Proposal for a Monitoring Concept for Veterinary Medicinal Products with PBT Properties, Using Parasiticides as a Case Study
Abstract
:1. Introduction
- The detection of these parasiticides in the selected environmental compartments, i.e., in soils, surface waters including suspended particles and sediments;
- The accumulation of the parasiticides in organisms living in these compartments;
- The effects of the parasiticides on exposed organisms, mainly arthropods, which are most likely to be affected by parasiticides, in particular dung flies and beetles, but also soil (e.g., Collembola) or sediment (e.g., insect larvae) insects.
2. Methodological Approach
- its physico-chemical properties;
- its application rate and frequency for the treatment of farm animals;
- its concentrations and fate in different environmental compartments, including predicted environmental concentrations (PECs);
- available analytical and extraction methods (including, e.g., detection limits);
- its excretion pathway, excreted amounts and major metabolites;
- results of bioaccumulation and ecotoxicity tests with the parasiticide.
3. Results
3.1. Short Overview on Existing Monitoring Concepts
- Background monitoring (i.e., determination of environmental reference conditions);
- Impact monitoring (i.e., measurement of the effects of anthropogenic activities);
- Trend monitoring (i.e., identification of long-term and/or large-scale changes).
- Ecotoxicological monitoring: determination of the toxicity of chemicals in the respective environmental compartment, either by exposing organisms directly in the field (e.g., in cages) or by evaluating water, sediment or soil samples brought from the field to the laboratory;
- Chemical monitoring: measurement of the concentrations of specific chemicals in different environmental compartments (so far, most often in surface waters);
- Ecological monitoring: detection of effects on biological entities (e.g., populations, species or communities) in the field and their assessment in relation to site-specific environmental parameters, including concentrations of chemicals.
3.2. Presentation and Evaluation of Monitoring Data
- Identification of an uncontaminated site close to the monitoring sites, with both sites being as similar as possible in their properties. This is possible but very difficult, especially because it is often unknown, which factors influence the occurrence, diversity or abundance of certain organisms at a given site.
