1. Introduction
Flavor is one of the most important attributes that affects food consumption. In the food industry, various core compounds, such as volatile compounds, essential oils, and oleoresins, are encapsulated to prevent volatilization, oxidation, or degradation. Spray drying is one of the most widely used processes for producing powders containing emulsified flavor in the food industry. The encapsulation by spray drying is a technique to entrap emulsified oily flavor within a carrier matrix of wall material to prevent the deterioration [
1]. In the flavor encapsulation, the important factor is the selection of wall material to get high retention of flavor during spray drying and to control flavor release from spray-dried powder. Zuidan and Heinlich [
2] summarized encapsulation of aroma, and Soottitantawata et al. [
3] also summarized the encapsulation of hydrophilic and hydrophobic flavors by spray drying. Yoshii et al. [
4] investigated flavor release from spray-dried maltodextrin/gum arabic or soy matrices as a function of storage relative humidity and showed that the flavor release rate is strongly affected by environmental humidity and temperature. They used Avrami (Weibull)’s equation [
5] for describing the release time-course of a spray-dried ethyl-
n-butylate powder during storage. Wall material influences barrier properties against flavor diffusion in spray-dried powder.
d-limonene is the major component in the oil of citrus fruit peels and a useful model flavor as a hydrophobic flavor oil.
A dynamic vapor sorption (DVS) instrument is a useful method to measure the release time course of flavor from spray-dried powder. Burnett et al. [
6] developed a DVS instrument to identify the critical RH value that induces the glass transition process at a specified temperature. Yamamoto et al. [
7] applied this DVS method to investigate the release of
d-limonene encapsulated in spray-dried cyclodextrin powder. However, there have been few investigations of flavor release from spray-dried powder with various wall materials.
The aim of this study is to investigate the effect of wall material on the flavor release behavior of spray-dried powders containing emulsified d-limonene and various wall materials such as sucrose, lactose, and maltodextrin (dextrose equivalent = 25 and 19) by using the ramping method to humidity at a constant temperature.
2. Materials and Methods
2.1. Materials
α-lactose (Lac) was purchased from Sigma-Aldrich Japan (Tokyo, Japan). Sucrose (Suc), d-limonene, and sodium ascorbate were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Maltodextrin (MD, with dextrose equivalent (DE) = 19, 25) was a kind gift from Matsutani Chemical Industry Co., Ltd. (Hyogo, Japan). Hydrolyzed casein protein (Emul-Up) was purchased from Morinaga Milk Industry Co., Ltd. (Tokyo, Japan). Other chemicals used were of analytical grade from FUJIFILM Wako Pure Chemical Corporation.
2.2. Preparation of Emulsion
d-limonene as a model flavor was dissolved in medium chain triacylglycerol (MCT) oil, with a weight ratio (d-limonene/oil) of 0.20. Suc, Lac, or MD (DE = 19 and 25) as carrier material was dissolved in distilled water at 50 °C. Hydrolyzed casein protein as an emulsifier was dissolved in distilled water and mixed with the carrier solution. Oil mixture was blended with the carrier solution. The solid content of the feed solution was 60 wt%, the composition of which was the oil mixture (53.9 wt%), carrier material (35.7 wt%), hydrolyzed casein protein (7.0 wt%), and sodium ascorbate (antioxidant, 3.4 wt%), in terms of solids. The solution was homogenized using a polytron homogenizer (PT-6100, Kinematica, Littau, Switzerland) at 8000 rpm for 3 min with a 30-s interval every 1 min. Then, this homogenized solution was further homogenized using a high-pressure homogenizer, Starburst Mini (HJP-25001K; Sugino, Uozu, Japan) at 100 MPa (two cycles).
2.3. Microencapsulation by Spray Drying
A pilot-scale spray dryer (L-8 type, Ohkawara Kakohki Co. Ltd., Yokohama, Tokyo, Japan) was used to form spray-dried powder under the following conditions: an atomizer speed of 10,000 rpm, air flow rate of 110 kg/h, the temperature and flow rate of the infeed solution at 50 °C and 30 mL/min, inlet-air temperature 140 °C, and outlet-air temperature from 82 to 92 °C, respectively. In the coating of corn starch, the corn starch was supplied near atomizer. The spray-dried powders were stored at −30 °C in an aluminum bag under nitrogen atmosphere until their use for analysis.
