An Integrated Downstream Process Development Strategy along QbD Principles
Abstract
:1. Introduction
1.1. Process Development along QbD Principles
1.2. Parameter Interactions
1.3. Design of Experiments
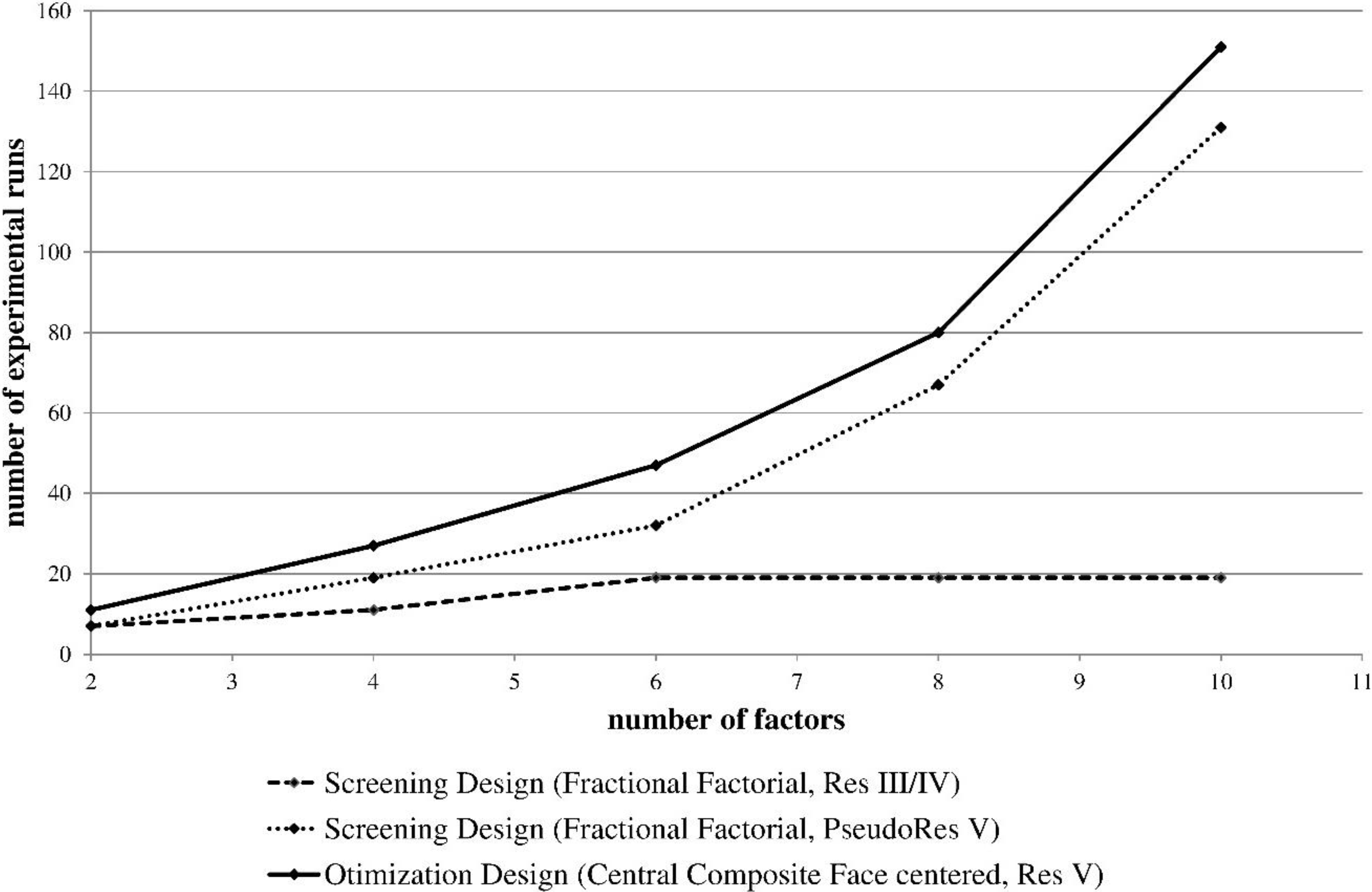
1.4. Risk Assessments
1.5. Goal
2. Experimental Section
2.1. Strain and Media
2.2. Harvest and Inclusion Body (IB) Processing
| Exp No | Exp Name | Run Order | Triton X-100 concentration | Passages | Time centrifugation | g-number centrifugation |
|---|---|---|---|---|---|---|
| 1 | N1 | 2 | 0 | 2 | 10 | 3000 |
| 2 | N2 | 15 | 1 | 2 | 10 | 3000 |
| 3 | N3 | 1 | 0 | 6 | 10 | 3000 |
| 4 | N4 | 23 | 1 | 6 | 10 | 3000 |
| 5 | N5 | 6 | 0 | 2 | 30 | 3000 |
| 6 | N6 | 11 | 1 | 2 | 30 | 3000 |
