Application of Response Surface Methodology for H2S Removal from Biogas by a Pilot Anoxic Biotrickling Filter
Abstract
:1. Introduction
2. Materials and Methods
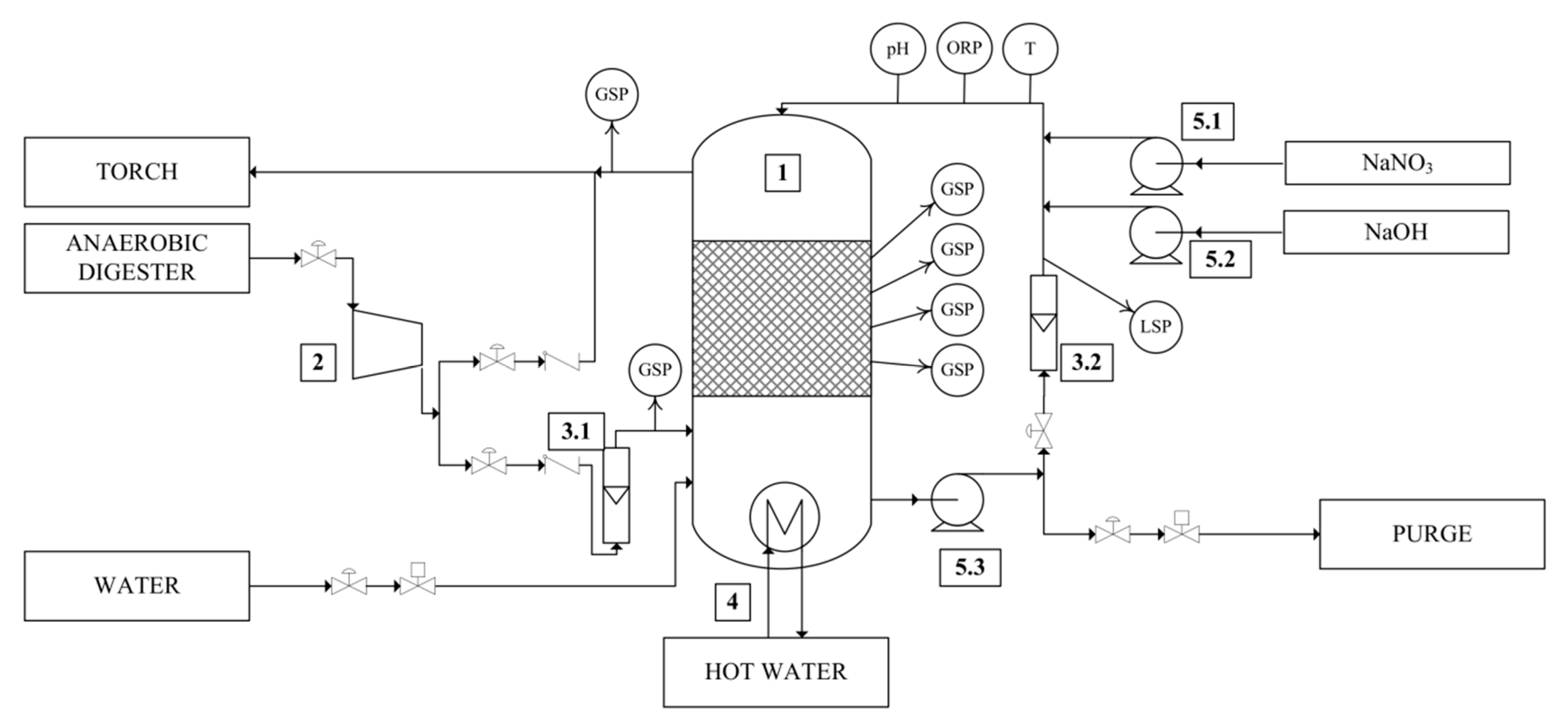
2.1. Experimental Set-up
2.2. Experimental Design
2.3. Ottengraf’s Model
- There is no diffusion limitation and the biofilm is fully active, and hence the conversion rate is controlled by a zero-order reaction rate. The solution is described by Equation (6). K0 is a pseudo zero-order rate (g·m−3·s−1) and is proportional to the zero-order reaction rate constant (k0, Equation (7)).
- There is diffusion limitation and therefore the mass transfer rate to the biofilm is insufficient compared to biological substrate utilization rate. The solution is described by Equation (8).