2.4. Gas Chromatographic Analysis of d-Limonene
About 100 mg of spray-dried powders were placed in a test tube (16.5ϕ × 105 mm), and 1 mL of distilled water was added to the tube to get a completely solubilized solution. Then, 4 mL of hexane, containing 1 µL/mL cyclohexanone as the internal standard, was further added to the solution.
d-limonene was extracted into the hexane by heating the tightly capped tube in a water bath at 90 °C for 90 min. Then, the tube was centrifuged at 3000 rpm for 10 min.
d-limonene was measured in duplicate by gas chromatography (GC-2010, Shimadzu, Kyoto, Japan) [
8]. Separation column was a DB-WAX fused silica capillary column (30 m × 0.32 mm i.d. 0.25 mm film, Agilent Technologies Inc., Santa Clara, CA, USA) with nitrogen as the carrier gas (70 kPa). Splitless injection was used with 1-min sampling time and a split ratio of 1:30. The column temperature was programmed from 50 °C (1-min initial hold time) to 130 °C at 10 °C/min. Retention of
d-limonene was evaluated by dividing the content of
d-limonene in the stored powder by that of
d-limonene before the storage.
2.5. Sieving of the Spray-Dried Powders
Stainless steel sieves with mesh opening sizes of 212, 106, and 75 µm (70, 140, and 200 mesh of the sieve tray, Φ300 × 100 mm; As One Corp., Osaka, Japan) were used to roughly separate the powders into different sizes by hand shaking. After sieving, the spray-dried powders were further sieved using stainless steel sieves with mesh opening sizes of 300, 212, 150, 106, 75, and 53 µm (50, 70, 100, 140, 200, and 270 mesh of the sieve tray, ϕ75 × 20) by using an electromagnetic vibration sieve unit (M-2; Tsutsui Scientific Instruments, Tokyo, Japan). In this study, the 104 mesh sieved powders were used to the release experiment. The average diameter of the sieved powder was 140, 128, 136, and 107 μm for Suc, Lac, MD (DE = 25), and MD (DE = 19), respectively.
2.6. Analysis of Flavor Release Flux from the Encapsulated Powder Using the DVS–GC Method
The release of
d-limonene from the spray-dried powders was studied by measuring the concentration change in head space of the flavor compound in a vessel of controlled RH. The home-built DVS instrument was the same instrument used by Yamamoto et al. [
7], as shown in
Figure 1. The vessel volume of spray-dried powder was 15 mL (16 mm internal diameter, 80 mm height). One-tenth of a gram of the powder was spread in a sample holder and packed by vibration. The release vessel was set in an air bath at constant temperature of 40, 50, or 60 °C humidity in the vessel, and was controlled by altering water bath temperature using a temperature controller (model DSSP93, Shimaden, Tokyo, Japan) to achieve a linear humidity ramping from 10 to 90%. Humidity-controlled nitrogen was flowed through the vessel at 100 mL/min, and the effluent nitrogen from the vessel was sampled using a 5.0-mL sampling loop and injected every 5 min in to a Shimadzu GC-14B gas chromatograph (Kyoto, Japan) by a timer-automated switching valve (Valco A6-G6 W, Valco Instruments Co. Inc., Houston, TX, USA). The gas chromatograph was fitted with a glass column (2.1 m 3.2 mm i.d.) packed with PEG-20 M (20% on Chromosorb W 80 = 100 AW mesh; Shinwa Chemical Industries, Kyoto, Japan) and equipped with a flame ionization detector. The humidity of the effluent nitrogen was continuously monitored with a humidity and temperature transmitter (model HMP233, Vaisala KK, Tokyo, Japan).