| 7 | N7 | 8 | 0 | 6 | 30 | 3000 |
| 8 | N8 | 19 | 1 | 6 | 30 | 3000 |
| 9 | N9 | 9 | 0 | 2 | 10 | 13,000 |
| 10 | N10 | 25 | 1 | 2 | 10 | 13,000 |
| 11 | N11 | 20 | 0 | 6 | 10 | 13,000 |
| 12 | N12 | 12 | 1 | 6 | 10 | 13,000 |
| 13 | N13 | 24 | 0 | 2 | 30 | 13,000 |
| 14 | N14 | 14 | 1 | 2 | 30 | 13,000 |
| 15 | N15 | 27 | 0 | 6 | 30 | 13,000 |
| 16 | N16 | 22 | 1 | 6 | 30 | 13,000 |
| 17 | N17 | 3 | 0 | 4 | 20 | 8000 |
| 18 | N18 | 5 | 1 | 4 | 20 | 8000 |
| 19 | N19 | 7 | 0.5 | 2 | 20 | 8000 |
| 20 | N20 | 21 | 0.5 | 6 | 20 | 8000 |
| 21 | N21 | 10 | 0.5 | 4 | 10 | 8000 |
| 22 | N22 | 26 | 0.5 | 4 | 30 | 8000 |
| 23 | N23 | 18 | 0.5 | 4 | 20 | 3000 |
| 24 | N24 | 17 | 0.5 | 4 | 20 | 13,000 |
| 25 | N25 | 13 | 0.5 | 4 | 20 | 8000 |
