CrossRef]
- Caminal, P.; Sola, F.; Gomis, P.; Guasch, E.; Perera, A.; Soriano, N.; Mont, L. Validity of the Polar V800 monitor for measuring heart rate variability in mountain running route conditions. Eur. J. Appl. Physiol. 2018, 118, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Hernando, D.; Garatachea, N.; Almeida, R.; Casajús, J.A.; Bailón, R. Validation of Heart Rate Monitor Polar RS800 for Heart Rate Variability Analysis During Exercise. J. Strength Cond. Res. 2018, 32, 716–725. [Google Scholar] [CrossRef]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the Polar H10 Sensor for Heart Rate Variability Analysis during Resting State and Incremental Exercise in Recreational Men and Women. Sensors 2022, 22, 6536. [Google Scholar] [CrossRef]
- Pasadyn, S.R.; Soudan, M.; Gillinov, M.; Houghtaling, P.; Phelan, D.; Gillinov, N.; Bittel, B.; Desai, M.Y. Accuracy of commercially available heart rate monitors in athletes: A prospective study. Cardiovasc. Diagn. Ther. 2019, 9, 379–385. [Google Scholar] [CrossRef]
- Chhetri, P.; Shrestha, L.; Mahotra, N.B. Validity of Elite-HRV Smartphone Application for Measuring Heart Rate Variability Compared to Polar V800 Heart Rate Monitor. J. Nepal Health Res. Counc. 2022, 19, 809–813. [Google Scholar] [CrossRef]
- Hettiarachchi, I.T.; Hanoun, S.; Nahavandi, D.; Nahavandi, S. Validation of Polar OH1 optical heart rate sensor for moderate and high intensity physical activities. PLoS ONE 2019, 14, e0217288. [Google Scholar] [CrossRef] [Green Version]
- Fokkema, T.; Kooiman, T.J.M.; Krijnen, W.P.; VAN DER Schans, C.P.; DE Groot, M. Reliability and Validity of Ten Consumer Activity Trackers Depend on Walking Speed. Med. Sci. Sport. Exerc. 2017, 49, 793–800. [Google Scholar] [CrossRef]
- Ho, W.T.; Yang, Y.J.; Li, T.C. Accuracy of wrist-worn wearable devices for determining exercise intensity. Digit. Health 2022, 8, 20552076221124393. [Google Scholar] [CrossRef]
- Hernández-Vicente, A.; Hernando, D.; Marín-Puyalto, J.; Vicente-Rodríguez, G.; Garatachea, N.; Pueyo, E.; Bailón, R. Validity of the Polar H7 Heart Rate Sensor for Heart Rate Variability Analysis during Exercise in Different Age, Body Composition and Fitness Level Groups. Sensors 2021, 21, 902. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, A.M.; Fuentes-Guajardo, M.; Latorre, K.; León, D.; Martín-Escudero, P. Skin Pigmentation Influence on Pulse Oximetry Accuracy: A Systematic Review and Bibliometric Analysis. Sensors 2022, 22, 3402. [Google Scholar] [CrossRef] [PubMed]
- Degroote, L.; De Bourdeaudhuij, I.; Verloigne, M.; Poppe, L.; Crombez, G. The Accuracy of Smart Devices for Measuring Physical Activity in Daily Life: Validation Study. JMIR mHealth uHealth 2018, 6, e10972. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Menghini, L.; Gianfranchi, E.; Cellini, N.; Patron, E.; Tagliabue, M.; Sarlo, M. Stressing the accuracy: Wrist-worn wearable sensor validation over different conditions. Psychophysiology 2019, 56, e13441. [Google Scholar] [CrossRef]
- Nelson, B.W.; Low, C.A.; Jacobson, N.; Areán, P.; Torous, J.; Allen, N.B. Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. NPJ Digit. Med. 2020, 3, 90. [Google Scholar] [CrossRef]




| ID | Age (years) | Size (m) | Weight (Kg) | BMI (Kg/m) | (mL/kg/min) | Sport | Tested Devices | Test Type |
|---|---|---|---|---|---|---|---|---|
| 00 | 40 | 1.85 | 81.6 | 23.84 | 52.10 | Athletics | TT+FB | TM |