- There is no diffusion limitation and the biofilm is fully active, and hence the conversion rate is controlled by a first-order reaction rate. The solution is described by Equation (6). K1 is a pseudo first-order rate constant (s−1) (Equation (10)) and is proportional to the first-order rate constant (k1, Equation (10)).
3. Results and Discussion
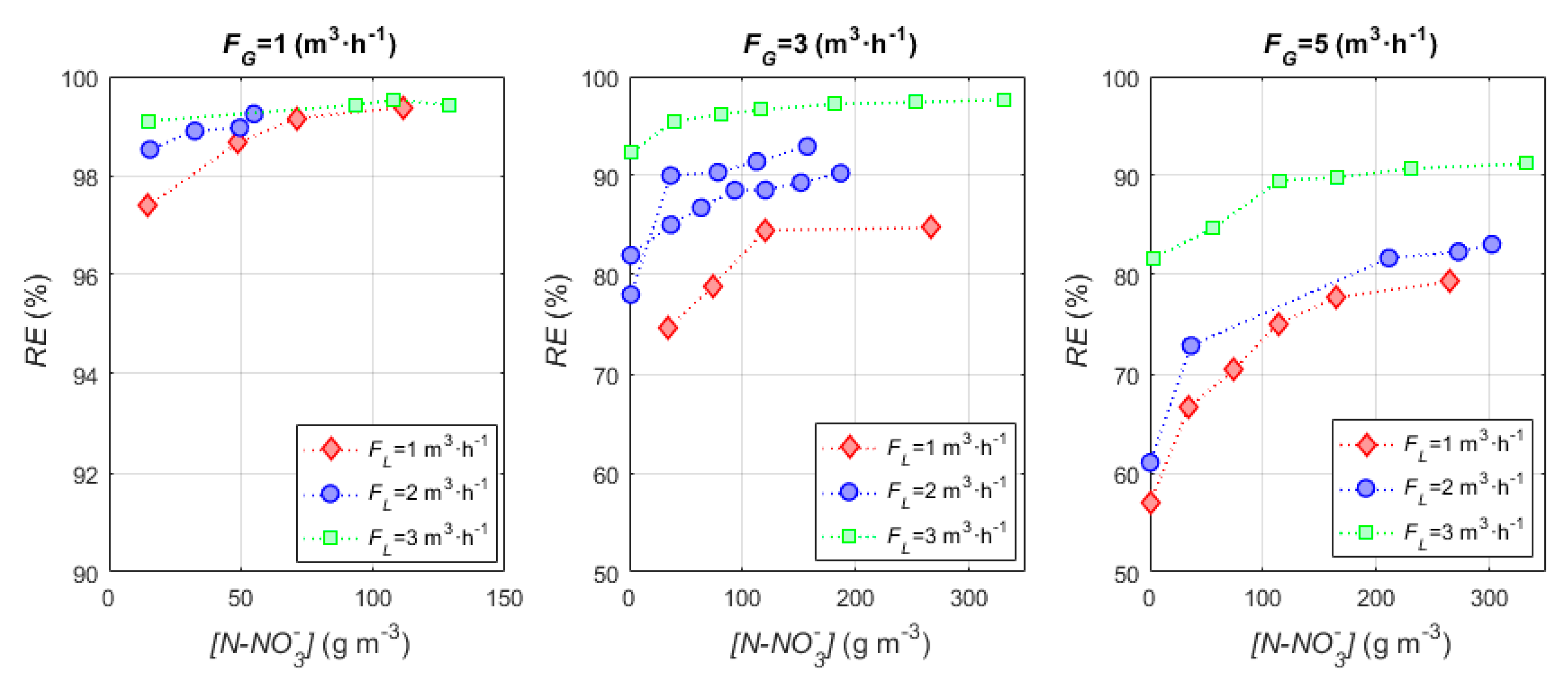
