Experimental Study on Fluorine Release from Photovoltaic Backsheet Materials Containing PVF and PVDF during Pyrolysis and Incineration in a Technical Lab-Scale Reactor at Various Temperatures
Abstract
1. Introduction
2. Materials and Methods
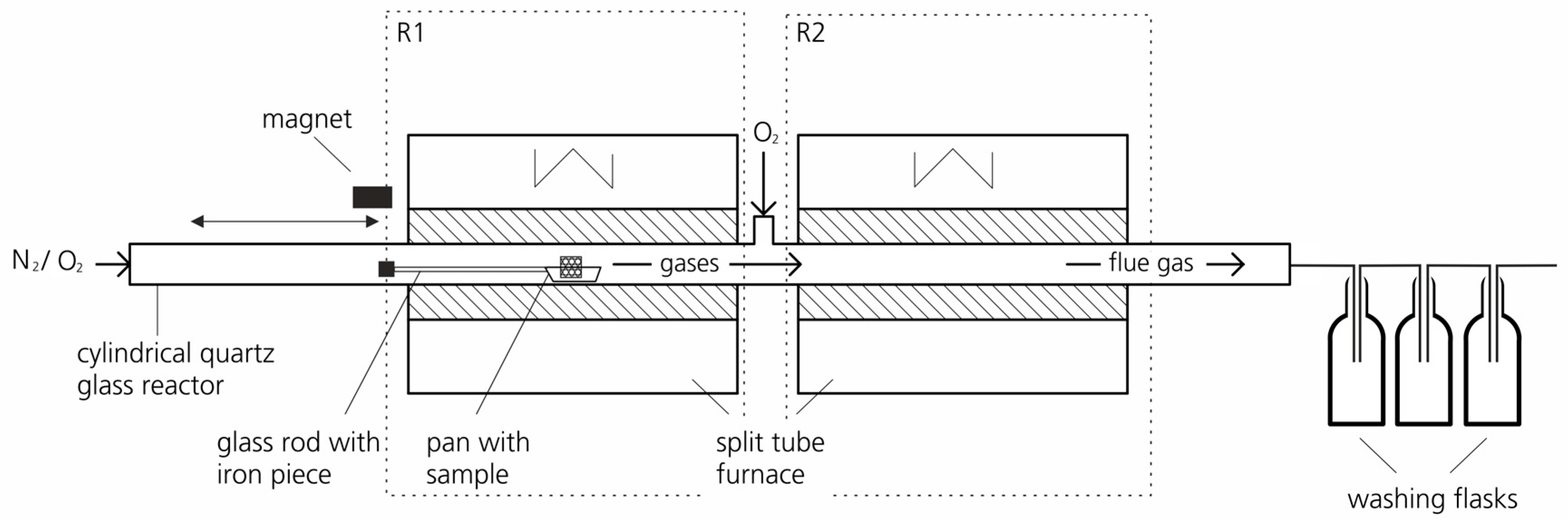
2.1. Experimental Setup and Methodology
2.2. Materials and Sample Preparation
2.3. Reference Experiments
2.4. Pyrolysis Experiments
2.5. Incineration Experiments