| 26 | N26 | 16 | 0.5 | 4 | 20 | 8000 |
| 27 | N27 | 4 | 0.5 | 4 | 20 | 8000 |
2.3. Experimental Design
3. Results and Discussion
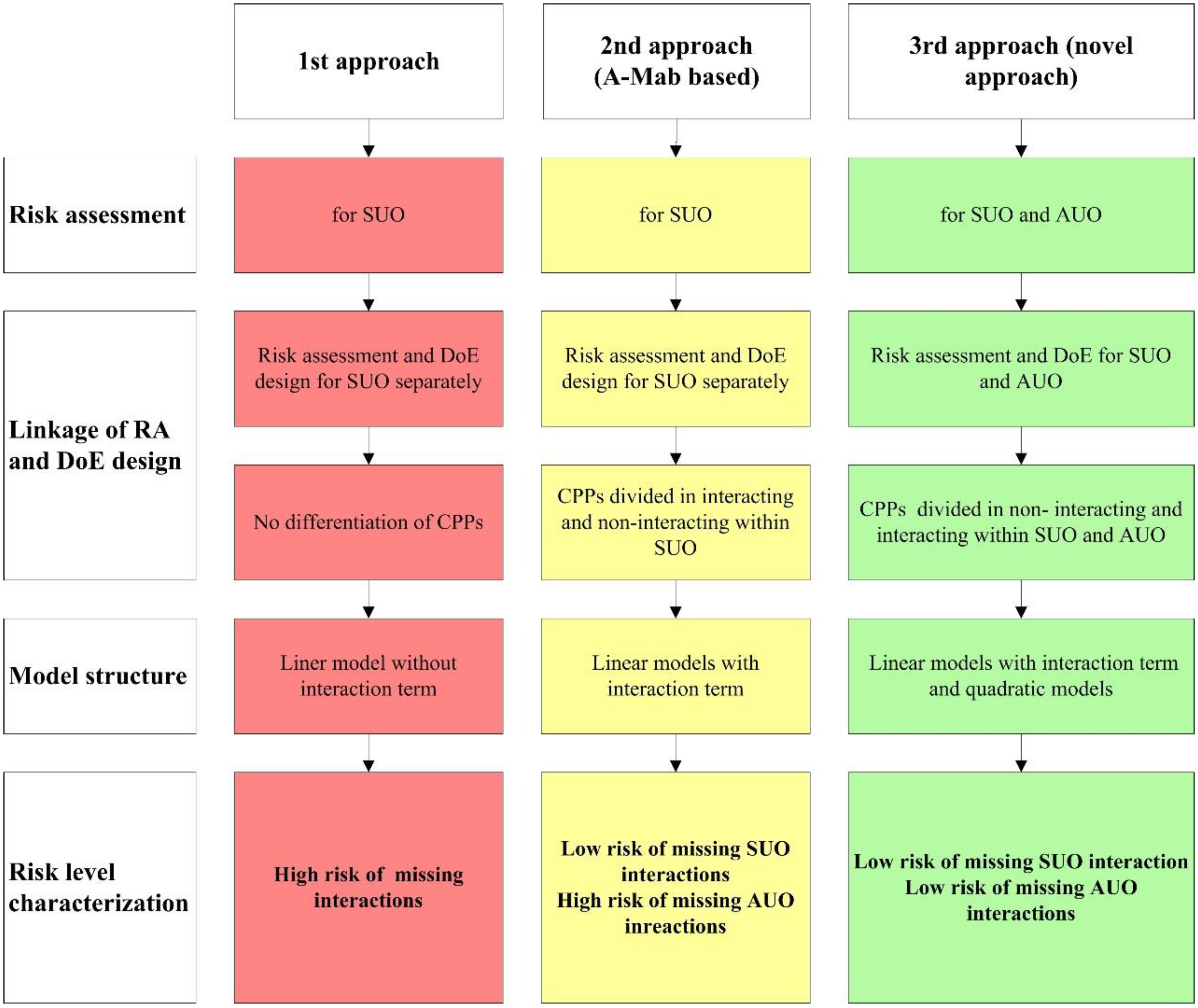
3.1. Strategy Overview

3.2. Risk Assessment 1—Identification of Parameters for Process Characterization
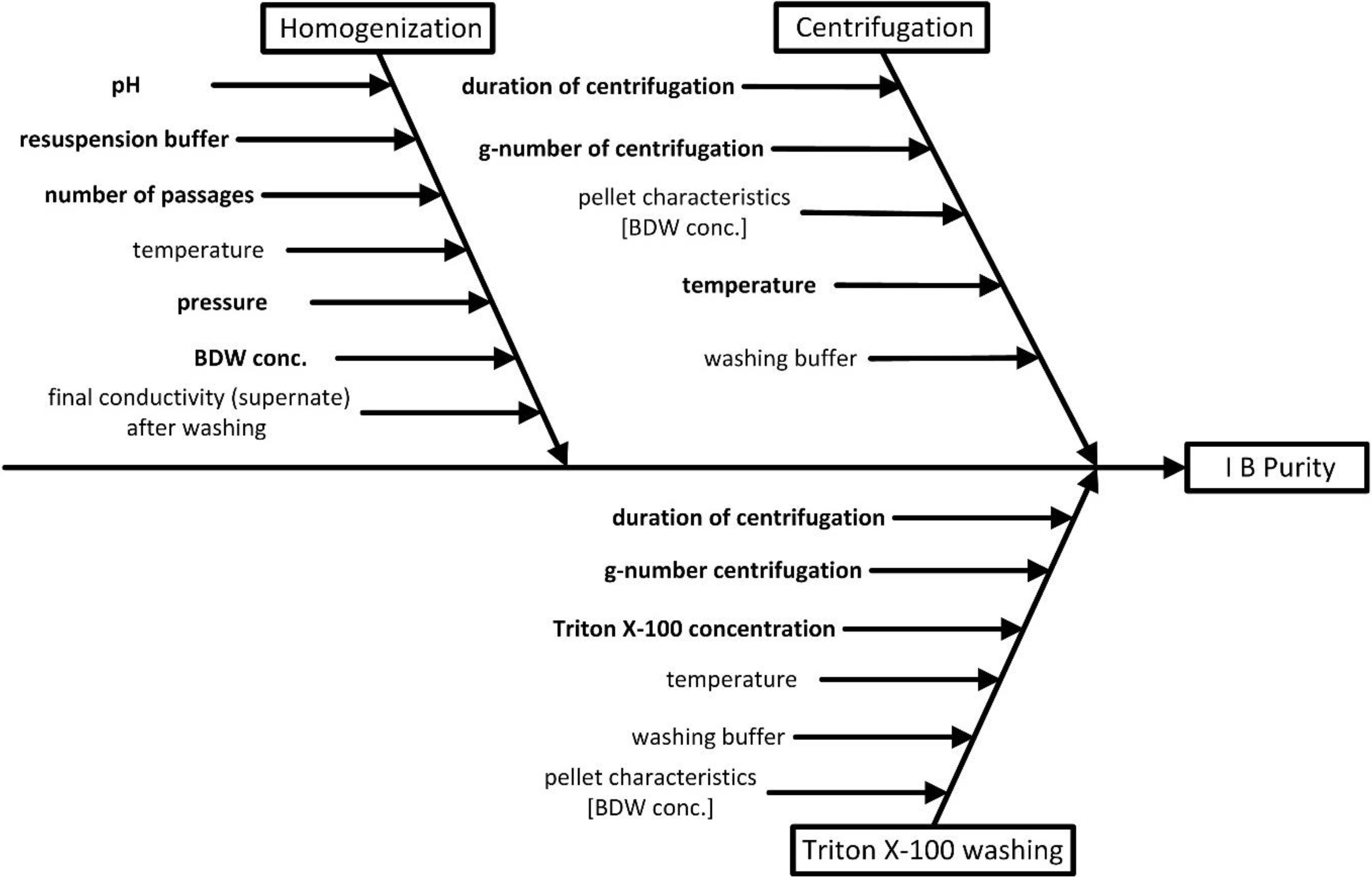
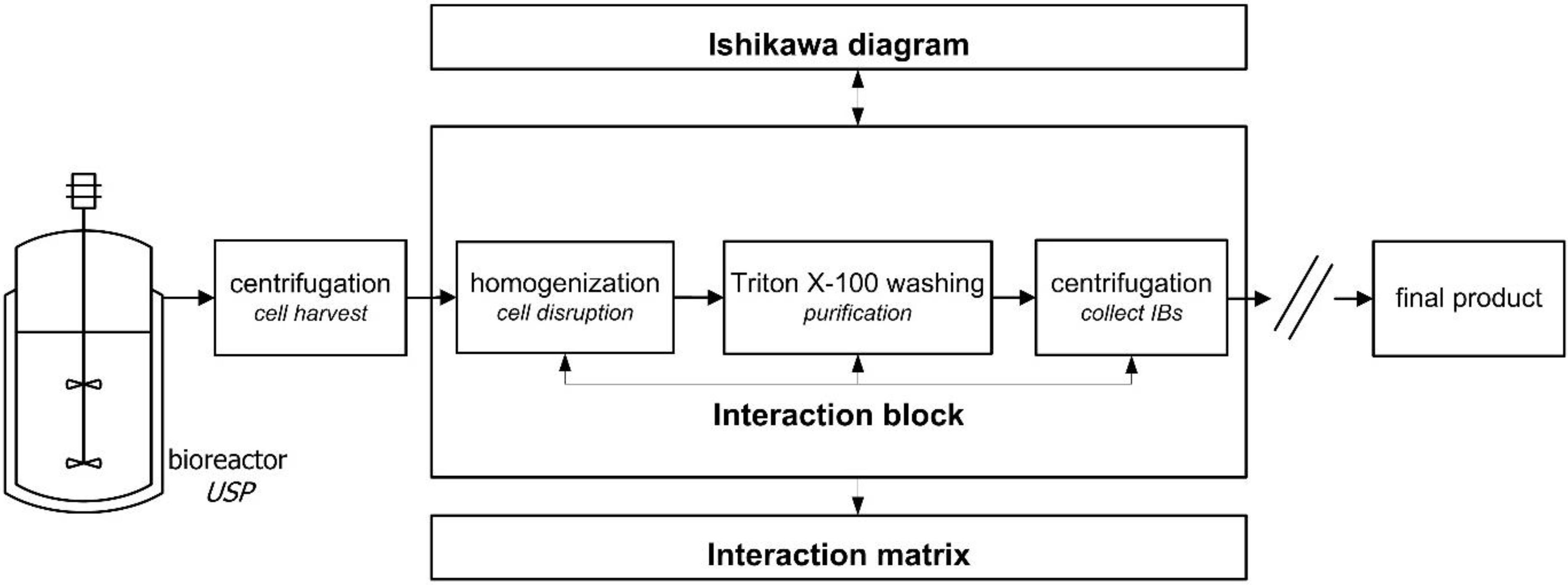
3.3. Risk Assessment 2—Interaction Matrix across Unit Operations (AUO)
| probability of interaction | washing buffer pH | washing buffer Triton X-100 concentration | washing buffer temperature | washing buffer DTT -concentration | number of passages homogenizer | temperature homogenizer | pressure homogenizer | BDW concentration homogenizer | duration centrifugation | g-number centrifugation | temperature centrifugation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| washing buffer pH | 0.1 | 0.3 | 0.1 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 |
| washing buffer Triton X-100 concentration | 0.3 | 0.1 | 0.1 | 0.1 | 0.5 | 0.3 | 0.3 | 0.3 | 0.7 | 0.7 | 0.1 |
| washing buffer temperature | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| washing buffer DTT - concentration | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| number of passages homogenizer | 0.3 | 0.5 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.5 | 0.5 | 0.1 |
| temperature homogenizer | 0.1 | 0.3 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| pressure homogenizer | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 |
| BDW concentration homogenizer | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 |
| duration centrifugation | 0.1 | 0.7 | 0.1 | 0.1 | 0.5 | 0.1 | 0.3 | 0.3 | 0.1 | 0.7 | 0.1 |
| g-number centrifugation | 0.1 | 0.7 | 0.1 | 0.1 | 0.5 | 0.1 | 0.3 | 0.3 | 0.7 | 0.1 | 0.3 |
| temperature centrifugation | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 |
3.4. Power of Approach is Demonstrated for Early Downstream Protein Isolation Steps for rhGF
3.4.1. DoE Design
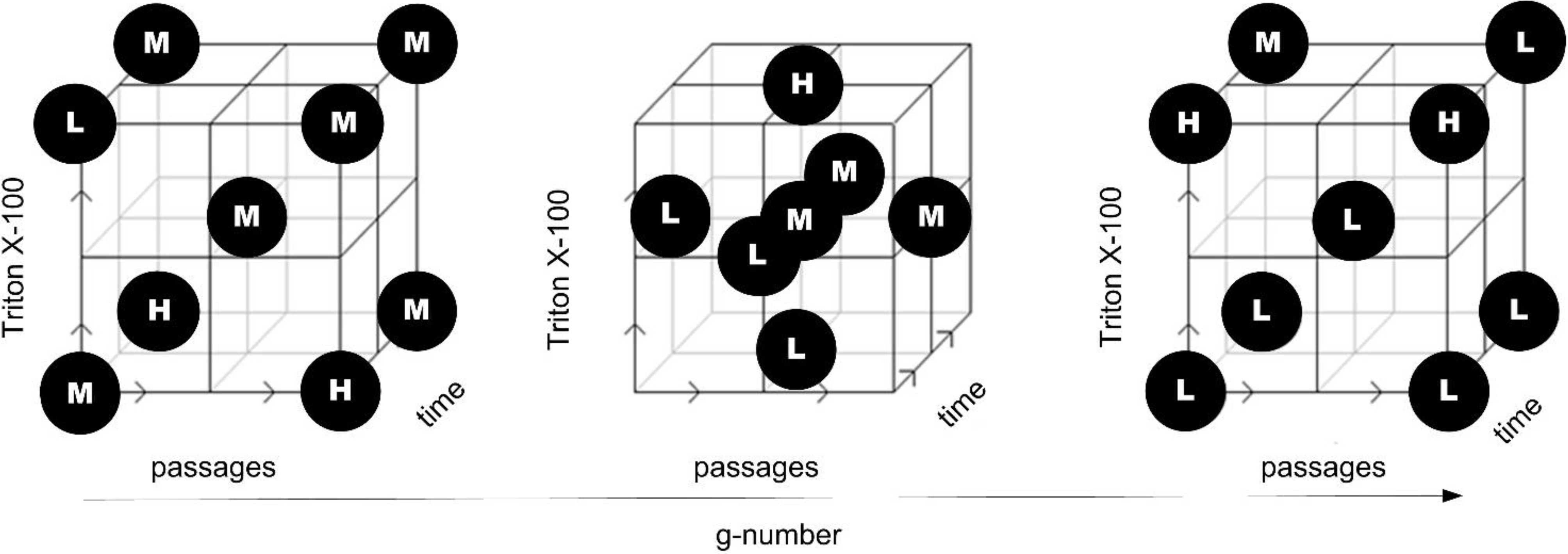
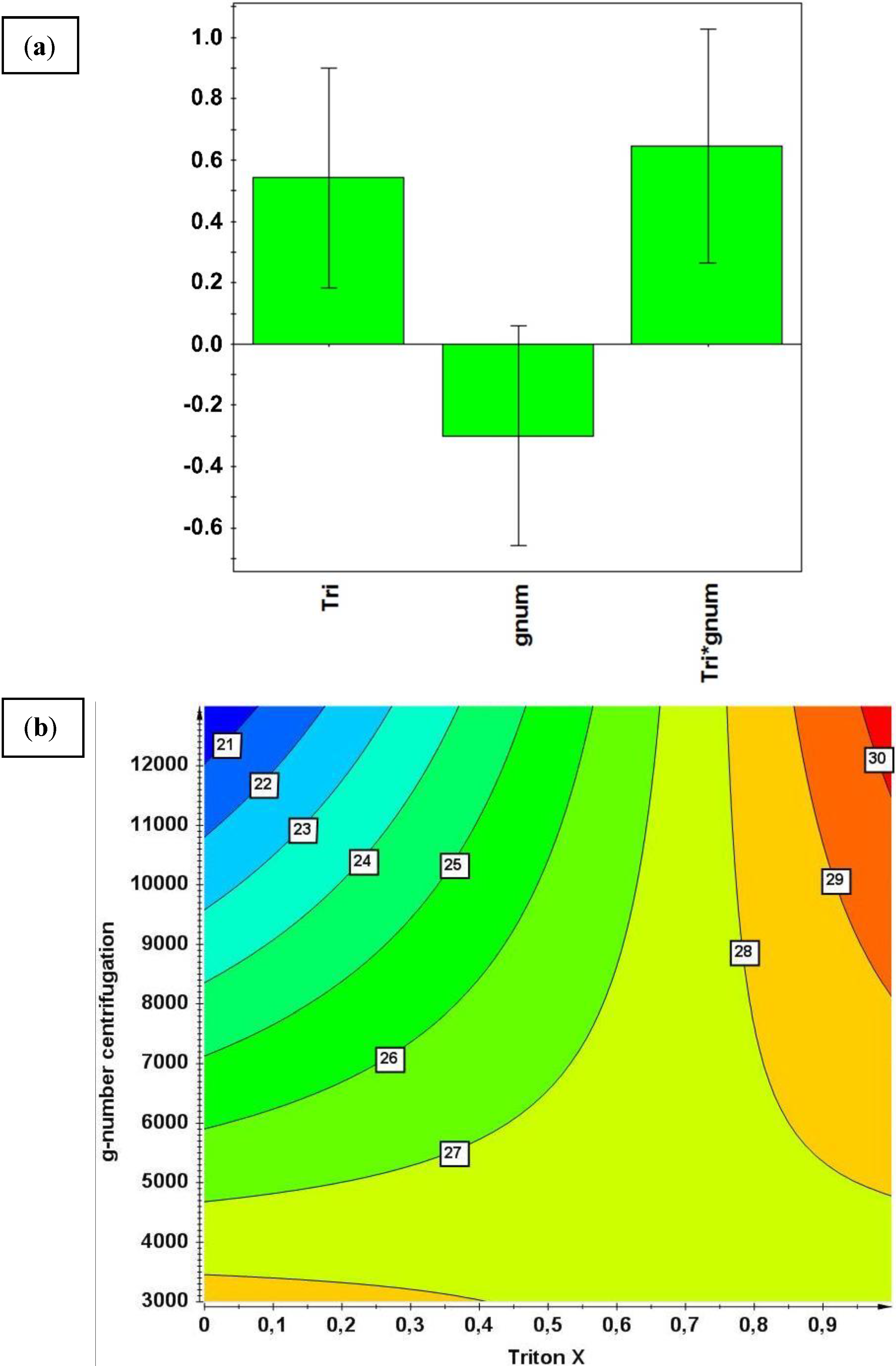
3.4.2. Multivariate DoE Study across Unit Operations
- The Triton X-100 concentration during the washing step showed a main effect on CQA IB purity
- A significant interaction effect between the process parameters Triton X-100 concentration and g-number of centrifugation was observed
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Gottschalk, U. The future of downstream processing-2013. BioPharm. Int. 2013, 24, S39–S45. [Google Scholar]
- Rathore, A.S.; Winkle, H. Quality by design for biopharmaceuticals. Nat. Biotechnol. 2009, 27, 26–34. [Google Scholar] [CrossRef] [PubMed]
- PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine, Office of Regulatory Affairs: Rockville, MD, USA, 2004.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Pharmaceutical Development Q8(R2). 2009. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf (accessed on 31 July 2014).