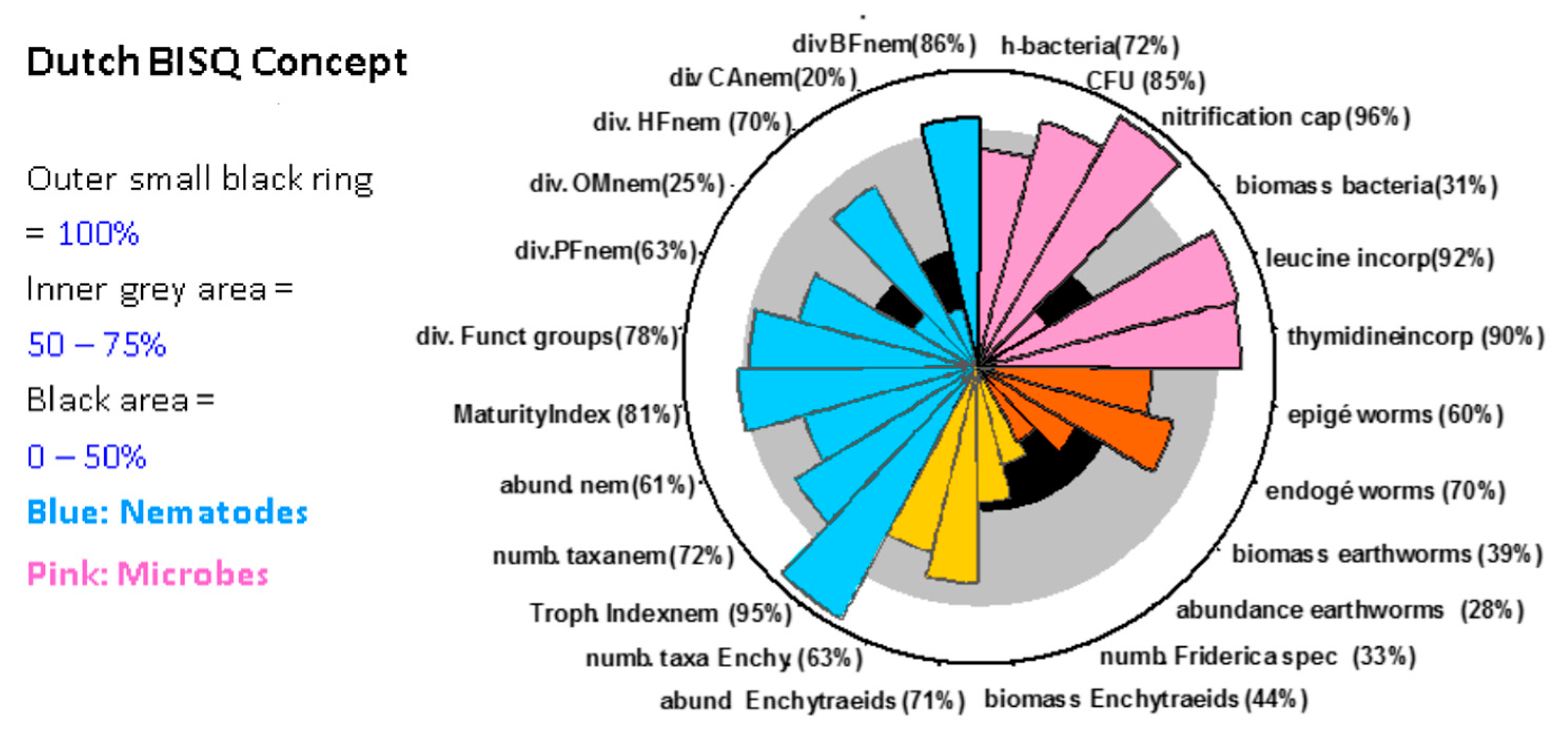
- A more robust approach is the identification of a number of uncontaminated sites with the same land-use (for parasiticides mainly grasslands) in the same biogeographic region, e.g., as defined by [40]. By statistical evaluation of such data (and with a suitable number of sites) reference ranges for different organism groups and endpoints could be defined (see Figure 1).
3.3. Veterinary Medical Products (VMPs), Especially Parasiticides
3.4. Issues to Be Considered for a PAM of Parasiticides
3.4.1. Study Design
3.4.2. Environmental Compartments and Residue Analysis
3.4.3. Bioaccumulation
3.4.4. Organisms to Be Sampled
3.4.5. Endpoints to Be Measured
3.4.6. Duration of the Monitoring
3.4.7. Target Farm Animals
3.4.8. Target and Control Sites
3.4.9. Conclusions Regarding the PAM for Parasiticides
- -
- The performance of a PAM with parasiticides is technically not a huge problem. Most of the methods to be used are well-known (partly even standardized), or could be adapted.
- -
- However, it is very difficult to define in detail where and when the monitoring should be performed. The main problem is to link parasiticide exposure with effects in a way that any impact can directly be traced back to a specific parasiticide.
- -
- In order to overcome this problem, the PAM for parasiticides has to be modified as described in the next section.
4. Discussion: Approach for a Targeted Environmental Monitoring (TEM) of Parasiticides
4.1. Control and Reference Sites
- -
- If the TEM is performed at sites without strong parasite pressure, one group of farm animals could be treated with the parasiticide, while another group could remain untreated. Both groups would be kept at the same site. This approach is similar to higher-tier field studies [21].
- -
- Ideally, sites could be selected that are farmed conventionally (test) or organically (control), preferably as pairs in the same region.
- -
- A detailed history of the individual sites including information on the treatments should be available. Theoretically, control sites would be close to the test sites but without treatment with the parasiticide under evaluation (or with similar mode-of-action), for at least e.g., five (?) years (as it is done when testing plant protection products). However, as mentioned above it is improbable to find such control sites.