3. Results and Discussion
3.1. Reference Test Experiments
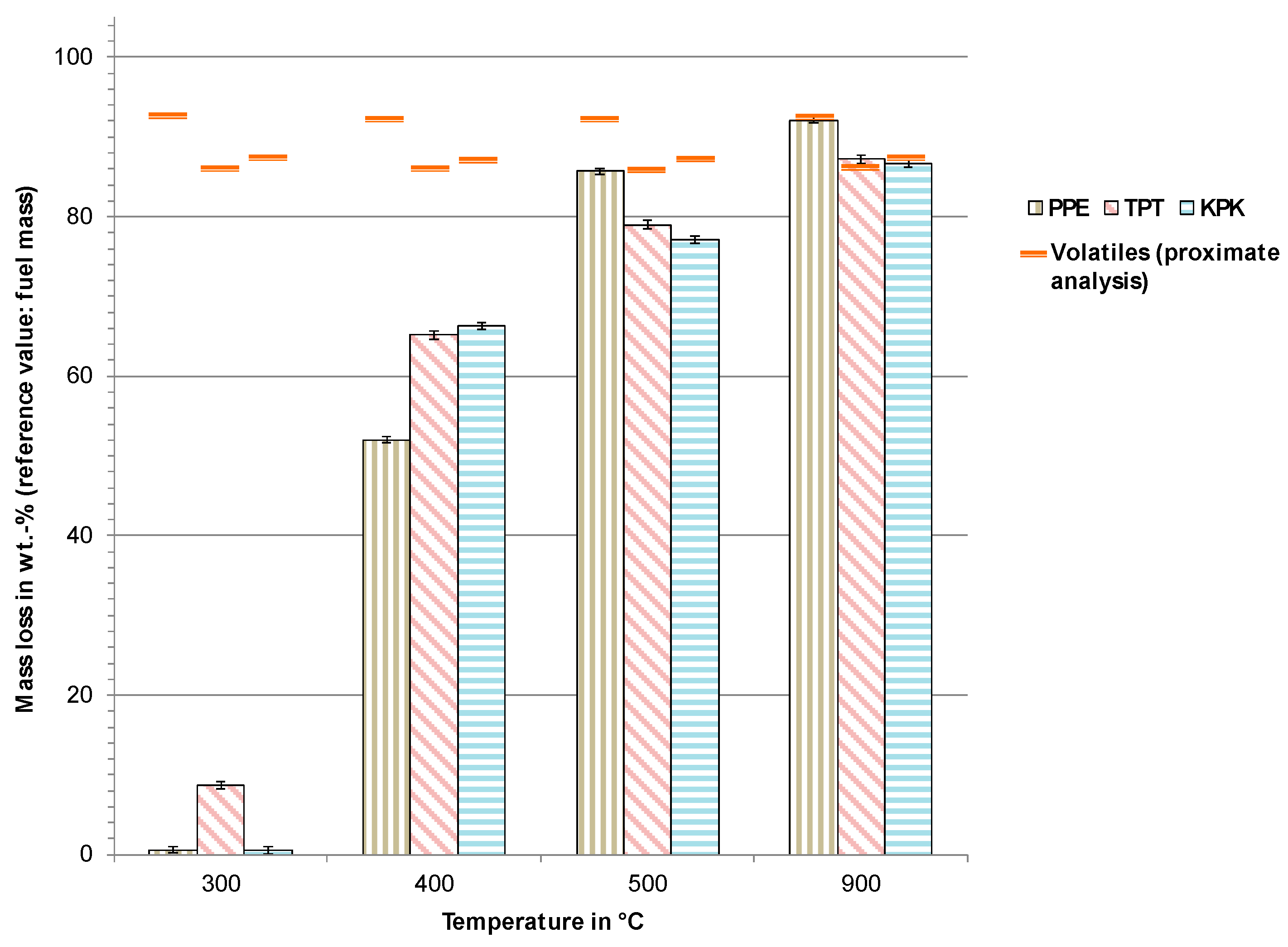
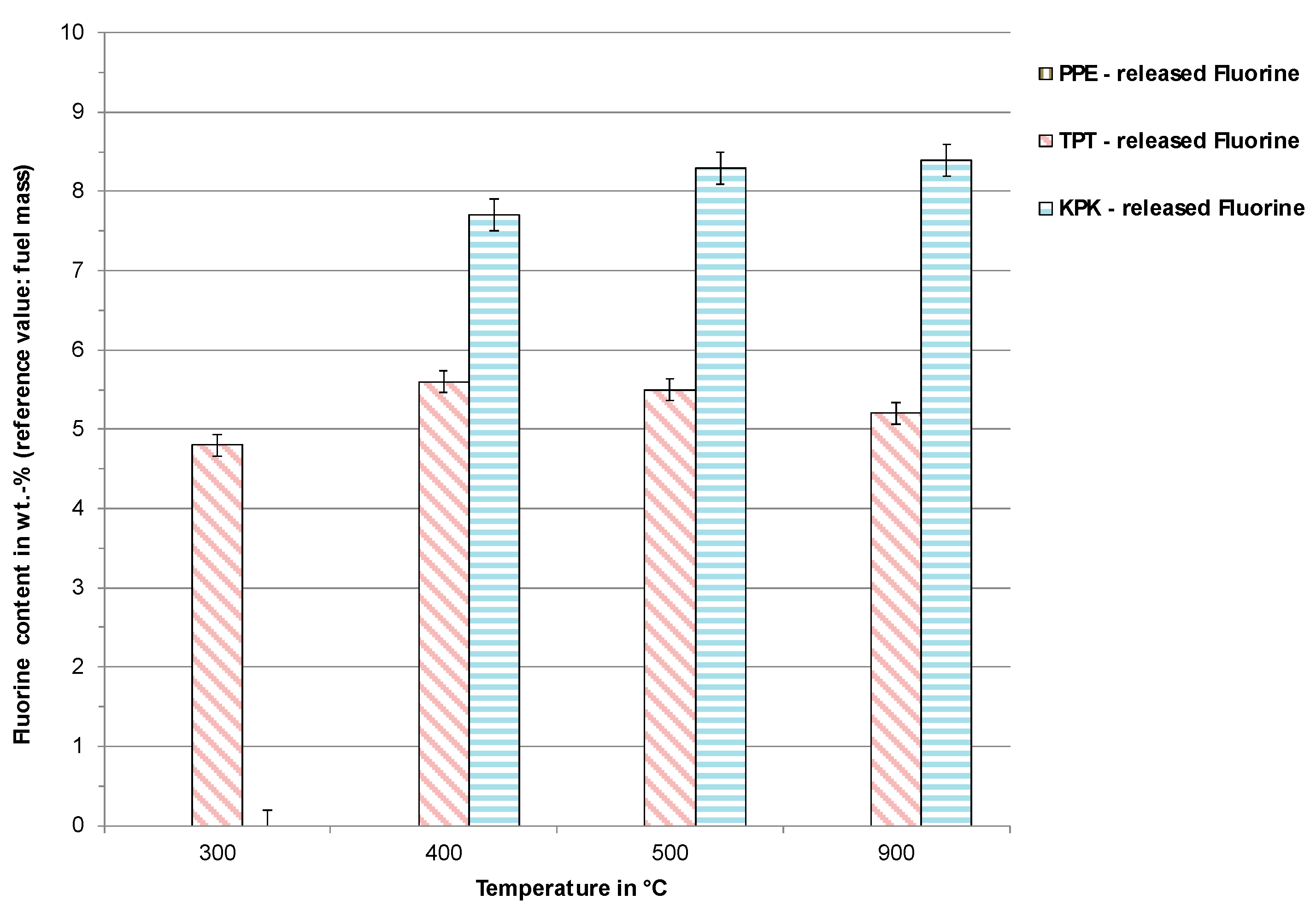
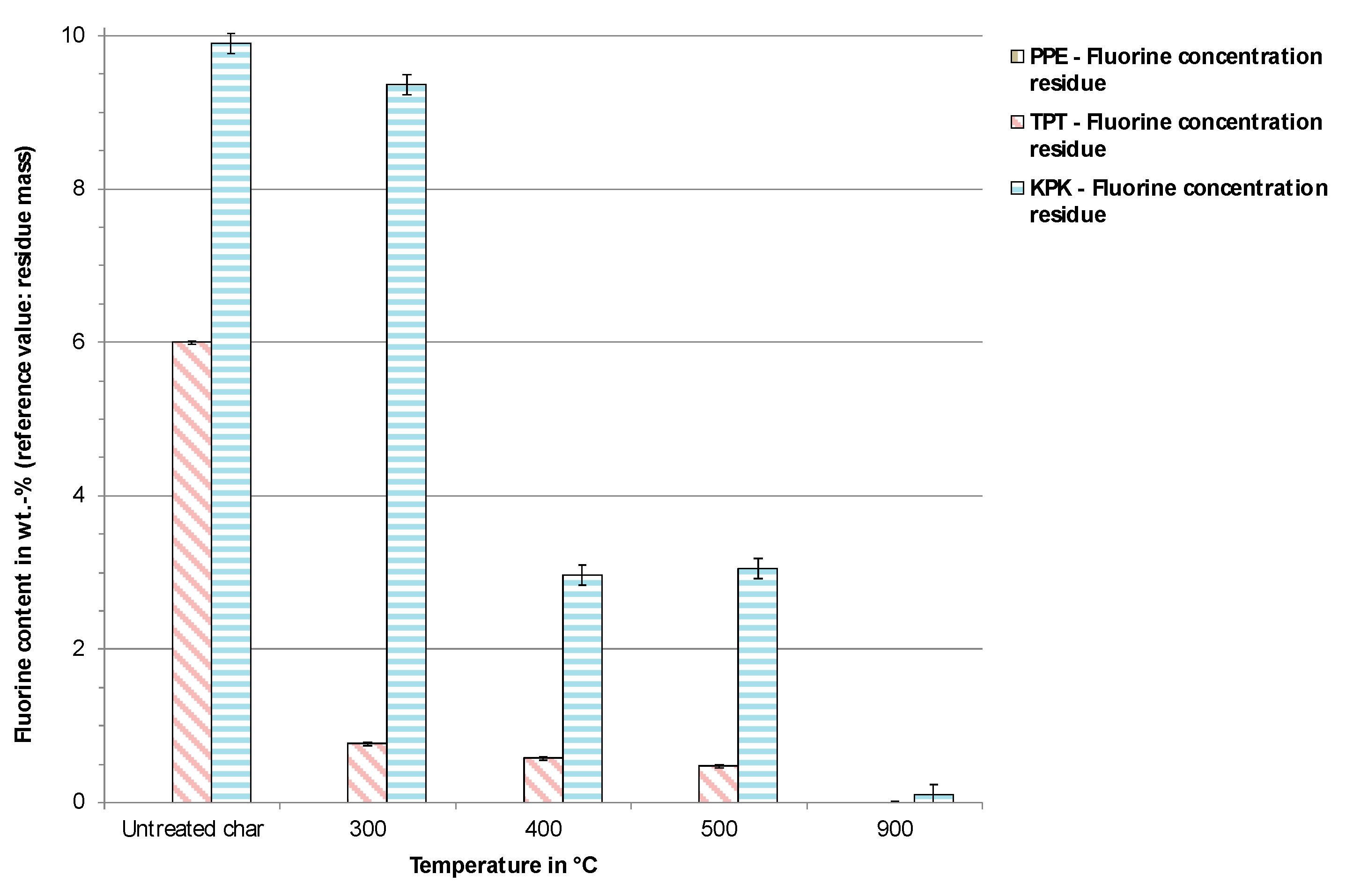
3.2. Pyrolysis Experiments
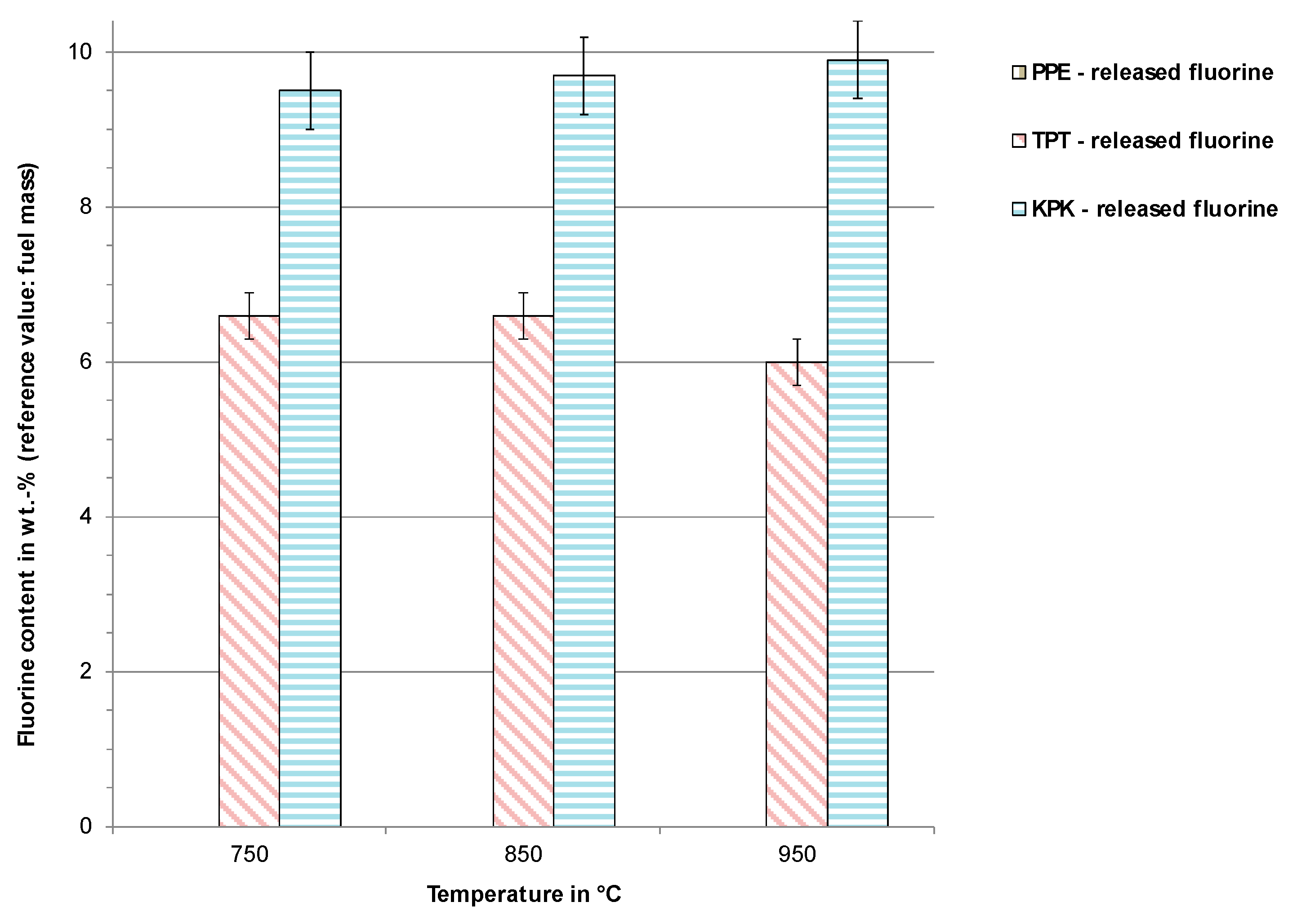
3.3. Incineration Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cucchiella, F.; D’Adamo, I.; Koh, S.C.L.; Rosa, P. Recycling of WEEEs: An economic assessment of present and future