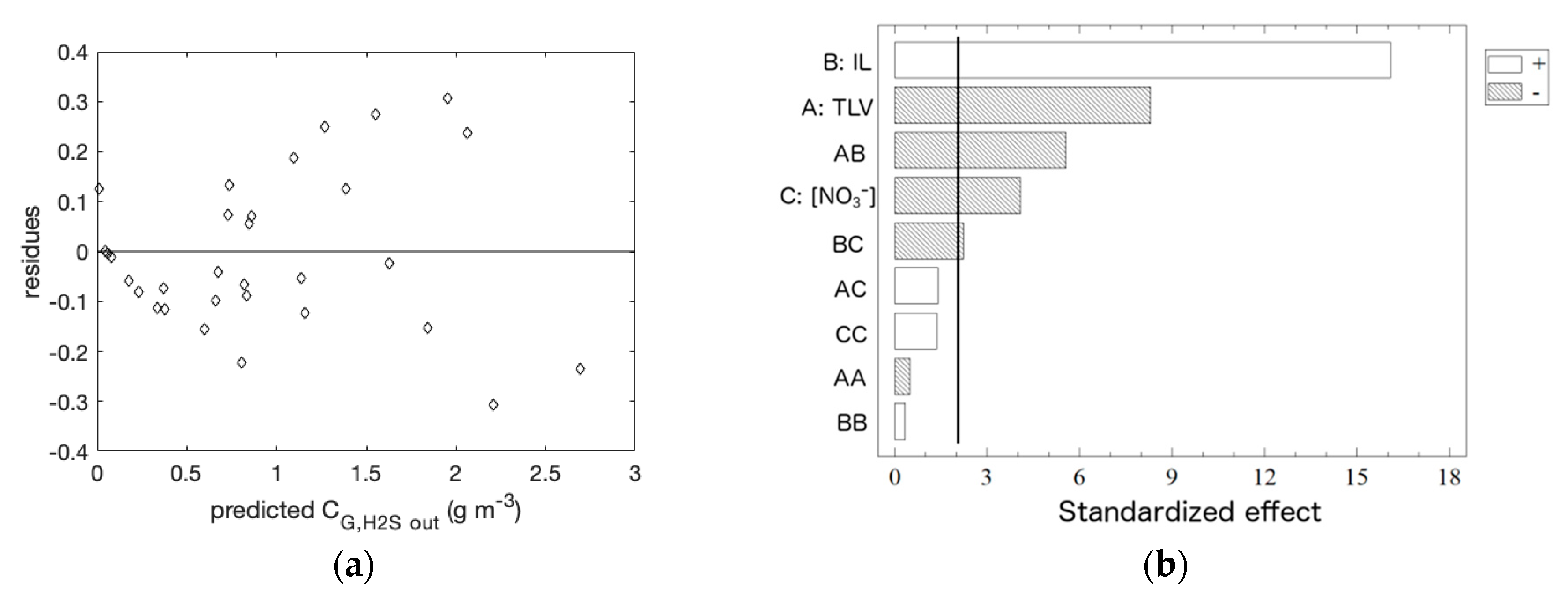
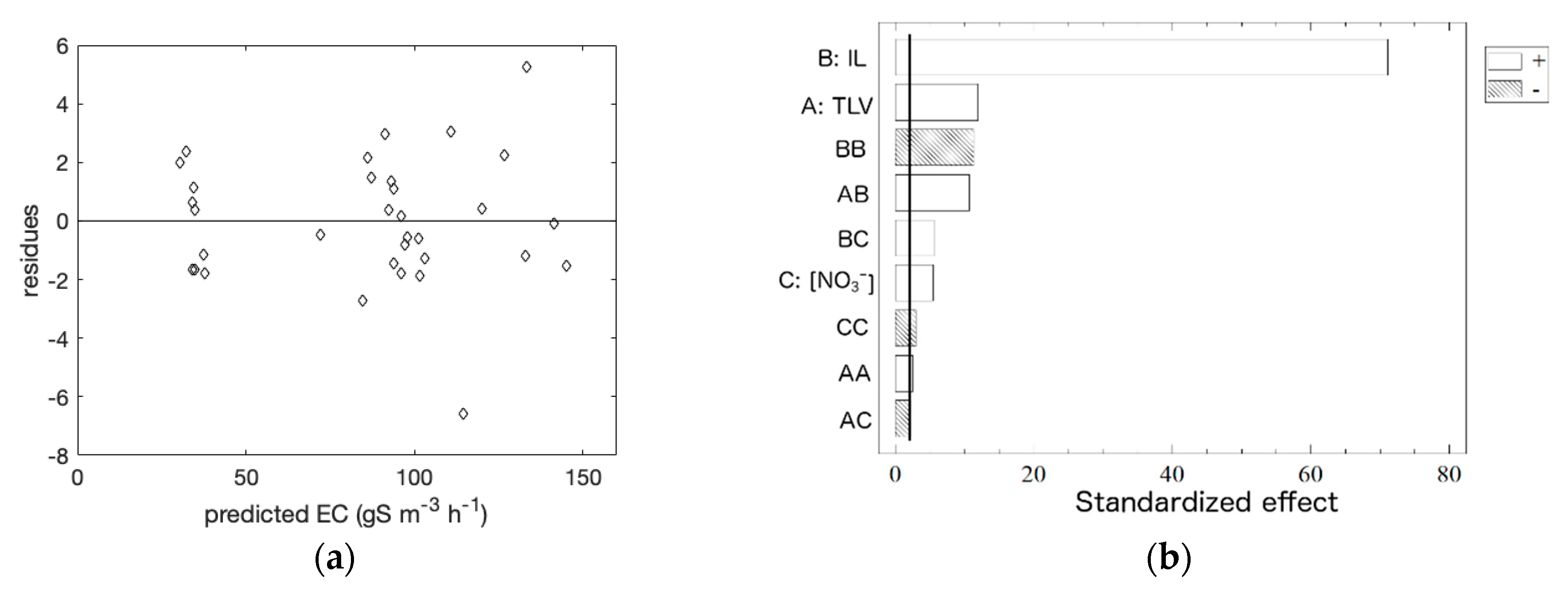
3.1. Empirical Model
3.2. Ottengraf’s Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. A Clean Planet for All a European Strategic Long-Term Vision for a Prosperous, Modern, Competitive and Climate Neutral Economy; COM (2018) 773 Final; European Commission: Brussels, Belgium, 2018; pp. 1–25.