- Rathore, A.S. Implementation of quality by design (QbD) for biopharmaceutical products. PDA J. Pharm. Sci. Technol. 2010, 64, 495–496. [Google Scholar] [PubMed]
- Rathore, A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Devine, R. PDA workshop on “Quality by Design for Biopharmaceuticals: Concepts and Implementation”, May 21–22, 2007, Bethesda, Maryland. PDA J. Pharm. Sci. Technol. 2008, 62, 380–390. [Google Scholar] [PubMed]
- Mandenius, C.F.; Graumann, K.; Schultz, T.W.; Premstaller, A.; Olsson, I.M.; Petiot, E.; Clemens, C.; Welin, M. Quality-by-design for biotechnology-related pharmaceuticals. Biotechnol. J. 2009, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Branning, R.; Cecchini, D. Quality: Design space for biotech products. Biopharm. Int. 2007, 20. Available online: http://www.biopharminternational.com/biopharm/Article/Quality-Design-Space-for-Biotech-Products/ArticleStandard/Article/detail/415832 (accessed on 31 July 2014).
- ICH Harmonised Tripartite Guideline. Quality Risk Management Q9. 2005. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf (accessed on 31 July 2014).
- ICH Harmonised Tripartite Guideline. Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) Q11. 2012. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Step_4.pdf (accessed on 31 July 2014).
- ICH Harmonised Tripartite Guideline. Pharmaceutical Quality System Q10. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf (accessed on 31 July 2014).
- Bade, P.D.; Kotu, S.P.; Rathore, A.S. Optimization of a refolding step for a therapeutic fusion protein in the quality by design (QbD) paradigm. J. Separ. Sci. 2012, 35, 3160–3169. [Google Scholar] [CrossRef]
- Bhambure, R.; Rathore, A.S. Chromatography process development in the quality by design paradigm I: Establishing a high-throughput process development platform as a tool for estimating “characterization space” for an ion exchange chromatography step. Biotechnol. Progr. 2013, 29, 403–414. [Google Scholar] [CrossRef]
- Rathore, A.S.; Yu, M.; Yeboah, S.; Sharma, A. Case study and application of process analytical technology (PAT) towards bioprocessing: use of on-line high-performance liquid chromatography (HPLC) for making real-time pooling decisions for process chromatography. Biotechnol. Bioeng. 2008, 100, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Rao Dasari, V.K.; Are, D.; Rao Joginapally, V.; Mangamoori, L.N.; Rao Adibhatla, K.S.B. Optimization of the downstream process for high recovery of rhG-CSF from inclusion bodies expressed in Escherichia coli. Process Biochem. 2008, 43, 566–575. [Google Scholar] [CrossRef]
- Gao, W.J.J.; Lin, H.J.; Leung, K.T.; Liao, B.Q. Influence of elevated pH shocks on the performance of a submerged anaerobic membrane bioreactor. Process Biochem. 2010, 45, 1279–1287. [Google Scholar] [CrossRef]
- Pieracci, J.; Perry, L.; Conley, L. Using partition designs to enhance purification process understanding. Biotechnol. Bioeng. 2010, 107, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Harms, J.; Wang, X.Y.; Kim, T.; Yang, X.M.; Rathore, A.S. Defining process design space for biotech products: Case study of Pichia pastoris fermentation. Biotechnol. Progr. 2008, 24, 655–662. [Google Scholar] [CrossRef]
- Looby, M.; Ibarra, N.; Pierce, J.J.; Buckley, K.; O’Donovan, E.; Heenan, M.; Moran, E.; Farid, S.S.; Baganz, F. Application of quality by design principles to the development and technology transfer of a major process improvement for the manufacture of a recombinant protein. Biotechnol. Progr. 2011, 27, 1718–1729. [Google Scholar] [CrossRef]
- Montgomery, D.C. Experimental design for product and process design and development. J. Royal Statistical Soc. Ser. D (The Statistician) 1999, 48, 159–177. [Google Scholar] [CrossRef]
- Czitrom, V. One-Factor-at-a-Time versus designed experiments. Am. Statistician 1999, 53, 126–131. [Google Scholar]
- Wahid, Z.; Nadir, N. Improvement of one factor at a time through design of experiments. WASJ 2013, 21, 56–61. [Google Scholar]
- NIST/SEMATECH e-Handbook of Statistical Methods. Available online: http://itl.nist.gov/div898/handbook/index.htm (accessed on 31 July 2014).