e-waste streams: An economic assessment of present and future e-waste streams. Renew. Sustain. Energy Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef]
- Singh, N.; Li, J.; Zeng, X. Global responses for recycling waste CRTs in e-waste. Waste Manag. 2016, 57, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.-W.; Born, M.; Wambach, K. Pyrolysis of EVA and its application in recycling of photovoltaic modules. J. Environ. Sci. 2004, 16, 889–893. [Google Scholar]
- Musson, S.E.; Jang, Y.C.; Townsend, T.G.; Chung, I.H. Characterization of Lead Leachability from Cathode Ray Tubes Using the Toxicity Characteristic Leaching Procedure. Energy Environ. Sci. 2000, 34, 4376–4381. [Google Scholar] [CrossRef]
- Innocent, C.; Nnorom, O.; Stanley, N. Achieving Resource Conservation in Electronic Waste Management: A Review of Options Available to Developing Countries. J. Appl. Sci. 2007, 7, 2918–2933. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Holuszko, M.; Espinosa, D.C.R. E-waste: An overview on generation, collection, legislation and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Weckend, S.; Wade, A.; Heath, G. End-of-Life Management: Solar Photovoltaic Systems. 2016. Available online: http://www.irena.org/menu/index.aspx?mnu=Subcat&PriMenuID=36&CatID=141&SubcatID=2734 (accessed on 1 July 2019).