- Cano, P.I.; Colón, J.; Ramírez, M.; Lafuente, J.; Gabriel, D.; Cantero, D. Life cycle assessment of different physical-chemical and biological technologies for biogas desulfurization in sewage treatment plants. J. Clean. Prod. 2018, 181, 663–674. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, Y.; Huan, C.; Shuai, Y.; Liu, Y.; Xu, L.; Ji, G.; Yan, Z. Anoxic biodesulfurization using biogas digestion slurry in biotrickling filters. J. Clean. Prod. 2019, 224, 88–99. [Google Scholar] [CrossRef]
- Valle, A.; Fernández, M.; Ramírez, M.; Rovira, R.; Gabriel, D.; Cantero, D. A comparative study of eubacterial communities by PCR-DGGE fingerprints in anoxic and aerobic biotrickling filters used for biogas desulfurization. Bioprocess Biosyst. Eng. 2018, 41, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Montebello, A.M.; Fernández, M.; Almenglo, F.; Ramírez, M.; Cantero, D.; Baeza, M.; Gabriel, D. Simultaneous methylmercaptan and hydrogen sulfide removal in the desulfurization of biogas in aerobic and anoxic biotrickling filters. Chem. Eng. J. 2012, 200–202, 237–246. [Google Scholar] [CrossRef]
- Fernández, M.; Ramírez, M.; Gómez, J.; Cantero, D. Biogas biodesulfurization in an anoxic biotrickling filter packed with open-pore polyurethane foam. J. Hazard. Mater. 2014, 264, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Fortuny, M.; Baeza, J.; Gamisans, X.; Casas, C.; Lafuente, J.; Deshusses, M.; Gabriel, D. Biological sweetening of energy gases mimics in biotrickling filters. Chemosphere 2008, 71, 10–17. [Google Scholar] [CrossRef]
- Wainwright, J.; Mulligan, M. Environmental Modelling: Finding Simplicity in Complexity, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-0-470-74911-1. [Google Scholar]
- Ottengraf, S.; Rehm, H.; Reed, G. Exhaust gas purification. In Biotechnology—A Comprehensive Treatise; Verlag Chemie: Weinheum, Germany, 1986; Volume 8, pp. 426–452. [Google Scholar]
- Shareefdeen, Z.; Baltzis, B.; Oh, Y.; Bartha, R. Biofiltration of Methanol Vapor. Biotechnol. Bioeng. 1993, 41, 512–524. [Google Scholar] [CrossRef]
- Baltzis, B.; Wojdyla, S.; Zarook, S. Modeling biofiltration of VOC mixtures under steady-state conditions. J. Environ. Eng. 1997, 123, 599–605. [Google Scholar] [CrossRef]
- Soreanu, G. Insights into siloxane removal from biogas in biotrickling filters via process mapping-based analysis. Chemosphere 2016, 146, 539–546. [Google Scholar] [CrossRef]
- Soreanu, G.; Falletta, P.; Béland, M.; Edmonson, K.; Ventresca, B.; Seto, P. Empirical modelling and dual-performance optimisation of a hydrogen sulphide removal process for biogas treatment. Bioresour. Technol. 2010, 101, 9387–9390. [Google Scholar] [CrossRef]
- López, L.; Dorado, A.; Mora, M.; Gamisans, X.; Lafuente, J.; Gabriel, D. Modeling an aerobic biotrickling filter for biogas desulfurization through a multi-step oxidation mechanism. Chem. Eng. J. 2016, 294, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Almenglo, F.; Ramírez, M.; Gómez, J.; Cantero, D.; Gamisans, X.; Dorado, A. Modeling and control strategies for anoxic biotrickling filtration in biogas purification. J. Chem. Technol. Biotechnol. 2016, 91, 1782–1793. [Google Scholar] [CrossRef]