4.2. Evaluation of Any Monitoring of Parasiticides
5. Open Issues Needing Further Research
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Adler, N.; Bachmann, J.; Römbke, J. New test strategy for dung beetles during the authorization process of parasiticides. Integr. Environ. Assess. Manag. 2013, 9, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Lumaret, J.-P.; Errouissi, F.; Floate, K.; Roembke, J.; Wardhaugh, K. A review on the toxicity and non-target effects of macrocyclic lactones in the terrestrial and aquatic environment. Curr. Pharm. Biotechnol. 2012, 13, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Liebig, M.; Fernandez, A.A.; Blübaum-Gronau, E.; Boxall, A.; Brinke, M.; Carbonell, G.; Egeler, P.; Fenner, K.; Fernandez, C.; Fink, G.; et al. Environmental Risk Assessment of Ivermectin—A Case Study. Integr. Environ. Assess. Manag. 2010, 6 (Suppl. 1), 567–587. [Google Scholar] [CrossRef] [PubMed]
- Puniamoorthya, N.; Schäfer, M.; Römbke, J.; Meier, R.; Blanckenhorn, W.U. Ivermectin sensitivity is an ancient trait affecting all Ecysozoa but shows phylogenetic clustering among sepsid flies. Evol. Appl. 2014, 7, 548–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küster, A.; Adler, N. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R. Soc. B 2014, 369, 20130587. [Google Scholar] [CrossRef] [PubMed]
- VICH—International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products. Environmental Impact Assessment (EIAs) for Veterinary Medicinal Products (VMPs)—Phase I. VICH GL 6, Ecotoxicity Phase I; Canary Wharf: London, UK, 2000. [Google Scholar]
- VICH—International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products. Environmental Impact Assessment for Veterinary Medicinal Products Phase II Guidance. VICH GL 38, Ecotoxicity Phase II; Canary Wharf: London, UK, 2004. [Google Scholar]
- EMA (European Medicines Agency). Revised Guideline on Environmental Impact Assessment for Veterinary Medicinal Products in Support of the VICH Guidelines GL6 and GL38; EMEA/CVMP/ERA/418282/2005-Rev.1; Committee for Medicinal Products for Veterinary Use (CVMP), EMEA: London, UK, 2008. [Google Scholar]
- EMA (European Medicines Agency). Guideline on the Assessment of Persistent, Bioaccumulative and Toxic (PBT) or Very Persistent and Very Bioaccumulative (vPvB) Substances in Veterinary Medicine; EMA/CVMP/ERA/52740/2012; Committee for Medicinal Products for Veterinary Use (CVMP, EMA): London, UK, 2015. [Google Scholar]
- ECHA (European Chemicals Agency). Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.11: PBT Assessment, version 1.1; ECHA: Helsinki, Finland, 2017. [Google Scholar]
- EC (European Commission). Commission Directive 2009/9/EC Amending Directive 2001/82/EC of the European Parliament and of the Council on the Community Code Relating to Medicinal Products for Veterinary Use; Official Journal of the European Union: Brussels, Belgium, 2009; Volume 44, pp. 1–52. [Google Scholar]
- EC (European Commission). Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Veterinary Medicinal Products; Official Journal of the European Union: Brussels, Belgium, 2001; pp. 1–66. [Google Scholar]
- BBodSchG (Bundes-Bodenschutzgesetz; Federal Soil Protection Act), 1998. Version dated 17 March 1998 (BGBl. I, p. 502), as most recently amended by Article 5, Paragraph 30 of the law of 24 February 2012 (BGBl. I, p. 212).