- Peeters, J.R.; Altamirano, D.; Dewulf, W.; Duflou, J.R. Forecasting the composition of emerging waste streams with sensitivity analysis: A case study for photovoltaic (PV) panels in Flanders. Resour. Conserv. Recycl. 2017, 120, 14–26. [Google Scholar] [CrossRef]
- Fthenakis, V.M. End-of-life management and recycling of PV modules. Energy Policy 2000, 28, 1051–1058. [Google Scholar] [CrossRef]
- Held, M.; Ilg, R. Update of environmental indicators and energy payback time of CdTe PV systems in Europe. Prog. Photovolt. Res. Appl. 2011, 19, 614–626. [Google Scholar] [CrossRef]
- Hense, P.; Reh, K.; Franke, M.; Aigner, J.; Hornung, A.; Contin, A. Pyrolysis of waste electrical and electronic equipment (WEEE) for recovering metals and energy: Previous achievements and current approaches. Environ. Eng. Manag. J. 2015, 14, 2661–2670. [Google Scholar] [CrossRef]
- Moskowitz, P.D.; Zweibel, K. Recycling of Cadmium and Selenium from Photovoltaic Modules and Manufacturing Wastes: A Workshop Report. 1992. Available online: http://webapp1.dlib.indiana.edu/virtual_disk_library/index.cgi/4298428/FID2707/m93002115.pdf (accessed on 1 July 2019).
- Choi, S.; Kim, Y. Analysis of pyrolysis products of poly(vinylidene fluoride-co-hexafluoropropylene) by pyrolysis-gas chromatography/mass spectrometry. J. Fluor. Chem. 2014, 165, 33–38. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Evangelisti, S.; Lettieri, P. Life cycle assessment of alternative technologies for municipal solid waste and plastic solid waste management in the Greater London area. Chem. Eng. J. 2014, 244, 391–402. [Google Scholar] [CrossRef]
- Alston, S.M. Environmental Impact of Pyrolysis of Mixed WEEE Plastics Part 2: Life Cycle Assessment. Environ. Sci. Technol. 2011, 45, 9386–9392. [Google Scholar] [CrossRef] [PubMed]
- Aryan, V.; Font-Brucart, M.; Maga, D. A comparative life cycle assessment of end-of-life treatment pathways for photovoltaic backsheets. Prog. Photovolt. Res. Appl. 2018, 26, 443–459. [Google Scholar] [CrossRef]
- Madorsky, S.L.; Hart, V.E.; Straus, S.; Sedlak, V.A. Thermal degradation of tetrafluoroethylene and hydrofluoroethylene polymers in a vacuum. J. Res. Natl. Bur. Stand. 1953, 51, 327–333. [Google Scholar] [CrossRef]
- Madorsky, S.L. Thermal Degradation of Organic Polymers; Huntingon: New York, NY, USA, 1975. [Google Scholar]
- Ellis, D.A.; Mabury, S.A.; Martin, J.W.; Muir, D.C.G. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001, 412, 321–324. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Introduction to Fluoropolymers; Elsevier Science: Amsterdam, The Netherlands, 2013; ISBN 9781455775514. [Google Scholar]
- Tewarson, A.; Chu, F.; Jiang, F.H. Combustion of Halogenated Polymers. 1993. Available online: http://www.iafss.org/publications/fss/4/563/view (accessed on 1 July 2019).
