Preparation and Photocatalytic/Photoelectrochemical Investigation of 2D ZnO/CdS Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
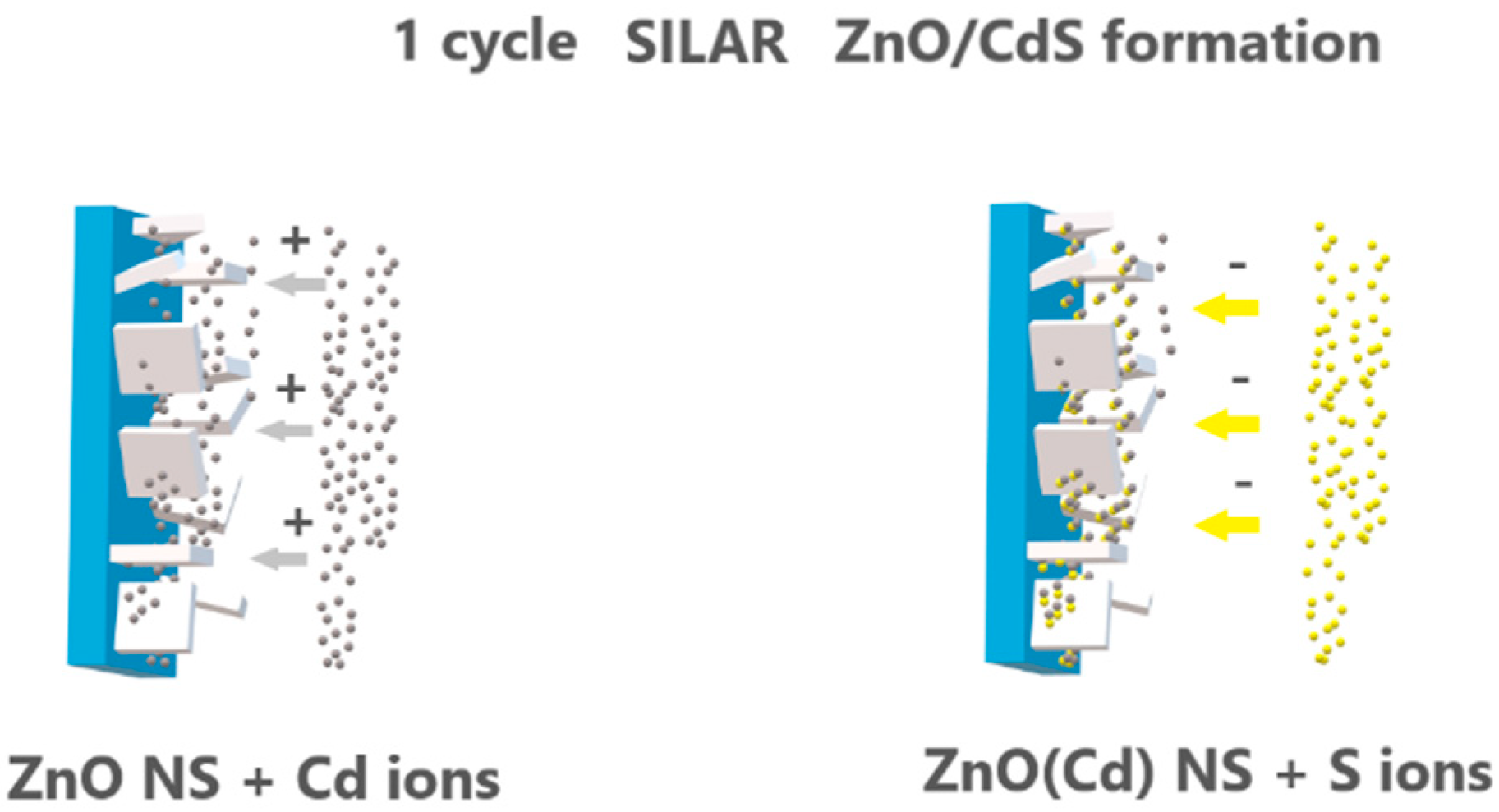
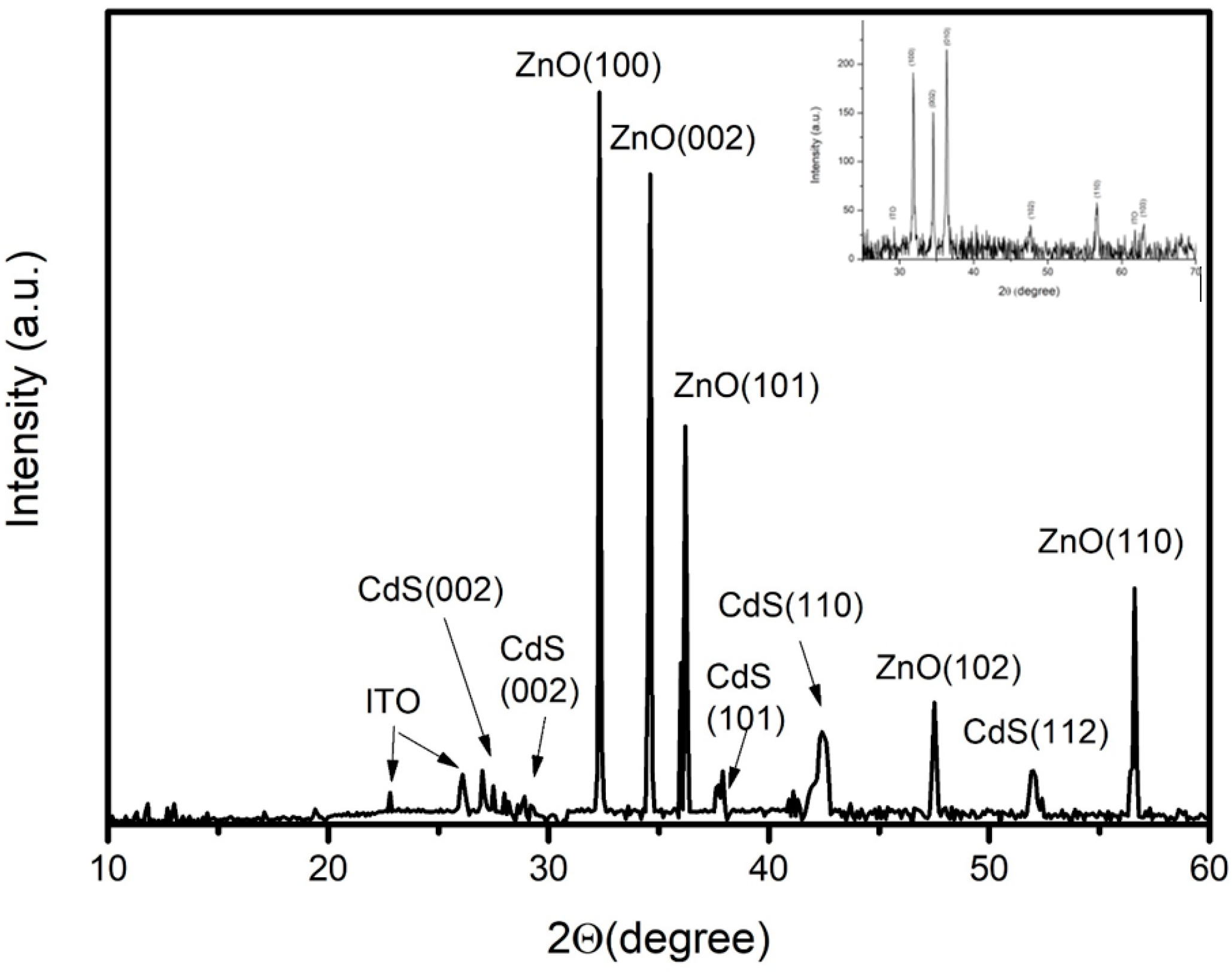
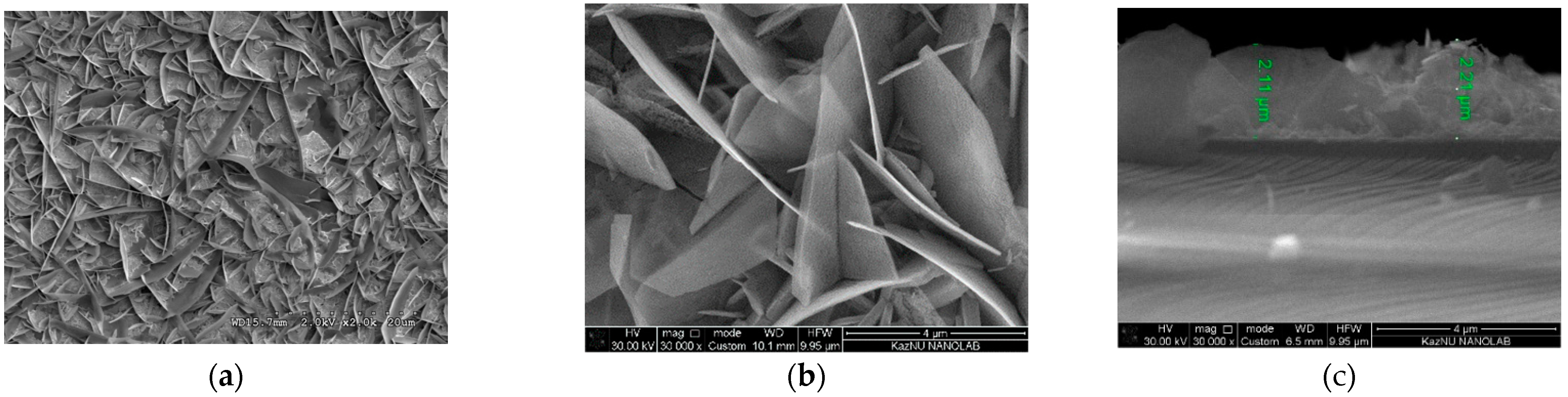
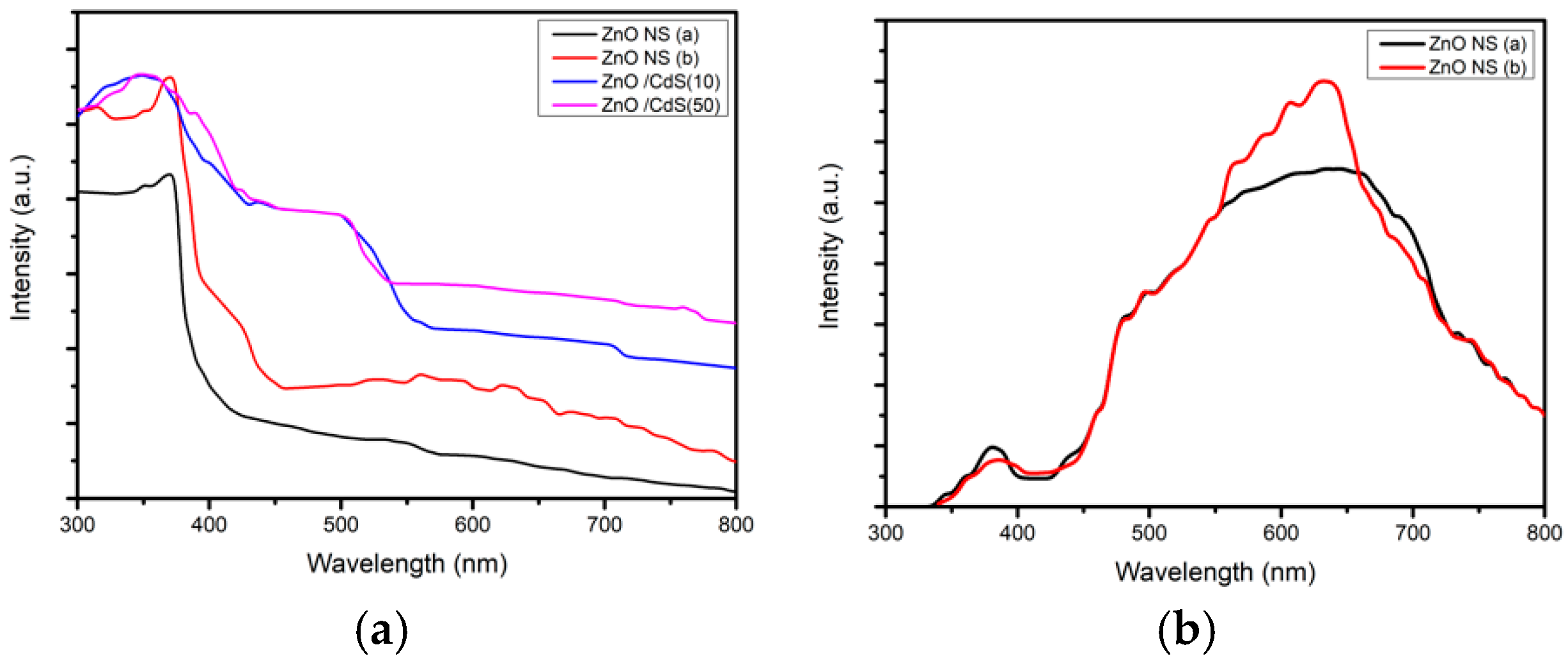
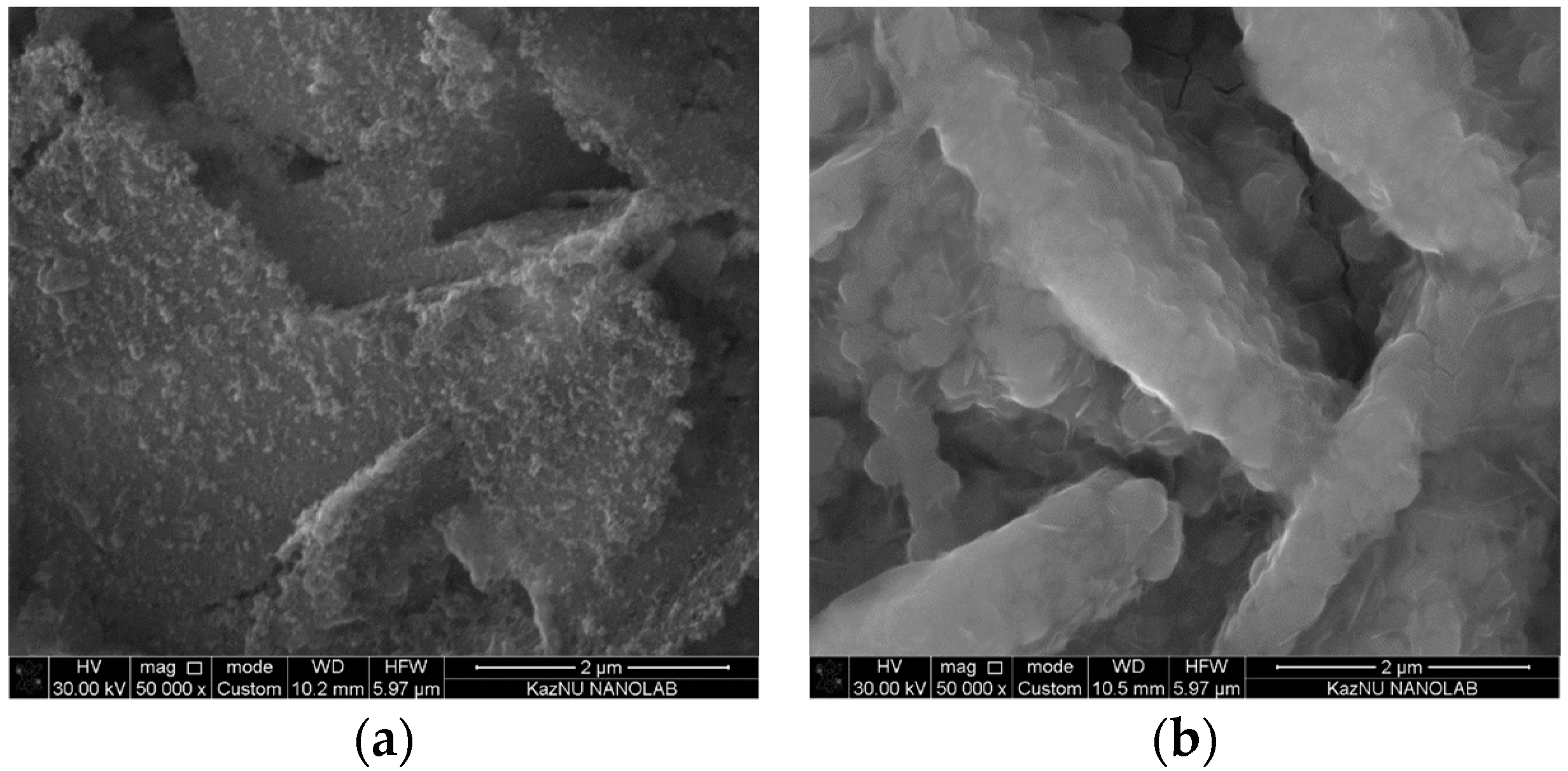
3.1. Properties of Low-Dimensional ZnO NS and ZnO/CdS Nanocomposites
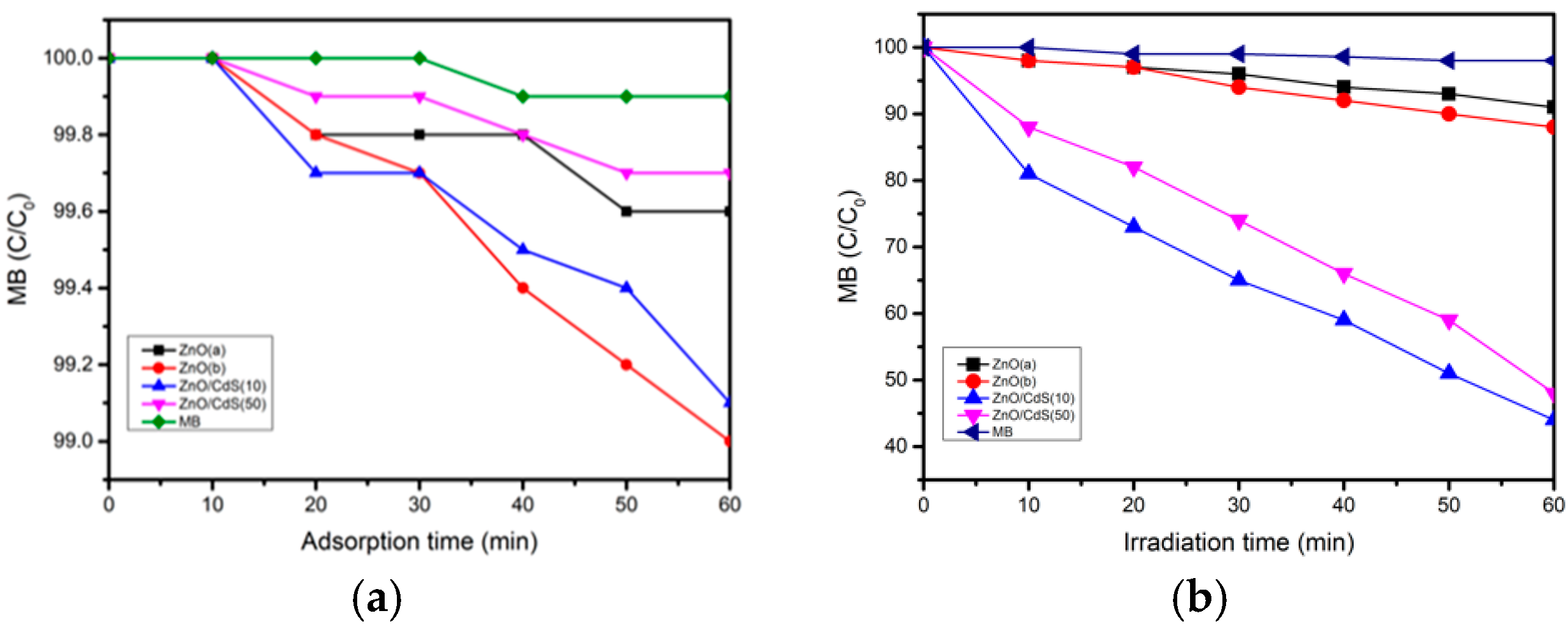
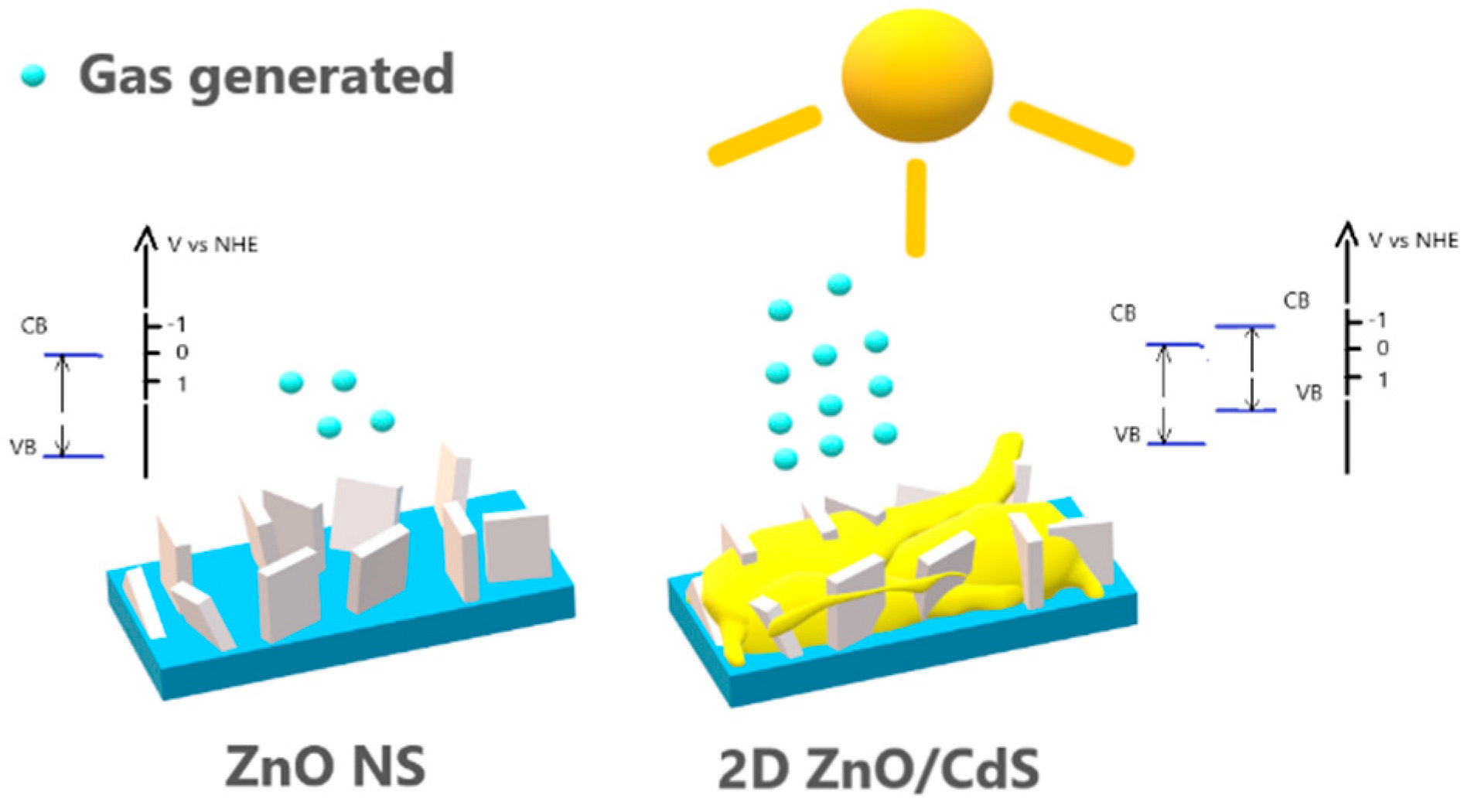
3.2. Photocatalytic and Photoelectrochemical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Volta, P.; Jeppesen, E. Impacts of Human Activities and Climate Change on Freshwater Fish. Water 2021, 13, 3068. [Google Scholar] [CrossRef]
- Fujishima, A. Electrochemical evidence for the mechanism of the primary stage of photosynthesis. In Book Electrochemical Evidence for the