- EU (European Union). Proposal for a Directive of the European Parliament and of the Council Establishing a Framework for the Protection of Soil and Amending Directive 2004/35/EC; COM 232; EU (European Union): Brussels, Belgium, 2006; pp. 1–30. [Google Scholar]
- EFSA (European Food Safety Authority). Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion on the Development of Specific Protection Goal Options for Environmental Risk Assessment of Pesticides, in Particular in Relation to the Revision of the Guidance Documents on Aquatic and Terrestrial Ecotoxicology (SANCO/3268/2001 and SANCO/10329/2002). EFSA J. 2010, 8, 1–55. [Google Scholar]
- EC (European Commission). Regulation (EC) No. 1107/2009 of the European parliament and the council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, L309, 1–50. [Google Scholar]
- MEA (Millennium Ecosystem Assessment). Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Manning, P.; Beynon, S.A.; Lewis, O.T. Quantifying immediate and delayed effects of anthelmintic exposure on ecosystem functioning supported by a common dung beetle species. PLoS ONE 2016, 12, e0182730. [Google Scholar] [CrossRef] [PubMed]
- ISO (International Organization for Standardization). Soil Quality––Sampling of Soil Invertebrates Part 1: Hand-Sorting and Formalin Extraction of Earthworms; ISO 23611-1:2006; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO (International Organization for Standardization). Soil Quality—Sampling of Soil Invertebrates Part 6: Guidance for the Design of Sampling Programs with Soil Invertebrates; ISO 23611-6:2012; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Floate, K.D.; Dühring, R.-A.; Hanafi, J.; Jud, P.; Lahr, J.; Lumaret, J.-P.; Scheffczyk, A.; Tixier, T.; Wohde, M.; Römbke, J.; et al. Validation of a standard field test method in four countries to assess the toxicity of residues in dung of cattle treated with veterinary medical products. Environ. Toxicol. Chem. 2016, 35, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.; Bachmann, J.; Blanckenhorn, J.; Floate, K.F.; Jensen, J.; Römbke, J. Effects of ivermectin application on the diversity and function of dung and soil fauna: Regulatory and scientific background information. Environ. Toxicol. Chem. 2016, 35, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Bänsch-Baltruschat, B.; Claus, E.; Coors, A.; Duis, K.; Hommen, U.; Rüdel, H.; Keller, M. Nutzung des Umweltmonitorings für das Risikomanagement bedenklicher Stoffe unter Besonderer Berücksichtigung von PBT-Stoffen (NUMoRi); Bericht für das Umweltbundesamt (FKZ 371063420); German Environmental Agency: Dessau-Roßlau, Germany, 2014; p. 334.
- Hommen, U.; Schäfers, C.; Ross-Nickoll, M.; Ratte, T. Auswertung der wichtigsten in Deutschland durchgeführten Monitoringstudien zu Auswirkungen von Pflanzenschutzmitteln auf Nichtzielorganismen; Report; Fraunhofer-Gesellschaft (FhG-IME): Schmallenberg, Germany, 2004; p. 97. [Google Scholar]
- Toschki, A.; Jänsch, S.; Roß-Nickoll, M.; Römbke, J.; Züghart, W. Possibilities of using the German federal states’ permanent soil monitoring program for the monitoring of GMO. Environ. Sci. Eur. 2015, 26, 1–13. [Google Scholar]
- Hellawell, J.M. Development of a rationale for monitoring. In Monitoring for Conservation and Ecology; Goldsmith, F.B., Ed.; Chapman and Hall: Londonm, UK, 1991; pp. 1–14. [Google Scholar]
- Kolkwitz, R.; Marsson, M. Grundsätze für die biologische Beurteilung des Wassers nach seiner Flora und Fauna. Mitteilungen der Königlichen Prüfanstalt für Wasserversorgung und Abwasserbeseitigung (Berlin-Dahlem) 1902, 1, 33–72. [Google Scholar]
- Reynoldson, T.B.; Day, K.E.; Pascoe, T. The development of the BEAST: A predictive approach for assessing sediment quality in the North American Great Lakes. In Assessing the Biological Quality of Fresh Waters: RIVPACS and Other Techniques; Wright, J.F., Sutcliffe, D.W., Furse, M.T., Eds.; Freshwater Biological Association: Ambleside, UK, 2000; pp. 165–180. [Google Scholar]
