Process Intensification in a Double-Pipe Reactor with Additively Manufactured Internal Inserts
Abstract
:1. Introduction
2. Materials and Methods
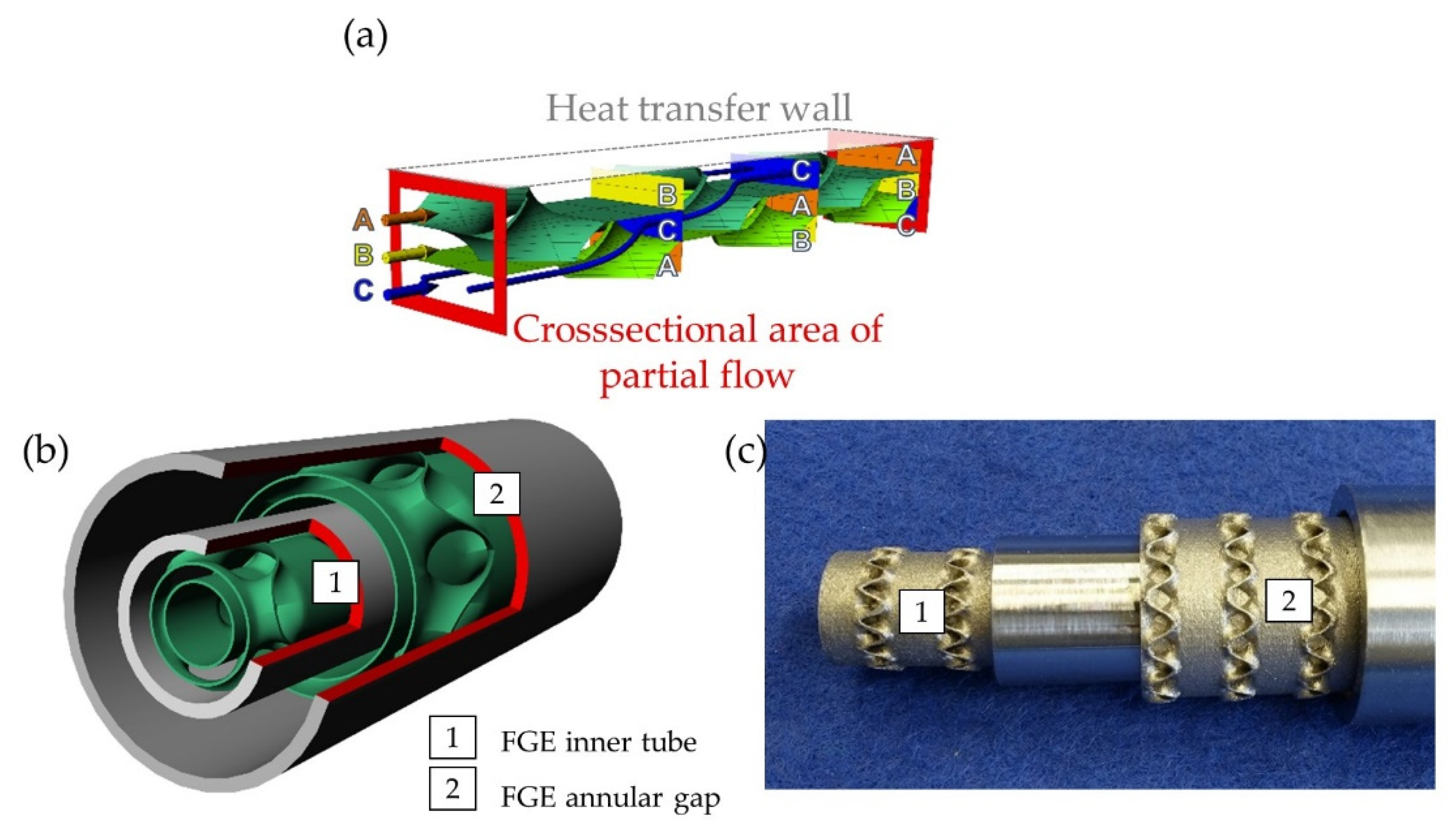
2.1. Double-Pipe Reactors Equipped with Additively Manufactured Internal Inserts
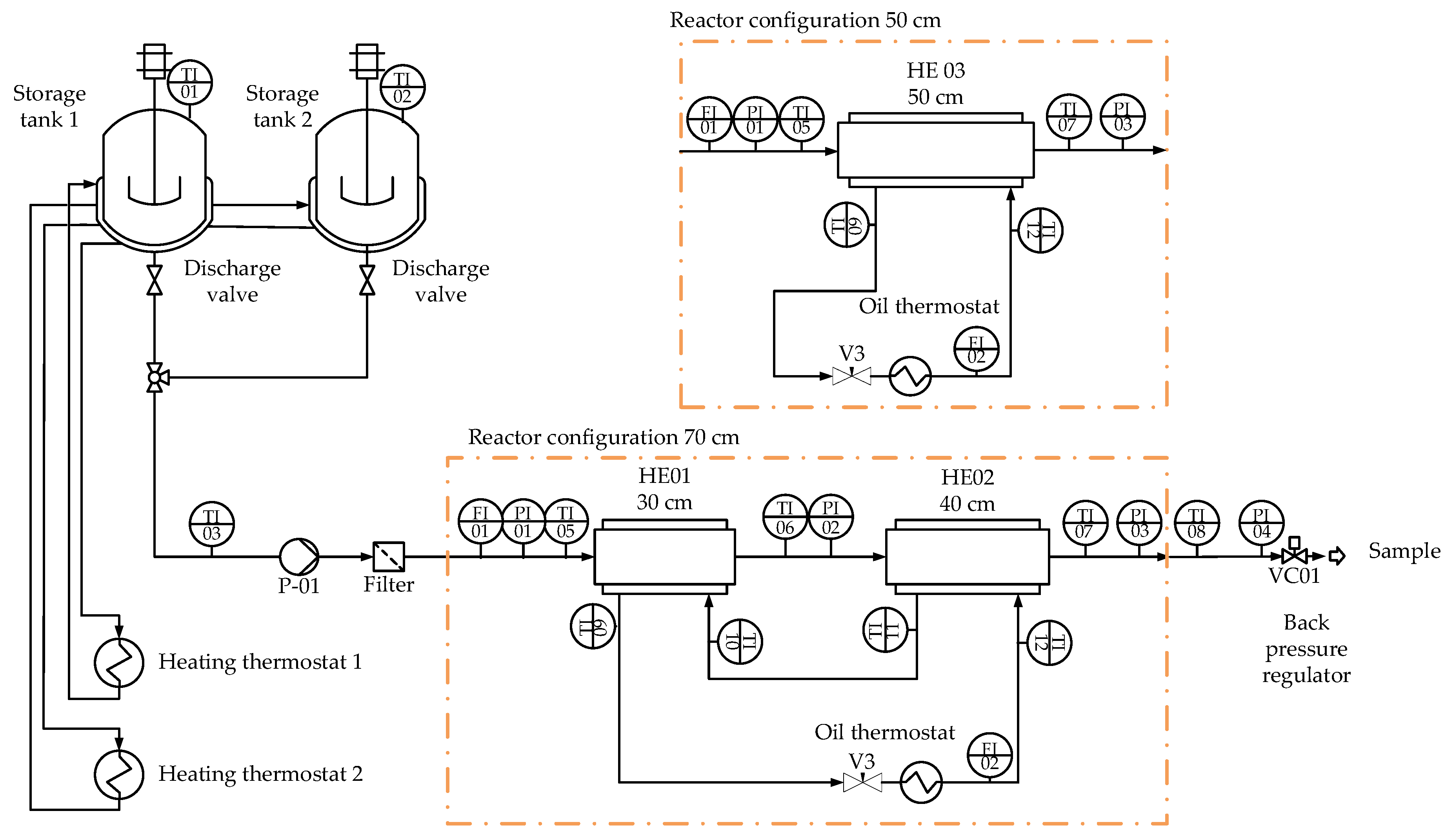
2.2. Experimental Plant for the Production of Polydextrose
2.3. Experimental Procedure
- Solution preparation. The first step was to prepare the feed solution with glucose and deionized water. For each experiment, the solutions were prepared in storage ank BR01 and heated to 80 °C.
