Visible-Light-Driven Photocatalytic Fuel Cell with an Ag-TiO2 Carbon Foam Anode for Simultaneous 4-Chlorophenol Degradation and Energy Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Nanosize Ag-TiO2
2.2. Preparation and Photocatalytic of the Ag-TiO2 Photoanode
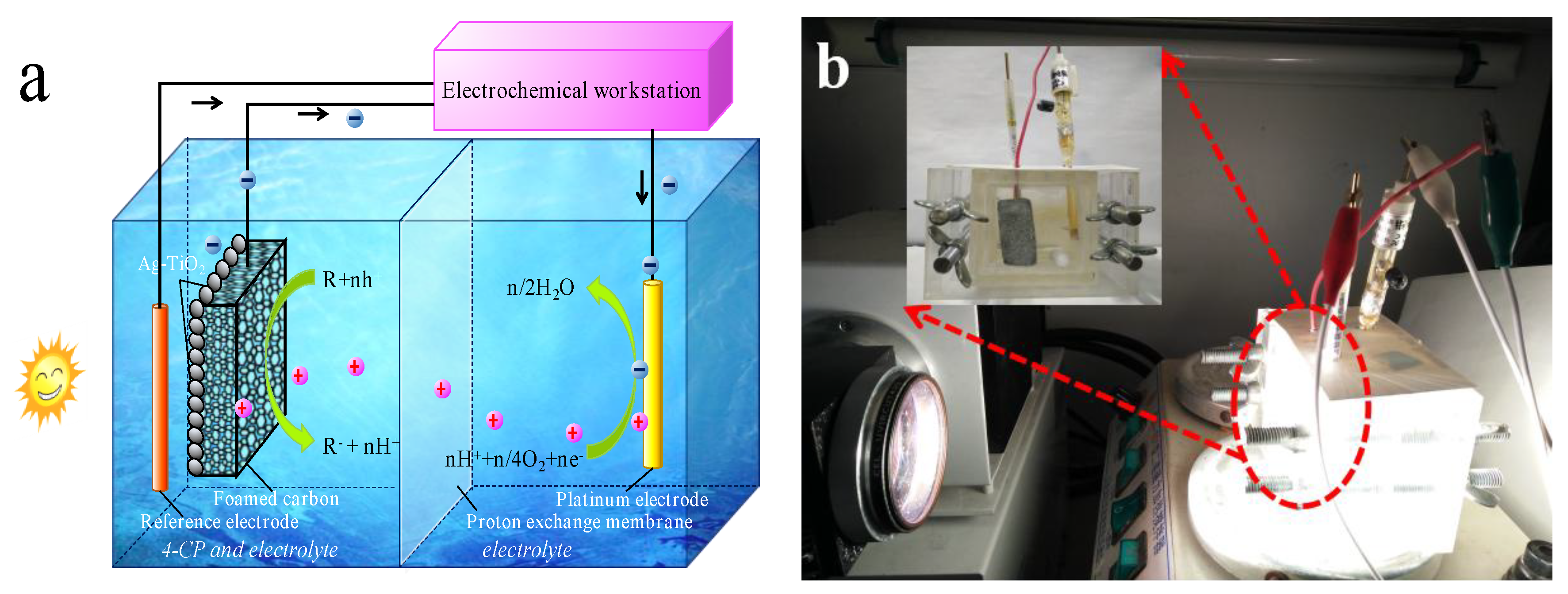
2.3. Photocatalytic Fuel Cell Set-Up and Operation
2.4. Photocatalytic Fuel Cell Set-Up and Operation
3. Results and Discussion
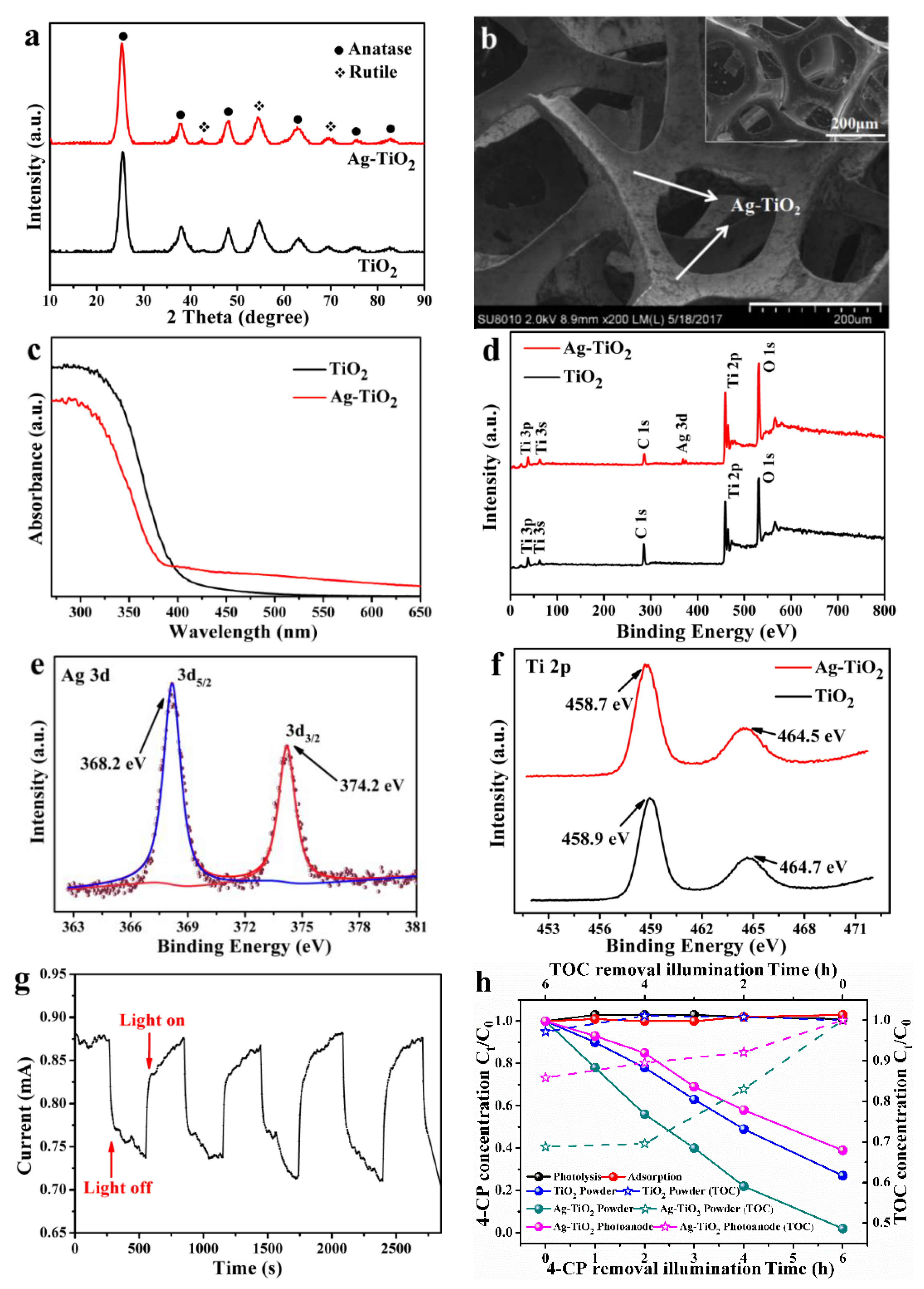
3.1. Characterization and Performance of the as-Prepared Ag-TiO2
3.2. Effect of Electrolyte on PFC Performance
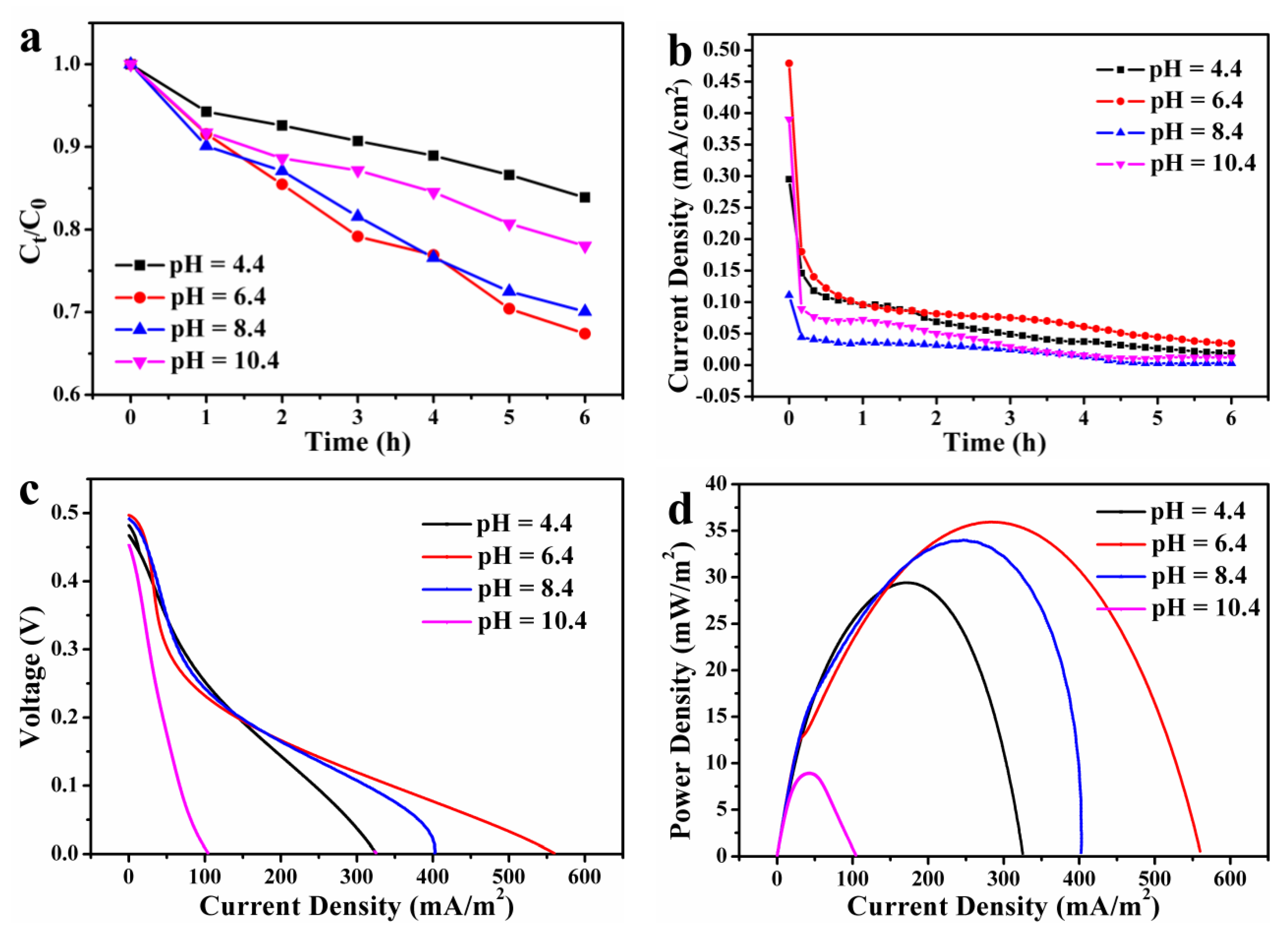
3.3. Effect of pH on PFC Performance
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gomez-Acata, S.; Vital-Jacome, M.; Perez-Sandoval, M.V.; Navarro-Noya, Y.E.; Thalasso, F.; Luna-Guido, M.; Conde-Barajas, E.; Dendooven, L. Microbial community structure in aerobic and fluffy granules formed in a sequencing batch reactor supplied with 4-chlorophenol at different settling times. J. Hazard. Mater. 2018, 342, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.; Bhunia, B.; Dutta, S.; Dey, A. Enhanced biodegradation of 4-chlorophenol by Candida tropicalis PHB5 via optimization of physicochemical parameters using Taguchi orthogonal array approach. Int. Biodeterior. Biodegrad. 2013, 78, 17–23. [Google Scholar] [CrossRef]
- Lim, J.W.; Lim, P.E.; Seng, C.E.; Adnan, R. Simultaneous 4-chlorophenol and nitrogen removal in moving bed sequencing batch reactors packed with polyurethane foam cubes of various sizes. Bioresour. Technol. 2013, 129, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Miran, W.; Nawaz, M.; Jang, J.; Lee, D.S. Chlorinated phenol treatment and in situ hydrogen peroxide production in a sulfate-reducing bacteria enriched bioelectrochemical system. Water Res. 2017, 117, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.D.; Dojčinović, B.P.; Obradović, B.M.; Nešić, J.; Natić, M.M.; Tosti, T.B.; Kuraica, M.M.; Manojlović, D.D. Degradation and detoxification of the 4-chlorophenol by non-thermal plasma-influence of homogeneous catalysts. Sep. Purif. Technol. 2015, 154, 246–254. [Google Scholar] [CrossRef]
