Electrospinning Chitosan/Fe-Mn Nanofibrous Composite for Efficient and Rapid Removal of Arsenite from Water
Abstract
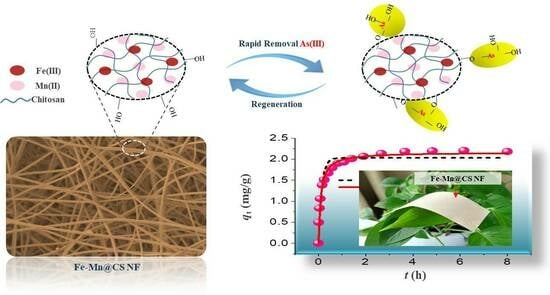
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sorbents
2.3. Characterization
2.4. Adsorption Tests
2.5. Regeneration Tests
3. Results and Discussion
3.1. Optimization of Fe-Mn@CS NF
3.2. Characterization of CTS/Fe-Mn NF
3.3. Adsorption Kinetics and Isotherms
3.4. Effects of Iron Strength and Common Substances
3.5. Regeneration Tests
3.6. Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, G.; Jia, Y.; Meharg, A.A.; Zhu, Y. A review on completing arsenic biogeochemical cycle: Microbial volatilization of arsines in environment. J. Environ. Sci. 2014, 26, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wei, D.; Tan, Z.; Lin, A.; Du, Y. The potential risk assessment for different arsenic species in the aquatic environment. J. Environ. Sci. 2015, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater Arsenic Contamination Throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Yamani, J.S.; Miller, S.M.; Spaulding, M.L.; Zimmerman, J.B. Enhanced arsenic removal using mixed metal oxide impregnated chitosan beads. Water Res. 2012, 46, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Väisänen, A.; Patil, A.B.; Lahtinen, M. The effect of synthesis conditions on the in situ grown MIL-100(Fe)-chitosan beads: Interplay between structural properties and arsenic adsorption. J. Hazard. Mater. 2023, 463, 132893. [Google Scholar] [CrossRef]
- Giri, D.D.; Jha, J.M.; Srivastava, N.; Hashem, A.; Abd_Allah, E.F.; Shah, M.; Pal, D.B. Sustainable removal of arsenic from simulated wastewater using solid waste seed pods biosorbents of Cassia fistula L. Chemosphere 2022, 287, 132308. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, L.; Wang, X.; Chen, Z.; Yang, W. Efficient adsorption of arsenic in groundwater by hydrated iron oxide and ferromanganese oxide chitosan gel beads. Sep. Purif. Technol. 2023, 315, 123692. [Google Scholar] [CrossRef]
- Min, L.; Yuan, Z.; Zhong, L.; Liu, Q.; Wu, R.; Zheng, Y. Preparation of chitosan based electrospun nanofiber membrane and its adsorptive removal of arsenate from aqueous solution. Chem. Eng. J. 2015, 267, 132–141. [Google Scholar] [CrossRef]
- Wang, Z.; Koh, K.Y.; Yang, Y.; Chen, J.P. Design and optimization of an innovative lanthanum/chitosan bead for efficient phosphate removal and study of process performance and mechanisms. Chemosphere 2022, 306, 135468. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Yang, L.; Wu, R.; Zhong, L.; Yuan, Z.; Zheng, Y. Enhanced adsorption of arsenite from aqueous solution by an iron-doped electrospun chitosan nanofiber mat: Preparation, characterization and performance. J. Colloid Interface Sci. 2019, 535, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Zhou, Y.; Liu, Y.; Yu, D.-G. Recent progress in electrospun nanofibers and their applications in heavy metal wastewater treatment. Front. Chem. Sci. Eng. 2023, 17, 249–275. [Google Scholar] [CrossRef]
- Dou, S.; Ke, X.-X.; Zhong, L.-B.; Fan, J.-J.; Chen, J.; Zheng, Y.-M. Novel ultraporous polyimide-based hollow nanofiber mat: Its polymer-blend electrospinning preparation strategy and efficient dynamic adsorption for ciprofloxacin removal. Sep. Purif. Technol. 2022, 295, 121341. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.B.; Yun, H.J.; Won, S.W. Efficient removal of arsenate from water using electrospun polyethylenimine/polyvinyl chloride nanofiber sheets. React. Funct. Polym. 2023, 184, 105514. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, J.; Hartley, W.; Ren, L.; Wang, M.; Tu, S.; Tan, W. As(III) adsorption on Fe-Mn binary oxides: Are Fe and Mn oxides synergistic or antagonistic for arsenic removal? Chem. Eng. J. 2020, 389, 124470. [Google Scholar] [CrossRef]
- Cai, G.; Li, L.; Li, D.; Wang, Q.; Zhang, L.; Zhang, J.; Zuo, W.; Tian, Y. Rapid purification of As(III) in water using iron–manganese composite oxide coupled with sulfite: Importance of the SO5•− radicals. Water Res. 2022, 222, 118839. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, F.; Liu, H.; Qu, J.; Liu, R. Respective Role of Fe and Mn Oxide Contents for Arsenic Sorption in Iron and Manganese Binary Oxide: An X-ray Absorption Spectroscopy Investigation. Environ. Sci. Technol. 2014, 48, 10316–10322. [Google Scholar] [CrossRef]
- Min, L.; Zhong, L.; Zheng, Y.; Liu, Q.; Yuan, Z.; Yang, L. Functionalized chitosan electrospun nanofiber for effective removal of trace arsenate from water. Sci. Rep. 2016, 6, srep32480. [Google Scholar] [CrossRef]
- Lee, S.; Hassan, M.; Ryu, H.J. Dual functional amorphous aluminosilicate sorbents for removing and cold-immobilizing cesium/cobalt/nickel-ions. Sustain. Mater. Technol. 2021, 30, e00356. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J.; Rawal, A.; Luca, V.; Hanley, T.L. Zirconium phosphonate sorbents with tunable structure and function. Microporous Mesoporous Mater. 2017, 252, 90–104. [Google Scholar] [CrossRef]