- Warm-up phase. The heating cords and the oil thermostat were switched on to ensure that the reactors were brought to the target temperature. Simultaneously, heated deionized water from storage tank 2 was pumped through the system. When the target-set temperature was reached, 1%wt. (regarding the dry mass) of catalyst(citric acid) was added to storage tank 1. Next, the pump feed was switched from storage tank 2 (DI water) to storage tank 1 (reacting glucose solution).
- Reaction conditions. Different experiments were set to determine the effect of the glucose solution in the feed (50–80%). In addition, the reactor setting was varied, using the 70 cm or the 50 cm reactor configuration. The influence of the type of inserts was also investigated, as well as the effect when no inserts (Type 0) were placed inside the reactor. The oil set temperature varied between 200 and 235 °C, and the system pressure was set between 1 and 3 bar g. Oscillations in pressure could be observed due to the ongoing evaporation. The pump was set to deliver feed mass flow rates between 1.5 and 2.5 kgh−1. A summary of the operating conditions is given in Table 3.
- Samples. Each operation point was held between 15 and25 min to reach a steady state. When sampling, the product was mixed with cold deionized water and neutralized with sodium hydroxide up to a pH value >5 to prevent further reactions.
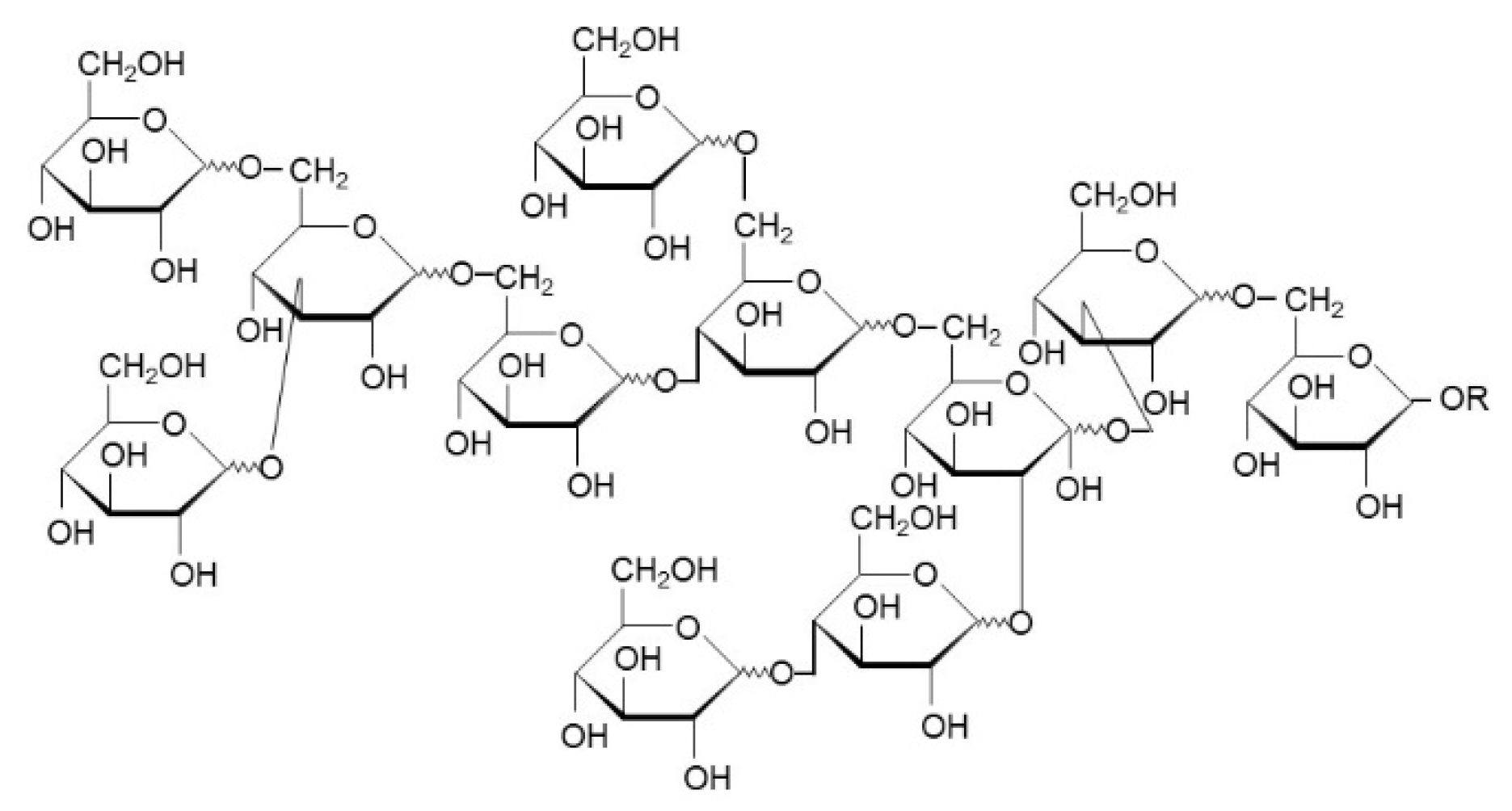
- Analysis. The samples were analyzed using HPLC according to the established method [19]. The product yield was determined as the total number of oligomers that had a polymerization degree ⩾2(polymerized glucose).
- Calculations. Due to the evaporation in the reactor, the density of the flowing medium along the reacting path was not constant. For an exact determination of the residence time, the course of the density of the reaction mixture over the length should be known. However, the kinetics of evaporation in the reactor were difficult to determine. Considering the known parameters, a Mass Hourly Volumetric Velocity (in kgh−1m−3) can be defined for a comparison of the experiments, as follows:
3. Results
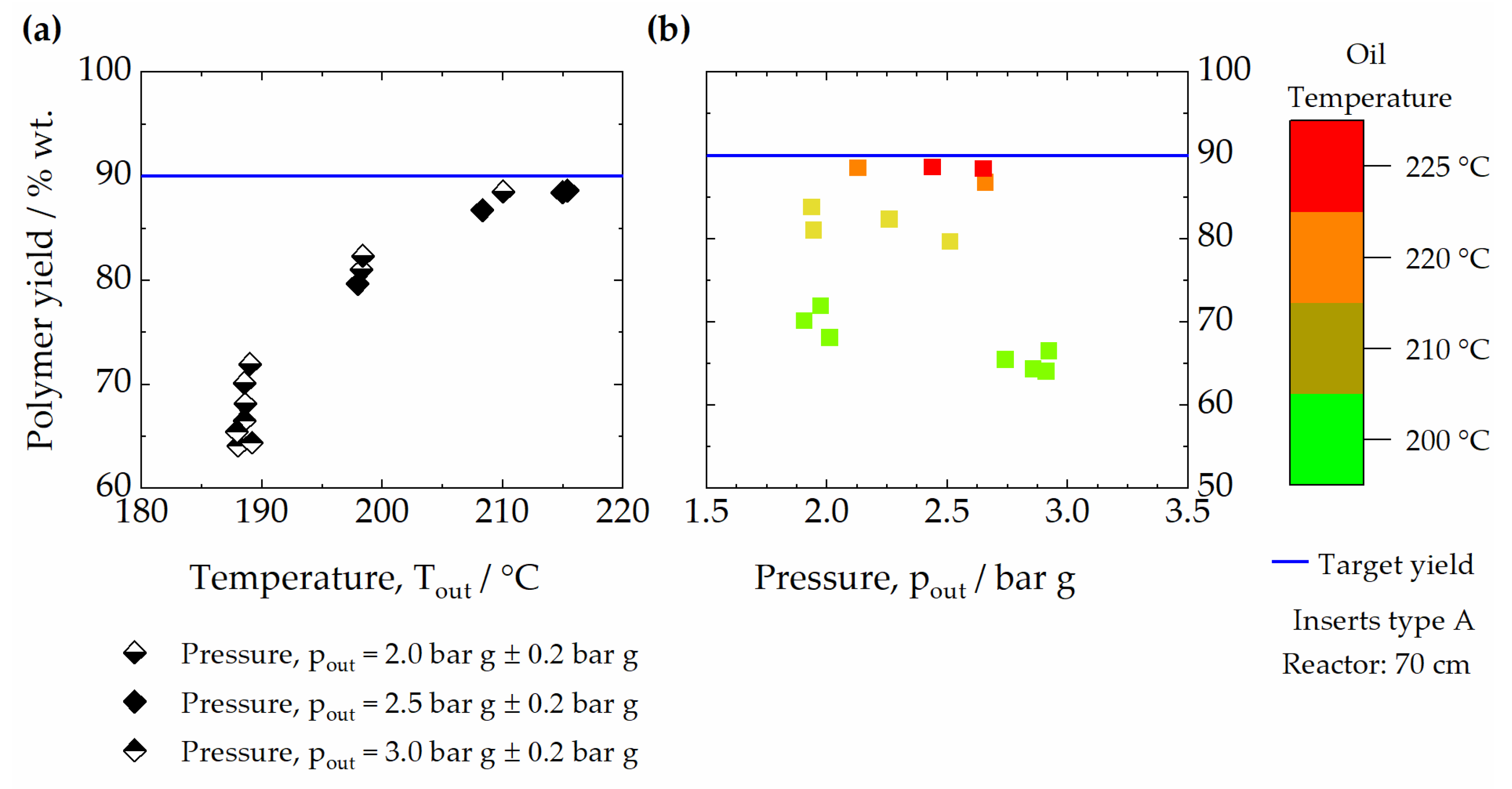
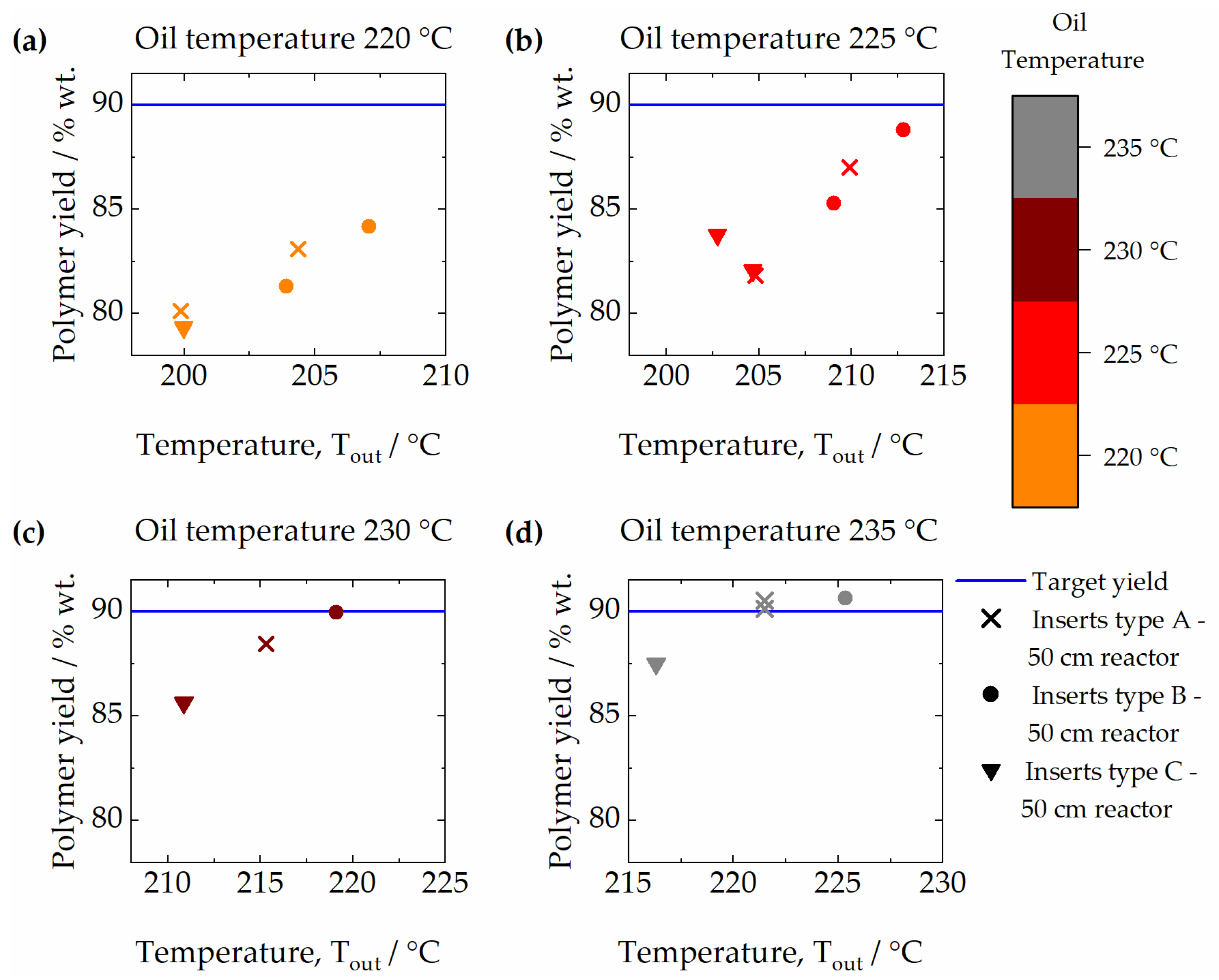
3.1. Effect of Temperature and Pressure
3.2. Effect of Glucose Concentration in the Feed
3.3. Effect of Residence Time
3.4. Effect of Internal Inserts Geometry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuesters, C.F.; Stengel, B.F.; Benzinger, W.; Brandner, J. Microprocessing for Preparing a Polycondensate. WO 2011/091962, 19th January 2011. Available online: https://data.epo.org/publication-server/document?iDocId=5859464&iFormat=0 (accessed on 15 June 2022).
- do Carmo, M.M.; Walker, J.C.; Novello, D.; Caselato, V.M.; Sgarbieri, V.C.; Ouwehand, A.C.; Andreollo, N.A.; Hiane, P.A.; Dos Santos, E.F. Polydextrose: Physiological Function, and Effects on Health. Nutrients 2016, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A.S.; Holden, J.F.; Troup, J.P.; Auerbach, M.H.; Frier, H.I. Polydextrose as Soluble Fiber: Physiological and Analytical Aspects. Cereal Foods World 1998, 43, 370–376. [Google Scholar]
- Auerbach, M.; Craig, S.; Mitchell, H. Bulking Agents: Multi-Functional Ingredients. In Sweeteners and Sugar Alternatives in Food Technology; Mitchell, H., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 435–467. [Google Scholar] [CrossRef]
- Auerbach, M.; Mitchell, H.; Moppett, F.K. Polydextrose. In Alternative Sweeteners; O’Brien-Nabors, L., Ed.; Taylor & Francis Group: Baton Rouge, LA, USA, 2011; pp. 489–505. [Google Scholar]
- EU-Commission. Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Text with EEA Relevance. 22 March 2012. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32012R0231 (accessed on 12 June 2022).
- The United States Pharmacopeial Convention, Food Chemicals Codex. 8. Ed. Rockville Md.: USP 2012. ISBN 9781936424054. Available online: http: //swb.bsz-bw.de/DB=2.1/PPN?PPN=1442229853 (accessed on 15 September 2022).