- Wright, J.F. An introduction to RIVPACS. In Assessing the Biological Quality of Fresh Waters: RIVPACS and Other Techniques; Wright, J.F., Sutcliffe, D.W., Furse, M.T., Eds.; Freshwater Biological Association: Ambleside, UK, 2000; pp. 1–24. [Google Scholar]
- Sundermann, A.; Lohse, S. Bestimmungsschlüssel für Die Aquatischen Zweiflügler (Diptera) in Anlehnung an Die Operationelle Taxaliste für Fließgewässer in Deutschland; Forschungsinstitut Senckenberg: Gelnhausen, Germany, 2005. [Google Scholar]
- Braun-Blanquet, J. Die Alpinen Pflanzengesellschaften. In Vegetationsentwicklung und Bodenbildung in der Alpinen Stufe der Zentralalpen; Braun-Blanquet, J., Jenny, H., Eds.; Denkschriften der Schweizerischen Naturforschenden Gesellschaft: Bern, Switzerland, 1926; Volume 63 (I-VIII), pp. 183–294. [Google Scholar]
- Jänsch, S.; Steffens, L.; Höfer, H.; Horak, F.; Roß-Nickoll, M.; Russell, D.; Toschki, A.; Römbke, J. State of knowledge of earthworm communities in German soils as a basis for biological soil quality assessment. Soil Org. 2013, 85, 215–232. [Google Scholar]
- Floate, K.D.; Colwell, D.D.; Fox, A.S. Reductions of non-pest insects in dung of cattle treated with endectocides: A comparison of four products. Bull. Entomol. Res. 2002, 92, 471–481. [Google Scholar] [CrossRef] [PubMed]
- ISO (International Organization for Standardization). Soil Quality—Effects of Pollutants on Earthworms—Part 3: Guidance on the Determination of Effects in Field Situations; ISO 11268-3; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- Burkhardt, U.; Russell, D.J.; Buryn, R.; Decker, P.; Döhler, M.; Höfer, H.; Römbke, J.; Trog, C.; Vorwald, J.; Wurst, E.; et al. The Edaphobase Project of GBIF-Germany—A new online soil—organism zoological data warehouse. Appl. Soil Ecol. 2014, 83, 5–12. [Google Scholar] [CrossRef]
- Pey, B.; Laporte, M.-A.; Nahmani, J.; Auclerc, A.; Capowiez, Y.; Caro, G.; Cluzeau, D.; Cortet, J.; Decaens, T.; Dubs, F.; et al. A Thesaurus for Soil Invertebrate Trait-Based Approaches. PLoS ONE 2014, 9, e108985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutgers, M.; Schouten, A.J.; Bloem, J.; Van Eekeren, N.; De Goede, R.G.M.; Jagers op Akkerhuis, G.A.J.M.; Van der Wal, A.; Mulder, C.; Brussaard, L.; Breure, A.M. Biological measurements in a nationwide soil monitoring network. Eur. J. Soil Sci. 2009, 60, 820–832. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial iogeography of British soils. Environ. Microbiol. 2009, 13, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Blanckenhorn, W.U.; Rohner, P.; Bernasconi, M.V.; Haugstetter, J.; Buser, A. Is qualitative and quantitative metabarcoding of dung fauna biodiversity feasible? Environ. Toxicol. Chem. 2016, 35, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- [EEA] (European Environment Agency). Biogeographical regions in Europe. 2009. Available online: https://www.eea.europa.eu/data-and-maps/figures/biogeographical-regions-in-europe-2 (accessed on 7 February 2018).
- Ellenberg, H.; Weber, W.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa, 2nd ed.; Goltze: Göttingen, Germany, 1992; p. 18. [Google Scholar]
- EC (European Commission). Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy; European Environment Agency: Copenhague, Danmark, 2000; p. 73. [Google Scholar]
- Cardoso, A.C.; Solimini, A.G.; Premazzi, G.; Birk, S.; Hale, P.; Rafael, T.; Serrano, M.L. Report on Harmonization of Freshwater Biological Methods; Joint Research Center, Institute for Environment and Sustainability, Inland and Marine Waters Unit: Ispra, Italy, 2005; p. 124. [Google Scholar]
- Breure, A.M.; Rutgers, M.; Bloem, J.; Brussaard, L.; Didden, W.; Jagers op Akkerhuis, G.; Mulder, C.; Schouten, A.J.; Van Wijnen, H.J. Ecological Quality of the Soil; RIVM Report 607604005; Dutch National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2003.