2.7. Scanning Electron Microscopy
A scanning electron microscope (SEM; model JSM 6060, JEOL Co., Tokyo, Japan) was used to observe the microstructures of the spray-dried powders. To examine the inner structure, the powder particles were fractured as described by Soottitantawat et al. [
9]. The specimen was subsequently coated with Pt-Pd using a magnetron sputter coater (model MSP-1S, Vacuum Device Inc., Tokyo, Japan). The coated sample was then observed at an electron accelerating voltage of 2 kV.
2.8. Statistical Analysis
The data were analyzed statistically using Excel 2016, Microsoft office professional plus software. All measurements were replicated at least three times. One-way analysis of variance (ANOVA) with post hoc Tukay (HSD) test was done to identify the significance differences (p < 0.05) between data sets, and average values of the flavor release data were plotted.
3. Results and Discussion
Figure 2 shows scanning electron micrographs (SEM) of surface and cross-sectional structures of spray-dried powders for Suc (a and a’), Lac (b and b’), MD (DE = 25) (c and c’), and MD (DE = 19) (d and d’). All powders had a big vacuole and oil-droplets could not be observed at the cross-cut section of spray-dried powder. Average powder diameters of spray-dried powders were between 106 and 150 µm for various wall materials. Reconstituted oil-droplet diameters were obtained near 0.2 µm for Suc, Lac, and MD (DE = 25) and 0.4 µm for MD (DE = 19).
d-limonene contents in spray-dried powders were 90.6 ± 4.47 mg/g-powder for Suc, 901 ± 4.17 for Lac, 96.6 ± 1.77 for MD (DE = 25), and 90.1 ± 1.26 for MD (DE = 19). On the other hand, the contents of surface
d-limonene were 0.11 ± 0.03 mg/g-powder for Suc, 0.12 ± 0.01 for Lac, 0.24 ± 0.01 for MD (DE = 25), and 0.36 ± 0.01 for MD (DE = 19), respectively. The ratios of surface
d-limonene to total d-limonene were 0.12% for Suc, 0.13 for Lac, 0.25 for MD (DE = 25), and 0.40 for MD (DE = 19). Therefore, the release of surface
d-limonene could be neglected in
d-limonene release experiments. The release experiments were carried out using the sieved spray-dried powders within a range of 107 to 140 µm. These powders were used to investigate the release behaviors of
d-limonene while ramping humidity at constant temperatures. Water contents of those powders after sieving were 3.54 wt% for Suc, 5.45 for Lac, 4.14 for MD (DE = 25), and 4.08 for MD (DE = 19), respectively.
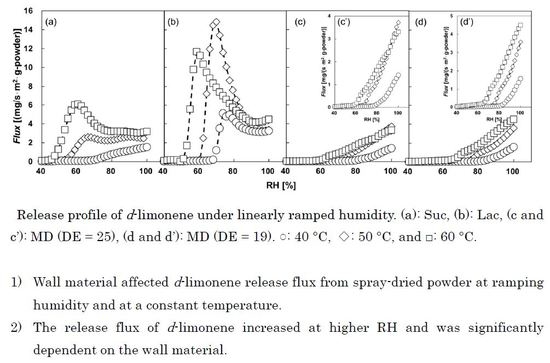
Figure 3 shows the release behaviors of
d-limonene at 40, 50, and 60 °C from 10 to 100%RH ramping humidity. These were plotted against the data sets within the significance differences (
p < 0.05). The release flux from spray-dried powder was affected by environmental humidity and wall materials. The flux for all the wall materials increased at a certain RH, which is defined as the release start humidity, s
RH. The values of s
RH for Suc were 67%RH at 40 °C, 53%RH at 50 °C, and 47%RH at 60 °C. The values for Lac were 69%RH at 40°C, 62%RH at 50 °C, and 52%RH at 60 °C. The values for MD (DE = 25) were 82%RH at 40 °C, 68%RH at 50 °C, and 59%RH at 60 °C. The values for MD (DE19) were 85%RH at 40° C, 75%RH at 50 °C, and 65%RH at 60 °C. The release of
d-limonene might be caused by the moisture adsorption on the surface of wall material. As mentioned above, the surface
d-limonene could be neglected and the released fluxes of
d-limonene were from the encapsulated
d-limonene in spray-dried powder. The release flux depended significantly on a kind of wall material. After the s
RH, the release flux increased gradually to the maximum values at 50 and 60 °C for Suc and 40, 50, and 60 °C for Lac. Then, the release flux decreased after the maximum of the flux for Suc and Lac. The decreases for Suc and Lac might be ascribed to the powder aggregation and surface hardening of powder by the partially crystallization, respectively. The decrement of flavor release fluxes was called the collapse of wall material. However, MD (DE = 19 and DE = 25) showed no decrease in flux due to collapse of the wall material. Roos [