- Zhou, D.; Dong, S.; Shi, J.; Cui, X.; Ki, D.; Torres, C.I.; Rittmann, B.E. Intimate coupling of an N-doped TiO2 photocatalyst and anode respiring bacteria for enhancing 4-chlorophenol degradation and current generation. Chem. Eng. J. 2017, 317, 882–889. [Google Scholar] [CrossRef]
- Deng, B.; Fu, S.; Zhang, Y.; Wang, Y.; Ma, D.; Dong, S. Simultaneous pollutant degradation and power generation in visible-light responsive photocatalytic fuel cell with an Ag-TiO2 loaded photoanode. Nano-Struct. Nano-Objects 2018, 15, 167–172. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, J.; Gao, L.; Wang, Y.; Zhou, D. Preparation of spherical activated carbon-supported and Er3+:YAlO3-doped TiO2 photocatalyst for methyl orange degradation under visible light. Trans. Nonferrous Met. Soc. China 2012, 22, 2477–2483. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Geng, J.; Jing, D. Photodecomposition of NOx on Ag/TiO2 composite catalysts in a gas phase reactor. Chem. Eng. J. 2017, 307, 181–188. [Google Scholar] [CrossRef]
- Ying, D.; Cao, R.; Li, C.; Tang, T.; Li, K.; Wang, H.; Wang, Y.; Jia, J. Study of the photocurrent in a photocatalytic fuel cell for wastewater treatment and the effects of TiO2 surface morphology to the apportionment of the photocurrent. Electrochim. Acta 2016, 192, 319–327. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhou, B.; Lv, S.; Li, X.; Chen, H.; Chen, Q.; Cai, W. Photoelectrocatalytic degradation of refractory organic compounds enhanced by a photocatalytic fuel cell. Appl. Catal. B 2012, 111–112, 485–491. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Ma, Y.; Cheng, H.; Fu, S.; Zhou, D.; Dong, S. Construction of Er3+:YAlO3 /RGO/TiO2 Hybrid Electrode with Enhanced Photoelectrocatalytic Performance in Methylene Blue Degradation Under Visible Light. Photochem. Photobiol. 2017, 93, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Lianos, P. Production of electricity by photoelectrochemical oxidation of ethanol in a PhotoFuelCell. Appl. Catal. B 2010, 99, 307–313. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Tang, T.; Xu, Y.; Ying, D.; Wang, Y.; Jia, J. Optimization and application of TiO2/Ti-Pt photo fuel cell (PFC) to effectively generate electricity and degrade organic pollutants simultaneously. Water Res. 2014, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Ho, L.; Ong, S.; Ibrahim, A.H.; Wong, Y.; Lee, S.; Oon, Y.; Oon, Y. Hybrid system of photocatalytic fuel cell and Fenton process for electricity generation and degradation of Reactive Black 5. Sep. Purif. Technol. 2017, 177, 135–141. [Google Scholar] [CrossRef]
- Koyama, M.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Kubo, M.; del Carpio, C.A.; Miyamoto, A. Development of Three-Dimensional Porous Structure Simulator for Optimizing Microstructure of SOFC Anode. ECS Trans. 2007, 7, 2057–2064. [Google Scholar] [CrossRef]
- Guo, D.; Song, R.B.; Shao, H.H.; Zhang, J.R.; Zhu, J.J. Visible-light-enhanced power generation in microbial fuel cells coupling with 3D nitrogen-doped graphene. Chem. Commun. 2017, 53, 9967–9970. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Yunlan, X.; Yi, H.; Chen, Y.; Yalin, W.; Jinping, J. Photocatalytic Fuel Cell (PFC) and Self-Photosensitization Photocatalytic Fuel Cell (DSPFC) with BiOCl/Ti Photoanode under UV and Visible Light Irradiation. Environ. Sci. Technol. 2013, 47, 3490–3497. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhou, B.; Li, X.; Chen, H.; Chen, Q.; Wang, Z.; Li, L.; Wang, J.; Cai, W. Efficient electricity production and simultaneously wastewater treatment via a high-performance photocatalytic fuel cell. Water Res. 2011, 45, 3991–3998. [Google Scholar] [CrossRef] [PubMed]
- Yasmina, B.; Marjolein, C.F.M.P.; Peter, W.A.; Luuk, C.R. Electrochemically active biofilm and photoelectrocatalytic regeneration of the titanium dioxide composite electrode for advanced oxidation in water treatment. Electrochim. Acta 2015, 182, 604–612. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C 2005, 6, 186–205. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Liu, B.; Xie, Y.; Han, G.; Li, Y.; Zhang, L.; Zhang, X. Preparation of nano-sized mixed crystal TiO2-coated Er3+:YAlO3 by sol-gel method for photocatalytic degradation of organic dyes under visible light irradiation. Water Sci. Technol. 2009, 60, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Teruhisa, O.; Koji, S.; Michio, M. Photocatalytic Activities of Pure Rutile Particles Isolated from TiO2 Powder by Dissolving the Anatase Component in HF Solution. J. Phys. Chem. B 2001, 105, 2417–2420. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A 2003, 244, 383–391. [Google Scholar] [CrossRef]
- Fresno, F.; Coronado, J.M.; Tudela, D.; Soria, J. Influence of the structural characteristics of Ti1−xSnxO2 nanoparticles on their photocatalytic activity for the elimination of methylcyclohexane vapors. Appl. Catal. B 2005, 55, 159–167. [Google Scholar] [CrossRef]
- Xin, B.; Jing, L.; Ren, Z.; Wang, J.; Yu, H.; Fu, H. Preparation and Activity of Ag-TiO2 Photocatalyst with Multi-valency State. Acta Chim. Sin. 2004, 62, 1110–1114. [Google Scholar]
- Jina, C.; Hyunwoong, P.; Michael, R.H. Effects of Single Metal-Ion Doping on the Visible-Light Photoreactivity of TiO2. J. Phys. Chem. 2010, 114, 783–792. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and characterization of Ag–TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Cheng, B.; Ong, H.C. Microwave-hydrothermal preparation and visible-light photoactivity of plasmonic photocatalyst Ag-TiO2 nanocomposite hollow spheres. Chem. Asian J. 2010, 5, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Shi, H.; Wang, D. Photoelectrocatalytic Degradation of Phenol Using a TiO2/Ni Thin-film Electrode. Korean J. Chem. Eng. 2003, 20, 679–684. [Google Scholar]
- Liu, H.; Cheng, S.; Logan, B.E. Power Generation in Fed-Batch Microbial Fuel Cells as a Function of Ionic Strength, Temperature and Reactor Configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xiong, H.; Dong, S.; Zhang, J.; Ma, D.; Zhou, D. Identifying the role of reactive oxygen species (ROSs) in Fusarium solani spores inactivation. AMB Express 2016, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Ejhieh, A.N.; Khorsandi, M. Photodecolorization of Eriochrome Black T using NiS-P zeolite as a heterogeneous catalyst. J. Hazard. Mater. 