Mechanism of the Primary Stage of Photosynthesis; EditorBulletin of the Chemical Society of Japan: Tokyo, Japan, 1971; pp. 1148–1150. [Google Scholar]
- Shabdan, Y.; Markhabayeva, A.; Bakrano, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Markhabayeva, A.A.; Moniruddin, M.; Dupre, R.; Abdullin, K.A.; Nuraje, N. Designing of WO3@Co3O4 Heterostructures to Enhance Photoelectrochemical Performances. J. Phys. Chem. A 2020, 124, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 12. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of zinc-oxide varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Nanto, H.; Minami, T.; Takata, S. Zinc-oxide thin-film ammonia gas sensors with high-sensitivity and excellent selectivity. J. Appl. Phys. 1986, 60, 482–484. [Google Scholar] [CrossRef]
- Becheri, A.; Durr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Xie, Y.P.; He, Y.P.; Irwin, P.L.; Jin, T.; Shi, X.M. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Goux, A.; Pauporte, T.; Chivot, J.; Lincot, D. Temperature effects on ZnO electrodeposition. Electrochim. Acta 2005, 50, 2239–2248. [Google Scholar] [CrossRef]
- Xu, F.; Lu, Y.N.; Xie, Y.; Liu, Y.F. Controllable morphology evolution of electrodeposited ZnO nano/micro-scale structures in aqueous solution. Mater. Des. 2009, 30, 1704–1711. [Google Scholar] [CrossRef]
- Yin, Y.; Jin, Z.; Hou, F. Enhanced solar water-splitting efficiency using core/sheath heterostructure CdS/TiO(2) nanotube arrays. Nanotechnology 2007, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Yuan, J.A.; Han, B.Y.; Jiang, L.; Shangguan, W.F. TiO2 nanotubes incorporated with CdS for photocatalytic hydrogen production from splitting water under visible light irradiation. Int. J. Hydrogen Energy 2010, 35, 7073–7079. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Sadtler, B.; Demchenko, D.O.; Zheng, H.; Hughes, S.M.; Merkle, M.G.; Dahmen, U.; Wang, L.W.; Alivisatos, A.P. Selective Facet Reactivity during Cation Exchange in Cadmium Sulfide Nanorods. J. Am. Chem. Soc. 2009, 131, 5285–5293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Synthesis of ultrathin CdS nanosheets as efficient visible-light-driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.Y.; Mao, C.B.; Gao, X.X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mews, A.; Eychmuller, A.; Giersig, M.; Schooss, D.; Weller, H. Preparation, characterization, and photophysics of the quantum-dot quantum-well system CdS/HgS/CdS. J. Phys. Chem. 1994, 98, 934–941. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Yoshida, A. Photocatalytic hydrogen evolution from water on nanocomposites incorporating cadmium sulfide into the interlayer. J. Phys. Chem. B 2002, 106, 12227–12230. [Google Scholar] [CrossRef]
- Xu, J.; Cao, X.J. Characterization and mechanism of MoS2/CdS composite photocatalyst used for hydrogen production from water splitting under visible light. Chem. Eng. J. 2015, 260, 642–648. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P. Alternate synthetic strategy for the preparation of CdS nanoparticles and its exploitation for water splitting. Int. J. Hydrogen Energy 2006, 31, 891–898. [Google Scholar] [CrossRef]
- Henglein, A. Photo-degradation and fluorescence of colloidal-cadmium sulfide in aqueous-solution. Ber. Bunsen-Ges.-Phys. Chem. Chem. Phys. 1982, 86, 301–305. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, H.; Lou, S.; Ding, J.; Zhang, D.; Zhu, H.X.; Fan, T.X. Butterfly wing architecture