| 01 | 39 | 1.69 | 69.9 | 24.47 | 57.42 | Triathlon | TT+FB | CE |
| 02 | 25 | 1.59 | 53 | 20.96 | 48.77 | Athletics | AW+FB | CE |
| 03 | 42 | 1.73 | 60 | 20.05 | 57.95 | Athletics | AW+FB | CE |
| 04 | 25 | 1.7 | 57.5 | 19.90 | 49.81 | Soccer | AW+G2 | CE |
| 05 | 26 | 1.78 | 65.2 | 20.58 | 67.36 | Athletics | AW+G2 | CE |
| 06 | 32 | 1.74 | 72 | 23,78 | 60.15 | Cycling | AW+FB | CE |
| 07 | 51 | 1.74 | 81 | 26.75 | 42.00 | Athletics | AW+FB | TM |
| ± | 35 ± 9.53 | 1.73 ± 0.07 | 67.53 ± 10.55 | 22.54 ± 2.51 | 54.45 ± 7.88 |
| ECG vs. Device | N | Mean ECG (SD) | Mean Device (SD) | Mean Difference ECG-Device (CI 95%) | p | ICC (CI 95%) |
|---|---|---|---|---|---|---|
| All Measurements | ||||||
| ECG vs. FB | 377 | 127.5 (28.6) | 115.5 (26.5) | 11.9 (9.9; 14.0) | <0.001 | 0.675 (0.434; 0.798) |
| ECG vs. TT | 321 | 134.9 (27.5) | 133.9 (27.2) | 1.1 (0.2; 1.9) | 0.013 | 0.961 (0.952; 0.969) |
| ECG vs. AW | 440 | 129.7 (29.2) | 129.4 (29.5) | 0.3 (−0.3; 1.0) | 0.301 | 0.970 (0.96; 0.97) |
| EECG vs. G2 | 148 | 143.3 (25.4) | 80.5 (14.2) | 62.7 (58.1; 67.4) | <0.001 | 0.005 (−0.022; 0.040) |
| Interval <100 HR | ||||||
| ECG vs. FB | 117 | 94.3 (12.4) | 89.7 (14.8) | 4.5 (2.9; 6.1) | <0.001 | 0.746 (0.568; 0.844) |
| ECG vs. TT | 75 | 98.2 (9.9) | 98.3 (13.3) | −0.1 (−2.2; 2.1) | 0.937 | 0.683 (0.540; 0.787) |
| ECG vs. AW | 123 | 93.9 (12.3) | 94.4 (14.8) | −0.5 (−2.0; 1.0) | 0.514 | 0.799 (0.724; 0.855) |
| ECG vs. G2 | 16 | 103.6 (4.6) | 76.8 (1.36) | 26.7 (24.5–29.0) | <0.001 | 0.007 (−0.009; 0.052) |
| Interval 100–150 | ||||||
| ECG vs. FB | 167 | 130.0 (11.6) | 119.7 (18.6) | 10.3 (7.2; 13.3) | <0.001 | 0.148 (0.004; 0.288) |
| ECG vs. TT | 132 | 129.5 (11.07) | 129.2 (11.6) | 0.3 (−0.4; 0.9) | 0.449 | 0.938 (0.914; 0.956) |
| ECG vs. AW | 196 | 129.8 (11.6) | 130.2 (11.9) | 0.4 (−0.2; 1.0) | 0.239 | 0.930 (0.908; 0.946) |
| ECG vs. G2 | 70 | 130.2 (11.9) | 80.35 (15.3) | 49.9 (45.8–53.9) | <0.001 | 0.030 (−0.030; 0.126) |
| Interval >150 HR | ||||||
| ECG vs. FB | 93 | 164.8 (8.7) | 140.3 (21.9) | 24.5 (18.5; 9.4) | <0.001 | −0.019 (−0.111; 0.094) |
| ECG vs. TT | 114 | 165.5 (8.4) | 162.7 (10.4) | 2.7 (1.0–4.4) | 0.002 | 0.528 (0.374; 0.653) |
| ECG vs. AW | 121 | 166 (10.7) | 164.8 (13.8) | 1.2(−0.4; 2.8) | 0.146 | 0.729 (0.634; 0.803) |
| ECG vs. G2 | 62 | 168.3 (12.2) | 81.7 (14.6) | 86.6 (80.6–92.5) | <0.001 | −0.024 (−0.035; 0.065) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Escudero, P.; Cabanas, A.M.; Dotor-Castilla, M.L.; Galindo-Canales, M.; Miguel-Tobal, F.; Fernández-Pérez, C.; Fuentes-Ferrer, M.; Giannetti, R. Are Activity Wrist-Worn Devices Accurate for Determining Heart Rate during Intense Exercise? Bioengineering 2023, 10, 254. https://doi.org/10.3390/bioengineering10020254
Martín-Escudero P, Cabanas AM, Dotor-Castilla ML, Galindo-Canales M, Miguel-Tobal F, Fernández-Pérez C, Fuentes-Ferrer M, Giannetti R. Are Activity Wrist-Worn Devices Accurate for Determining Heart Rate during Intense Exercise? Bioengineering. 2023; 10(2):254. https://doi.org/10.3390/bioengineering10020254
Chicago/Turabian StyleMartín-Escudero, Pilar, Ana María Cabanas, María Luisa Dotor-Castilla, Mercedes Galindo-Canales, Francisco Miguel-Tobal, Cristina Fernández-Pérez, Manuel Fuentes-Ferrer, and Romano Giannetti. 2023. "Are Activity Wrist-Worn Devices Accurate for Determining Heart Rate during Intense Exercise?" Bioengineering 10, no. 2: 254. https://doi.org/10.3390/bioengineering10020254