- Telford, J.K. A brief introduction to design of experiments. Johns Hopkins Apl. Technical. Digest. 2007, 27, 224–232. [Google Scholar]
- Garcia, T.; Cook, G.; Nosal, R. PQLI key topics—Criticality, design space, and control strategy. J. Pharm. Innov. 2008, 3, 60–68. [Google Scholar] [CrossRef]
- Guebitz, B.; Schnedl, H.; Khinast, J.G. A risk management ontology for Quality-by-Design based on a new development approach according GAMP 5.0. Expert Syst. Appl. 2012, 39, 7291–7301. [Google Scholar] [CrossRef]
- Schmidt, R.; Riedel, G.J.; Kangas, K. Risk Assessment Using Design Review Based on Failure Mode. In Proceedings of 2011 Annual Reliability and Maintainability Symposium (RAMS), Lake Buena Vista, FL, USA, 24–27 January 2011; pp. 1–6.
- Dizon-Maspat, J.; Bourret, J.; D’Agostini, A.; Li, F. Single pass tangential flow filtration to debottleneck downstream processing for therapeutic antibody production. Biotechnol. Bioeng. 2012, 109, 962–970. [Google Scholar] [CrossRef] [PubMed]
- CMC Biotech Working Group. A-Mab: A case Study in Bioprocess Development. Available online: http://www.casss.org/?page=286 (accessed on 31 July 2014).
- Maachupalli-Reddy, J.; Kelley, B.D.; Clark, E.D.B. Effect of inclusion body contaminants on the oxidative renaturation of hen egg white lysozyme. Biotechnol. Progr. 1997, 13, 144–150. [Google Scholar] [CrossRef]
- DeLisa, M.P.; Li, J.; Rao, G.; Weigand, W.A.; Bentley, W.E. Monitoring GFP-operon fusion protein expression during high cell density cultivation of Escherichia coli using an on-line optical sensor. Biotechnol. Bioeng. 1999, 65, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Margreiter, G.; Messner, P.; Caldwell, K.D.; Bayer, K. Size characterization of inclusion bodies by sedimentation field-flow fractionation. J. Biotechnol. 2008, 138, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Batas, B.; Schiraldi, C.; Chaudhuri, J.B. Inclusion body purification and protein refolding using microfiltration and size exclusion chromatography. J. Biotechnol. 1999, 68, 149–158. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meitz, A.; Sagmeister, P.; Langemann, T.; Herwig, C. An Integrated Downstream Process Development Strategy along QbD Principles. Bioengineering 2014, 1, 213-230. https://doi.org/10.3390/bioengineering1040213
Meitz A, Sagmeister P, Langemann T, Herwig C. An Integrated Downstream Process Development Strategy along QbD Principles. Bioengineering. 2014; 1(4):213-230. https://doi.org/10.3390/bioengineering1040213
Chicago/Turabian StyleMeitz, Andrea, Patrick Sagmeister, Timo Langemann, and Christoph Herwig. 2014. "An Integrated Downstream Process Development Strategy along QbD Principles" Bioengineering 1, no. 4: 213-230. https://doi.org/10.3390/bioengineering1040213