assisted CdS/Au/TiO2 Z-scheme type photocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 8244–8253. [Google Scholar] [CrossRef]
- Matsumura, M.; Furukawa, S.; Saho, Y.; Tsubomura, H. Cadmium-sulfide photocatalyzed hydrogen-production from aqueous-solutions of sulfite—Effect of crystal-structure and preparation method of the catalyst. J. Phys. Chem. 1985, 89, 1327–1329. [Google Scholar] [CrossRef]
- Johnson, J.; Bakrano, N.; Moniruddin, M.; Iskako, R.; Kudaibergeno, S.; Nuraje, N. Spontaneous polarization field-enhanced charge separation for an iron oxide photo-catalyst. N. J. Chem. 2017, 41, 15528–15532. [Google Scholar] [CrossRef]
- Tak, Y.; Hong, S.J.; Lee, J.S.; Yong, K. Fabrication of ZnO/CdS core/shell nanowire arrays for efficient solar energy conversion. J. Mater. Chem. 2009, 19, 5945–5951. [Google Scholar] [CrossRef] [Green Version]
- Kudaibergeno, S.; Tatykhanova, G.; Bakrano, N.; Tursunova, R. Layer-by-Layer Thin Films and Coatings Containing Metal Nanoparticles in Catalysis. In Thin Film Processes—Artifacts on Surface Phenomena and Technological Facets; Thirumalai, J., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Fang, Y.L.; Li, Z.Y.; Xu, S.; Han, D.D.; Lu, D.Y. Optical properties and photocatalytic activities of spherical ZnO and flower-like ZnO structures synthesized by facile hydrothermal method. J. Alloys Compd. 2013, 575, 359–363. [Google Scholar] [CrossRef]
- Fu, H.B.; Xu, T.G.; Zhu, S.B.; Zhu, Y.F. Photocorrosion Inhibition and Enhancement of Photocatalytic Activity for ZnO via Hybridization with C-60. Environ. Sci. Technol. 2008, 42, 8064–8069. [Google Scholar] [CrossRef]
- Jawale, D.; Al-fahdawi, A.; Salve, S.; Pandit, S.; Dawange, G.; Gugale, G.; Chaskar, M.; Hammiche, D.; Arbuj, S. Pandit 6, 13-pentacenequinone/zinc oxide nanocomposites for organic dye degradation. Mater. Today-Proc. 2022, 52, 17–20. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakranova, D.; Seitov, B.; Bakranov, N. Preparation and Photocatalytic/Photoelectrochemical Investigation of 2D ZnO/CdS Nanocomposites. ChemEngineering 2022, 6, 87. https://doi.org/10.3390/chemengineering6060087
Bakranova D, Seitov B, Bakranov N. Preparation and Photocatalytic/Photoelectrochemical Investigation of 2D ZnO/CdS Nanocomposites. ChemEngineering. 2022; 6(6):87. https://doi.org/10.3390/chemengineering6060087
Chicago/Turabian StyleBakranova, Dina, Bekbolat Seitov, and Nurlan Bakranov. 2022. "Preparation and Photocatalytic/Photoelectrochemical Investigation of 2D ZnO/CdS Nanocomposites" ChemEngineering 6, no. 6: 87. https://doi.org/10.3390/chemengineering6060087