- Blume, R.R.; Younger, R.L.; Aga, A.; Myers, C.J. Effects of residues of certain anthelmintics in bovine manure on Onthophagus gazella, a non-target organism. Southwest Entomol. 1976, 2, 100–103. [Google Scholar]
- Boxall, A.B.A.; Fogg, L.A.; Blackwell, P.A.; Kay, P.; Pemberton, E.J.; Croxford, A. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol. 2004, 180, 1–91. [Google Scholar] [PubMed]
- Garric, J.; Vollat, B.; Duis, K.; Péry, A.; Junker, T.; Ramil, M.; Fink, G.; Ternes, T.A. Effects of the parasiticide ivermectin on the cladoceran Daphnia magna and the green alga Pseudokirchneriella subcapitata. Chemosphere 2007, 69, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Floate, K.D. Off-target effects of ivermectin on insects and on dung degradation in southern Alberta, Canada. Bull. Entomol. Res. 1998, 88, 25–35. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Errouissi, F. Use of anthelmintics in herbivores and evaluation of risks for the nontarget fauna of pastures. Vet. Res. 2002, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Römbke, J.; Coors, A.; Fernandez, A.A.; Fernandez, C.; Förster, B.; Jensen, J.; Lumaret, J.P.; Porcel Cots, M.A.; Liebig, M. Effects of the parasiticide ivermectin on the structure and function of dung and soil invertebrate communities in the field (Madrid; Spain). Appl. Soil Ecol. 2010, 45, 284–292. [Google Scholar] [CrossRef]
- Floate, K.D.; Bouchard, P.; Holroyd, G.; Poulin, R.; Wellicome, T.I. Does Doramectin Use on Cattle Indirectly Affect the Endangered Burrowing Owl? Rangel. Ecol. Manag. 2008, 61, 543–553. [Google Scholar] [CrossRef]
- OECD (Organization for Economic Co-Operation and Development). Aerobic and Anaerobic Transformation in Soil; Guideline for Testing of Chemicals 307; OECD: Paris, France, 2002. [Google Scholar]
- Krogh, K.A.; Jensen, G.G.; Schneider, M.K.; Fenner, K.; Halling-Sørensen, B. Analysis of the dissipation kinetics of ivermectin at different temperatures and in four different soils. Chemosphere 2009, 75, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Prasse, C.; Löffler, D.; Ternes, T.A. Environmental fate of the anthelmintic ivermectin in an aerobic sediment/water system. Chemosphere 2009, 77, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Slootweg, T.; Alvinrie, M.; Egeler, P.; Gilberg, D.; Kukkonen, J.V.; Oehlmann, J.; Prasse, C.; Somunen, A.J.; Liebig, M. Bioaccumulation of ivermectin from natural and artificial sediments in the benthic organism Lumbriculus variegatus. J. Soils Sediments 2010, 10, 1611–1622. [Google Scholar] [CrossRef]
- Environment Canada. Guidance Document on the Sampling and Preparation of Contaminated Soil for Use in Biological Testing; EPS 1/RM/53; Environment Canada: Ottawa, ON, Canada, 2012; p. 222.