- Almenglo, F.; Ramírez, M.; Gómez, J.; Cantero, D. Operational conditions for start-up and nitrate-feeding in an anoxic biotrickling filtration process at pilot scale. Chem. Eng. J. 2016, 285, 83–91. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.; Eaton, A. Standard Methods for the Examination of Water and Waste Water, 20th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1999; ISBN 0875532357. [Google Scholar]
- Veljković, V.B.; Veličković, A.V.; Avramović, J.M.; Stamenković, O.S. Modeling of biodiesel production: Performance comparison of box–Behnken, face central composite or full factorial design. Chin. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; p. 208. ISBN 0-471-31649-0. [Google Scholar]
- Ottengraf, S.; Oever, V.A. Kinetics of organic compound removal from waste gases with a biological filter. Biotechnol. Bioeng. 1983, 25, 3089–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onda, K.; Takeuchi, H.; Okumoto, Y. Mass transfer coefficients between gas and liquid phases in packed columns. J. Chem. Eng. Jpn. 1968, 1, 56–62. [Google Scholar] [CrossRef]
- López, L.R.; Brito, J.; Mora, M.; Almenglo, F.; Baeza, J.A.; Ramírez, M.; Lafuente, J.; Cantero, D.; Gabriel, D. Feedforward control application in aerobic and anoxic biotrickling filters for H2S removal from biogas. J. Chem. Technol. Biotechnol. 2018, 93, 2307–2315. [Google Scholar] [CrossRef]
- Brito, J.; Valle, A.; Almenglo, F.; Ramírez, M.; Cantero, D. Progressive change from nitrate to nitrite as the electron acceptor for the oxidation of H2S under feedback control in an anoxic biotrickling filter. Biochem. Eng. J. 2018, 139, 154–161. [Google Scholar] [CrossRef]
- López, L.R.; Bezerra, T.; Mora, M.; Lafuente, J.; Gabriel, D. Influence of trickling liquid velocity and flow pattern in the improvement of oxygen transport in aerobic biotrickling filters for biogas desulfurization. J. Chem. Technol. Biotechnol. 2016, 91, 1031–1039. [Google Scholar] [CrossRef]
- Fernández, M.; Ramírez, M.; Pérez, R.; Gómez, J.; Cantero, D. Hydrogen sulphide removal from biogas by an anoxic biotrickling filter packed with Pall rings. Chem. Eng. J. 2013, 225, 456–463. [Google Scholar] [CrossRef]
- Ordaz, A.; Figueroa-González, I.; San-Valero, P.; Gabaldón, C.; Quijano, G. Effect of the height-to-diameter ratio on the mass transfer and mixing performance of a biotrickling filter. J. Chem. Technol. Biotechnol. 2018, 93, 121–126. [Google Scholar] [CrossRef]
- Lebrero, R.; Gondim, A.; Pérez, R.; García-Encina, P.A.; Muñoz, R. Comparative assessment of a biofilter, a biotrickling filter and a hollow fiber membrane bioreactor for odor treatment in wastewater treatment plants. Water Res. 2014, 49, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Gómez, J.; Cantero, D.; Ramírez, M.; Gómez, J.; Cantero, D. Biogas: Sources, Purification and Uses. In Hydrogen and Other Technologies; Studium Press LLC: Houston, TX, USA, 2015; Volume 11, pp. 296–323. ISBN 978-1-626990-72-2. [Google Scholar]
- Koda, M.; Dogru, A.H.; Seinfeld, J.H. Sensitivity analysis of partial differential equations with application to reaction and diffusion processes. J. Comput. Phys. 1979, 30, 259–282. [Google Scholar] [CrossRef]