10] reviewed the glass transition temperature and its relevance in food processing and pointed out that plasticization of the noncrystalline structures by temperature or water reduce relaxation times exponentially above the glass transition, which results in rapid deterioration, and critical values for water activity and water content express the level of water plasticization leading to glass transition in food storage. Whorton and Reineccius [
11] and Soottitantawat et al. [
9] correlated the flavor release rate with the glass transition temperature and the collapse temperature of wall materials. As shown in
Figure 3, the release flux increased with increasing RH after reaching s
RH for MDs (DE = 19 and 25). In the investigation of
d-limonene release from same spray-dried powder at 50 °C and a constant RH [
8], the logarithm of release rate constants for MDs (DE = 19 and 25) at 50 °C were linearly proportional to RH. These results show that MD was a suitable wall material to control the flavor release from spray-dried powder.
Figure 4 shows that the release of
d-limonene from Suc and Lac powders at 50 °C and constant humidity using DVS. Suc powder could find clearly decrement in the flux at 55%RH due to collapse and the release flux at 60%RH decreased compared with that at 55%RH. For Lac powder, clear collapse phenomena of
d-limonene flux could be observed at 60%RH. In
d-limonene release experiments [
7] of same powders at the constant humidity and constant temperature, 50 °C, the release rate constant for Suc initially decreased from 44 to 58% RH, then increased from 58 to 68% RH followed by a constant value at higher RH. The release rate constant for Lac also decreased from 44 to about 60% RH followed by an increase at higher RH. These collapse phenomena were observed at the
d-limonene release experiment at 50 °C and various humidity conditions for 8-days experiments. This method requires a long measurement time, and the same sample powder could not be used because it is difficult to detect the change in release corresponding to the rapid changes in the powder, including aggregation and solubilization. However, DVS experiments were able to find the collapse phenomena of the decrement change of flavor release rate. After the ramping experiments at 50 °C, SEMs of powders for Suc, Lac, and MD (DE = 19 and 25) were observed (
Figure 5). SEM images indicated that Suc powder aggregated and grew the clusters and that crystals existed on the surface of Lac powder. On the other hand, the powder size might have increased with the moisture adsorption and Lac powder did not aggregate unlike Suc powder. The flux decrement for Lac increased significantly from 55% to 70%RH.
Figure 6 shows the relationship between the release start humidity, s
RH and glass transition temperature, and
Tg of dry wall material. The
Tg values of dry materials [
12] were 62 °C for Suc, 101 °C for Lac, 141 °C for MD (DE = 25), and 150 °C for MD (DE = 19). Values of s
RH for spray-dried powder were linearly proportional to
Tg. This fact suggests that glass transition enhances the mobility of molecules to promote the flavor release from spray-dried powder. The slopes of the straight lines are almost the same at the three temperatures. s
RH for Lac and Suc powders were lower than those for MDs (DE = 19 and 25). Regarding the comparison of Suc and Lac with lower
Tg, MD with higher molecular mass might be effective to control the flavor release from the spray-dried powder in humid conditions. The release flux of
d-limonene increased at higher RH and significantly depended on the wall material. The s
RH value reflected the moisture content when the wall material underwent glass transition. Therefore, the fact that the three straight lines had the same slope suggests that the glass transition curves of these wall materials are almost parallel in a narrow temperature range of 40 to 60 °C.