- Rennhard, H.H. Polysaccharides and Their. Preparation. Patent US3766165 (A), 16 October 1973. [Google Scholar]
- Stowell, J. Prebiotic Potential of Polydextrose. In Prebiotics and Probiotics Science and Technology; Charalampopoulos, D., Rastall, R.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 337–352. ISBN 978-0-387-79057-2. [Google Scholar]
- Zentel, K.M.; Fassbender, M.; Pauer, W.; Luinstra, G.A. Chapter Four-3D printing as chemical reaction engineering booster. In Advances in Chemical Engineering; Moscatelli, D., Sponchioni, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 56, pp. 97–137. [Google Scholar]
- Alimi, O.A.; Meijboom, R. Current and future trends of additive manufacturing for chemistry applications: A review. J. Mater. Sci. 2021, 56, 16824–16850. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.K.; Ramakrishna, S.; Brandt, M.; Selvakannan, P.R. Additive Manufacturing for Chemical Sciences and Engineering; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Grinschek, F.; Xie, D.; Klumpp, M.; Kraut, M.; Hansjosten, E.; Dittmeyer, R. Regular Microstructured Elements for Intensification of Gas-Liquid Contacting Made by Selective Laser Melting. Ind. Eng. Chem. Res. 2020, 59, 3736–3743. [Google Scholar] [CrossRef]
- Trinkies, L.L.; Düll, A.; Deschner, B.J.; Stroh, A.; Kraut, M.; Dittmeyer, R. Simulation of Fluid Flow During Direct Synthesis of H2O2 in a Microstructured Membrane Reactor. Chem. Ing. Tech. 2021, 93, 789–795. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies; Springer International Publishing: New York, NY, USA, 2021; ISBN 978-3-030-56127-7. [Google Scholar]
- Hansjosten, E.; Wenka, A.; Hensel, A.; Benzinger, W.; Klumpp, M.; Dittmeyer, R. Custom-designed 3D-printed metallic fluid guiding elements for enhanced heat transfer at low pressure drop. Chem. Eng. Processing Process Intensif. 2018, 130, 119–126. [Google Scholar] [CrossRef]
- Hensel, A.; Wenka, A.; Hansjosten, E.; Benzinger, W. Flow Guide in a Channel. DE 10 2015 113 432 A1. 2017. Available online: https://www.kit-technology.de/fileadmin/user_upload/DE102015113432A1_01.pdf (accessed on 13 July 2022).
- ISO 10504:2013; International Organization for Standardization (ISO) Starch Derivatives—Determination of the Composition of Glucose Syrups, Fructose Syrups and Hydrogenated Glucose Syrups—Method Using High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/50792.html (accessed on 13 July 2022).




| Tube | Outer Diameter | Inner Diameter | Wall Thickness | Contact Length |
|---|---|---|---|---|
| Inner | 12 mm | 10 mm | 1 mm | 30 cm/40 cm/50 cm |
| Outer | 18 mm | 15 mm | 1.5 mm |
| Tube | Number of Partial Streams | Repetition Length (FGU) | Length of an Element | Void Fraction |
|---|---|---|---|---|
| A | 3 | 9 mm | 89% | |
| B | 3 | 6 mm | 55 mm | 88% |
| C | 2 | 9 mm | 91% |
| Set Glucose Solution /%wt. | Reactor Configuration | Type of Inserts | Oil Set Temperature /°C | Pressure /Bar g | Feed Mass Flow Rate /kgh−1 |
|---|---|---|---|---|---|
| 50, 60, 70, 80 | 70 cm, 50 cm | A, B, C, 0 | 200, 210, 220, 225, 230, 235 | 1.5, 2, 2.5, 3, 4 | 2, 3.15, 3.50, 3.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claramunt, S.; Schell, P.V.; Kraut, M.; Stengel, B.F.; Kuesters, C.F.; Dittmeyer, R. Process Intensification in a Double-Pipe Reactor with Additively Manufactured Internal Inserts. ChemEngineering 2022, 6, 85. https://doi.org/10.3390/chemengineering6060085
Claramunt S, Schell PV, Kraut M, Stengel BF, Kuesters CF, Dittmeyer R. Process Intensification in a Double-Pipe Reactor with Additively Manufactured Internal Inserts. ChemEngineering. 2022; 6(6):85. https://doi.org/10.3390/chemengineering6060085
Chicago/Turabian StyleClaramunt, Sara, Paul V. Schell, Manfred Kraut, Bruno F. Stengel, Christof F. Kuesters, and Roland Dittmeyer. 2022. "Process Intensification in a Double-Pipe Reactor with Additively Manufactured Internal Inserts" ChemEngineering 6, no. 6: 85. https://doi.org/10.3390/chemengineering6060085