- Oyarzún, P.; Arancibia, F.; Canales, C.; Aroca, G.E. Biofiltration of high concentration of hydrogen sulphide using Thiobacillus thioparus. Process Biochem. 2003, 39, 165–170. [Google Scholar] [CrossRef]
- Jaber, M.; Couvert, A.; Amrane, A.; Rouxel, F.; Cloirec, P.; Dumont, E. Biofiltration of H2S in air—Experimental comparisons of original packing materials and modeling. Biochem. Eng. J. 2016, 112, 153–160. [Google Scholar] [CrossRef]
- Jin, Y.; Veiga, M.C.; Kennes, C. Effects of pH, CO2, and flow pattern on the autotrophic degradation of hydrogen sulfide in a biotrickling filter. Biotechnol. Bioeng. 2005, 92, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Shareefdeen, Z.M.; Ahmed, W.; Aidan, A. Kinetics and Modeling of H2S Removal in a Novel Biofilter. Adv. Chem. Eng. Sci. 2011, 1, 72–76. [Google Scholar] [CrossRef]



| Factor | Levels of Factors | ||
|---|---|---|---|
| (–1) | (0) | (+1) | |
| FG (m3·h−1) | 1 | 3 | 5 |
| FL (m3·h−1) | 1 | 2 | 3 |
| [N-NO3−] (mg·L−1) | 1.4 ± 1.1 | 35.3 ± 2.4 | 70.5 ± 10.2 |
| IL1 (gS·m3·h−1) | 35.1 ± 1.5 | 109.1 ± 11.7 | 172.4 ± 3.4 |
| TLV2 (m·h−1) | 5.09 | 10.18 | 15.27 |
| EBRT1 (s) | 600 | 200 | 120 |
| TLV (m·h−1) | EBRT (s) 1 | ||
|---|---|---|---|
| 600 | 200 | 120 | |
| 5.09 | 99.39 | 84.73 | 79.31 |
| 10.18 | 99.24 | 93.08 | 83.06 |
| 15.27 | 99.53 | 97.65 | 92.13 |
| Factor | ‘C Model’ | ‘EC Model’ | ||
|---|---|---|---|---|
| Maximum | Minimum | Maximum | Minimum | |
| TLV | 0.0078 | –0.1579 | 4.3303 | –1.252 |
| IL | 0.0177 | 0.0045 | 1.1553 | 0.1657 |
| [N-NO3−] | 0.0038 | –0.159 | 0.4363 | –0.1801 |
| IL (gS·m−3·h−1) | TLV (m·h−1) | Zero-Order Diffusion | Zero-Order Kinetic | First-Order Kinetic | |||
|---|---|---|---|---|---|---|---|
| r2 | k (s−1) | r2 | k (s−1) | r2 | k (s−1) | ||
| 36 | 5.09 | 0.62 | 0.0018 | 0.3 | 0.012 | 0.95 | 0.008 |
| 35 | 10.18 | 0.57 | 0.0018 | 0.26 | 0.012 | 0.92 | 0.0087 |
| 33 | 15.27 | 0.52 | 0.0018 | 0.22 | 0.011 | 0.93 | 0.0092 |
| 130 | 5.09 | 0.95 | 0.0011 | 0.94 | 0.011 | 0.93 | 0.0031 |
| 108 | 10.18 | 0.91 | 0.0012 | 0.82 | 0.01 | 0.93 | 0.0036 |
| 104 | 15.27 | 0.93 | 0.0014 | 0.77 | 0.01 | 0.96 | 0.0057 |
| 170 | 5.09 | 0.97 | 0.0009 | 0.96 | 0.0079 | 0.96 | 0.0025 |
| 176 | 10.18 | 0.88 | 0.001 | 0.79 | 0.0091 | 0.93 | 0.0029 |
| 167 | 15.27 | 0.86 | 0.0011 | 0.74 | 0.0092 | 0.95 | 0.0034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almenglo, F.; Ramírez, M.; Cantero, D. Application of Response Surface Methodology for H2S Removal from Biogas by a Pilot Anoxic Biotrickling Filter. ChemEngineering 2019, 3, 66. https://doi.org/10.3390/chemengineering3030066
Almenglo F, Ramírez M, Cantero D. Application of Response Surface Methodology for H2S Removal from Biogas by a Pilot Anoxic Biotrickling Filter. ChemEngineering. 2019; 3(3):66. https://doi.org/10.3390/chemengineering3030066
Chicago/Turabian StyleAlmenglo, Fernando, Martín Ramírez, and Domingo Cantero. 2019. "Application of Response Surface Methodology for H2S Removal from Biogas by a Pilot Anoxic Biotrickling Filter" ChemEngineering 3, no. 3: 66. https://doi.org/10.3390/chemengineering3030066