- Williams, P.T.; Williams, E.A. Recycling plastic waste by pyrolysis. J. Inst. Energy 1998, 71, 81–93. [Google Scholar]
- Scheirs, J.; Kaminsky, W. Converting waste plastics into diesel and other fuels. In Feedstock Recycling and Pyrolysis of Waste Plastics; Scheirs, J., Kaminsky, W., Eds.; Wiley: Chichester, UK, 2006; ISBN 9780470021521. [Google Scholar]
- Latunussa, C.E.L.; Ardente, F.; Blengini, G.A.; Mancini, L. Life Cycle Assessment of an innovative recycling process for crystalline silicon photovoltaic panels. Solar Energy Mater. Solar Cells 2016, 156, 101–111. [Google Scholar] [CrossRef]
- Dams, R.; Hintzer, K. Chapter 1: Industrial Aspects of Fluorinated Oligomers and Polymers. In Fluorinated Polymers; Améduri, B., Ed.; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 1–31. ISBN 978-1-78262-936-8. [Google Scholar]
- Huber, S.; Moe, M.K.; Schmidbauer, N.; Hansen, G.H.; Herzke, D. Emissions from Incineration of Fluoropolymer Materials: A Literature Survey; O-108099. 2009. Available online: http://www.nilu.no/Publikasjoner/tabid/62/ctl/PublicationDetails/mid/764/publicationid/24739/language/en-GB/Default.aspx (accessed on 1 July 2019).
- Wall, L.A.; Straus, S. Pyrolysis of fluorocarbon polymers. J. Res. Natl. Bur. Stand. 1961, 65A, 227–238. [Google Scholar] [CrossRef]
- Lonfei, J.; Jingling, W.; Shuman, X. Mechanisms of pyrolysis of fluoropolymers. J. Anal. Appl. Pyrolysis 1986, 10, 99–106. [Google Scholar] [CrossRef]
- ISO. ISO 10304-1:2007—Water Quality—Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate. Available online: https://www.iso.org/standard/46004.html (accessed on 1 July 2019).
- ISO. ISO 1171:2010(en), Solid Mineral Fuels—Determination of Ash. Available online: https://www.iso.org/obp/ui/#iso:std:iso:1171:ed-4:v1:en (accessed on 1 July 2019).
- DIN. DIN 51734: Testing of Solid Mineral Fuels—Proximate Analysis and Calculation of Fixed Carbon. Available online: https://www.din.de/en/getting-involved/standards-committees/nmp/wdc-beuth:din21:111469736?sourceLanguage&destinationLanguage (accessed on 1 July 2019).
- ISO. ISO 29541:2010(en), Solid Mineral Fuels—Determination of Total Carbon, Hydrogen and Nitrogen Content—Instrumental Method. Available online: https://www.iso.org/obp/ui/#iso:std:iso:29541:ed-1:v1:en (accessed on 1 July 2019).
- CEN. CEN—EN 14582—Characterization of Waste—Halogen and Sulfur Content—Oxygen Combustion in Closed Systems and Determination Methods | Engineering360. Available online: https://standards.globalspec.com/std/10039472/cen-en-14582 (accessed on 14 October 2018).
- ISO. ISO 1928:2009(en), Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value. Available online: https://www.iso.org/obp/ui/#iso:std:iso:1928:ed-3:v1:en (accessed on 1 July 2019).
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H. Recycling WEEE: Polymer characterization and pyrolysis study for waste of crystalline silicon photovoltaic modules. Waste Manag. 2017, 60, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, C.; He, Z.; Wang, Q. Pyrolysis Characteristic and kinetics of Polyvinylidene fluoride with and without Pine Sawdust. J. Anal. Appl. Pyrolysis 2017, 123, 402–408. [Google Scholar] [CrossRef]
- Ansah, E.; Wang, L.; Shahbazi, A. Thermogravimetric and calorimetric characteristics during co-pyrolysis of municipal solid waste components. Waste Manag. 2016, 56, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Boguski, J.; Przybytniak, G.; Łyczko, K. New monitoring by thermogravimetry for radiation degradation of EVA. Radiat. Phys. Chem. 2014, 100, 49–53. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Sun, F.; Gao, L.P.; Wang, L.N.; Shao, M.; Liu, J.Q. Thermodynamics Properties and Isothermal Crystallization Kinetics of Carbon Black/Poly(ethylene terephthalate) Composites. J. Compos. Mater. 2007, 41, 1477–1485. [Google Scholar] [CrossRef]
- Stapler, J.T.; Barnes, W.J.; Yelland, W.E.C. Thermal Degradation of Polyvinylidene Fluoride and Polyvinyl Fluoride by Oven Pyrolysis; U.S. Army Natick Laboratories: MA, USA, 1968; Available online: http://www.dtic.mil/dtic/tr/fulltext/u2/672509.pdf (accessed on 1 July 2019).