2010, 176, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kelm, D.; Schreiner, M.; Wollborn, T.; Madler, L.; Teoh, W.Y. Designing Photoelectrodes for Photocatalytic Fuel Cells and Elucidating the Effects of Organic Substrates. ChemSusChem 2015, 8, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Nezamzadeh-Ejhieh, A.; Karimi-Shamsabadi, M. Decolorization of a binary azo dyes mixture using CuO incorporated nanozeolite-X as a heterogeneous catalyst and solar irradiation. Chem. Eng. J. 2013, 228, 631–641. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Lu, X.-Y.; Xuan, J.; Leung, M.K.H. Solar photocatalytic fuel cell using CdS–TiO2 photoanode and air-breathing cathode for wastewater treatment and simultaneous electricity production. Chem. Eng. J. 2014, 253, 174–182. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Deng, B.; Ma, D.; Cheng, H.; Dong, S. Visible-Light-Driven Photocatalytic Fuel Cell with an Ag-TiO2 Carbon Foam Anode for Simultaneous 4-Chlorophenol Degradation and Energy Recovery. ChemEngineering 2018, 2, 20. https://doi.org/10.3390/chemengineering2020020
Fu S, Deng B, Ma D, Cheng H, Dong S. Visible-Light-Driven Photocatalytic Fuel Cell with an Ag-TiO2 Carbon Foam Anode for Simultaneous 4-Chlorophenol Degradation and Energy Recovery. ChemEngineering. 2018; 2(2):20. https://doi.org/10.3390/chemengineering2020020
Chicago/Turabian StyleFu, Shaozhu, Beiqi Deng, Dongmei Ma, Hanqing Cheng, and Shuangshi Dong. 2018. "Visible-Light-Driven Photocatalytic Fuel Cell with an Ag-TiO2 Carbon Foam Anode for Simultaneous 4-Chlorophenol Degradation and Energy Recovery" ChemEngineering 2, no. 2: 20. https://doi.org/10.3390/chemengineering2020020
APA StyleFu, S., Deng, B., Ma, D., Cheng, H., & Dong, S. (2018). Visible-Light-Driven Photocatalytic Fuel Cell with an Ag-TiO2 Carbon Foam Anode for Simultaneous 4-Chlorophenol Degradation and Energy Recovery. ChemEngineering, 2(2), 20. https://doi.org/10.3390/chemengineering2020020