- EFSA (European Food Safety Authority). Scientific Opinion on outline proposals for assessment of exposure of organisms to substances in soil. EFSA J. 2010, 8, 1442–1478. [Google Scholar]
- VDI (Verein Deutscher Ingenieure). Soil Quality—Biological Procedures to Determine Effects of Air Pollutants (bioindication)—Biomonitoring with Earthworms as Accumulation Indicators; VDI 4230-2; VDI: Düsseldorf, Germany, 2008. [Google Scholar]
- Koschorreck, J.; Heiss, C.; Wellmitz, J.; Fliedner, A.; Rüdel, H. The use of monitoring data in EU chemicals management-experiences and considerations from the German environmental specimen bank. Environ. Sci. Pollut. Res. Int. 2015, 22, 1597–1611. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Hering, D.; Haase, P.; Sundermann, A.; Böhmer, J. Die Bewertung von Fließgewässern mit dem Makrozoobenthos. Limnol. Akt. 2005, 11, 76–90. [Google Scholar]
- Jochmann, R.; Blanckenhorn, W.; Bussière, L.; Jensen, J.; Kryger, U.; Lahr, J.; Lumaret, J.-P.; Römbke, J.; Wardhaugh, K.; Floate, K. How to test non-target effects of veterinary pharmaceutical residues in livestock dung in the field. Integr. Environ. Assess. Manag. 2011, 7, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lemanceau, P.; Maron, P.A.; Mazurier, S.; Mougel, C.; Pivato, B.; Plassart, P.; Ranjard, L.; Revellin, C.; Tardy, V.; Wipf, D. Understanding and managing soil biodiversity: A major challenge in agroecology. Agron. Sustain. Dev. 2015, 35, 67–81. [Google Scholar] [CrossRef]
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991; p. 481. [Google Scholar]
- Bouché, M. Strategies lombriciennes. Ecol. Bull. 1977, 25, 122–132. [Google Scholar]
- Skidmore, P. Insects of the British Cow-Dung Community; Richmond Publishing Co. Ltd.: Slough, UK, 1991. [Google Scholar]
- Lumaret, J.-P. Atlas des Coléoptères Scarabéides Laparosticti de France; Ministère de l’Environnement, Secrétariat Faune Flore (édit.): Paris, France, 1990.
- Pont, A.C.; Meier, R. The Sepsidae (Diptera) of Europe. Fauna Entomol. Scand. 2002, 37, 1–221. [Google Scholar]
- Rössner, E. Die Hirschkäfer und Blatthornkäfer Ostdeutschlands (Coleoptera: Scarabaeoidea); Verein der Freunde und Förderer des Naturkundemuseums Erfurt: Erfurt, Germany, 2012; p. 505. [Google Scholar]
- ISO (International Organization for Standardization). Soil Quality—Sampling of Soil Invertebrates Part 2: Sampling and Extraction of Microarthropods (Collembola and Acarina); ISO 23611-2; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Subedi, B.; Du, B.; Chambliss, C.K.; Koschorreck, J.; Rüdel, H.; Quack, M.; Brooks, B.W.; Usenko, S. Occurrence of Pharmaceuticals and Personal Care Products in German Fish Tissue: A National Study. Environ. Sci. Technol. 2012, 46, 9047–9054. [Google Scholar] [CrossRef] [PubMed]
- Riecken, U.; Finck, P.; Raths, U.; Schröder, E.; Ssymank, A. Standard-Biotoptypenliste für Deutschland. In Schriftenreihe für Landschaftspflege u. Naturschutz, 2nd ed.; Federal Agency for Nature Conservation in Germany: Bonn, Germany, 2003. [Google Scholar]
- Williams, O. Impact of the Agricultural Use of Cattle Wormers and Insecticides on Dung-dependent Diptera. Ph.D. Thesis, University of Bristol, Bristol, UK, 2013; p. 165. [Google Scholar]
- Streloke, M. Member State Experience with Monitoring Data. View from Germany. In Proceedings of the the SETAC Europe Conference, Berlin, Germany, 20–24 May 2012. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Römbke, J.; Duis, K. Proposal for a Monitoring Concept for Veterinary Medicinal Products with PBT Properties, Using Parasiticides as a Case Study. Toxics 2018, 6, 14. https://doi.org/10.3390/toxics6010014
Römbke J, Duis K. Proposal for a Monitoring Concept for Veterinary Medicinal Products with PBT Properties, Using Parasiticides as a Case Study. Toxics. 2018; 6(1):14. https://doi.org/10.3390/toxics6010014
Chicago/Turabian StyleRömbke, Jörg, and Karen Duis. 2018. "Proposal for a Monitoring Concept for Veterinary Medicinal Products with PBT Properties, Using Parasiticides as a Case Study" Toxics 6, no. 1: 14. https://doi.org/10.3390/toxics6010014
APA StyleRömbke, J., & Duis, K. (2018). Proposal for a Monitoring Concept for Veterinary Medicinal Products with PBT Properties, Using Parasiticides as a Case Study. Toxics, 6(1), 14. https://doi.org/10.3390/toxics6010014