| Type of Backsheet | Fixed Carbon (wt. %) | Volatiles (wt. %) | Ash Content (wt. %) |
|---|---|---|---|
| PPE | 2.9 | 92.2 | 4.9 |
| TPT | 8.2 | 86.1 | 5.7 |
| KPK | 10.7 | 88.2 | 1.1 |
| Type of Backsheet | C (wt. %) | H (wt. %) | O (wt. %) | N (wt. %) | F (wt. %) | Lower Heating Value (MJ/kg) |
|---|---|---|---|---|---|---|
| PPE | 63.8 | 6.6 | 24.5 | 0.2 | 0.0 | 30.0 |
| TPT | 55.6 | 4.5 | 28.5 | 0.2 | 5.5 | 26.6 |
| KPK | 54.9 | 4.3 | 30.5 | 0.2 | 9.0 | 24.6 |
| Material | R1 Temperature during Pyrolysis, (°C) | Purge Gas | Absorption Solution | Repetitions |
|---|---|---|---|---|
| PPE, TPT, KPK | 300, 400, 500, 900 | N2 | Na2CO3/NaHCO3 | 3 |
| Material | R1 Temperature during Incineration, (°C) | Purge Gas | Absorption Solution | Repetitions |
|---|---|---|---|---|
| PPE, TPT, KPK | 750, 850, 950 | O2 | Na2CO3/NaHCO3 | 3 |
| Reference Material | Theoretical Fluorine Content, in wt. % | Measured Fluorine Content, Incineration at 950 °C in the two-Stage System, wt. % | Measured from Oxygen Bomb Combustion, in wt. % |
|---|---|---|---|
| PVDF | 59.4 | 59.7 | 57.6 |
| PVDF–cellulose mixture | 4.9 | 5.1 | 5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danz, P.; Aryan, V.; Möhle, E.; Nowara, N. Experimental Study on Fluorine Release from Photovoltaic Backsheet Materials Containing PVF and PVDF during Pyrolysis and Incineration in a Technical Lab-Scale Reactor at Various Temperatures. Toxics 2019, 7, 47. https://doi.org/10.3390/toxics7030047
Danz P, Aryan V, Möhle E, Nowara N. Experimental Study on Fluorine Release from Photovoltaic Backsheet Materials Containing PVF and PVDF during Pyrolysis and Incineration in a Technical Lab-Scale Reactor at Various Temperatures. Toxics. 2019; 7(3):47. https://doi.org/10.3390/toxics7030047
Chicago/Turabian StyleDanz, Philipp, Venkat Aryan, Edda Möhle, and Nicole Nowara. 2019. "Experimental Study on Fluorine Release from Photovoltaic Backsheet Materials Containing PVF and PVDF during Pyrolysis and Incineration in a Technical Lab-Scale Reactor at Various Temperatures" Toxics 7, no. 3: 47. https://doi.org/10.3390/toxics7030047
APA StyleDanz, P., Aryan, V., Möhle, E., & Nowara, N. (2019). Experimental Study on Fluorine Release from Photovoltaic Backsheet Materials Containing PVF and PVDF during Pyrolysis and Incineration in a Technical Lab-Scale Reactor at Various Temperatures. Toxics, 7(3), 47. https://doi.org/10.3390/toxics7030047