- Long, L.; Huang, N.; Liu, X.; Gong, L.; Xu, M.; Zhang, S.; Chen, C.; Wu, J.; Yang, G. Enhanced silicate remediation in cadmium-contaminated alkaline soil: Amorphous structure improves adsorption performance. J. Environ. Manag. 2023, 326, 116760. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, J.-H.; Liu, H.-J.; Liu, R.-P.; Li, G. Removal Mechanism of As(III) by a Novel Fe–Mn Binary Oxide Adsorbent: Oxidation and Sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef]
- Cumbal, L.; Sengupta, A. Arsenic Removal Using Polymer-Supported Hydrated Iron(III) Oxide Nanoparticles: Role of Donnan Membrane Effect. Environ. Sci. Technol. 2005, 39, 6508–6515. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Wu, Y.; Li, Y.; Zhao, J.; Na, P. Synthesis of magnetic orderly mesoporous α-Fe2O3 nanocluster derived from MIL-100(Fe) for rapid and efficient arsenic(III,V) removal. J. Hazard. Mater. 2018, 343, 304–314. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.-K. Application of magnesium ferrite nanomaterials for adsorptive removal of arsenic from water: Effects of Mg and Fe ratio. Chemosphere 2022, 307, 135817. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Copper, Nickel and Lead Single Component Systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Efficient removal of arsenic from aqueous solution by continuous adsorption onto iron-coated cork granulates. J. Hazard. Mater. 2022, 432, 128657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, J.; Cui, F.; Feng, H.; Zhang, L. One pot synthesis of tunable Fe3O4–MnO2 core–shell nanoplates and their applications for water purification. J. Mater. Chem. 2012, 22, 9052–9057. [Google Scholar] [CrossRef]
- Sahu, N.; Singh, J.; Koduru, J.R. Removal of arsenic from aqueous solution by novel iron and iron–zirconium modified activated carbon derived from chemical carbonization of Tectona grandis sawdust: Isotherm, kinetic, thermodynamic and breakthrough curve modelling. Environ. Res. 2021, 200, 111431. [Google Scholar] [CrossRef]
- Jain, N.; Maiti, A. Fe-Mn-Al metal oxides/oxyhydroxides as As(III) oxidant under visible light and adsorption of total arsenic in the groundwater environment. Sep. Purif. Technol. 2022, 302, 122170. [Google Scholar] [CrossRef]
- Tang, W.; Su, Y.; Li, Q.; Gao, S.; Shang, J.K. Superparamagnetic magnesium ferrite nanoadsorbent for effective arsenic (III, V) removal and easy magnetic separation. Water Res. 2013, 47, 3624–3634. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C.T. Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef]
- Wijnja, H.; Schulthess, C.P. Vibrational Spectroscopy Study of Selenate and Sulfate Adsorption Mechanisms on Fe and Al (Hydr)oxide Surfaces. J. Colloid Interface Sci. 2000, 229, 286–297. [Google Scholar] [CrossRef]
- Brechbühl, Y.; Christl, I.; Elzinga, E.J.; Kretzschmar, R. Competitive sorption of carbonate and arsenic to hematite: Combined ATR-FTIR and batch experiments. J. Colloid Interface Sci. 2012, 377, 313–321. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, R.; Xia, Y.; Wang, Z.; Yang, W.; Luo, J.; Liu, C. Enhanced arsenite adsorption from water by activated MOF embedded in macroporous chitosan bionanocomposite beads. Mater. Today Chem. 2022, 26, 101091. [Google Scholar] [CrossRef]
- Liu, R.; Wu, K.; Miao, B.; Sun, X.; Li, A.; Liu, T.; Duan, C.; Li, Z. The treatment of As(III)-contaminated water by using granular Fe-Mn-Cu composite oxide: Removing As(III) via the oxidation-adsorption process and handling the release of manganese ions. J. Water Process Eng. 2023, 56, 104396. [Google Scholar] [CrossRef]
- Ryu, S.-R.; Jeon, E.-K.; Yang, J.-S.; Baek, K. Adsorption of As(III) and As(V) in groundwater by Fe–Mn binary oxide-impregnated granular activated carbon (IMIGAC). J. Taiwan Inst. Chem. Eng. 2017, 72, 62–69. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, Y.; Xia, Y.; Wang, Z.; Tang, H.; Tan, M.; Liu, X.; Shi, J.; Liu, C. Rapid and effective removal of arsenite from water using a novel oxidation-sorption bifunctional, MOF. Chem. Eng. J. 2023, 476, 146787. [Google Scholar] [CrossRef]
- Xu, W.-H.; Wang, L.; Wang, J.; Sheng, G.-P.; Liu, J.-H.; Yu, H.-Q.; Huang, X.-J. Superparamagnetic mesoporous ferrite nanocrystal clusters for efficient removal of arsenite from water. CrystEngComm 2013, 15, 7895–7903. [Google Scholar] [CrossRef]
- Wang, J.; Sun, M.; Wang, L.; Xiong, X.; Yuan, W.; Liu, Y.; Liu, S.; Zhang, Q.; Liu, J.; Wang, Y.; et al. High-efficiency removal of arsenic(III) from wastewater using combined copper ferrite@biochar and persulfate. Chemosphere 2023, 336, 139089. [Google Scholar] [CrossRef] [PubMed]







| Pseudo-First-Order, qt = qe(1 − e−k1t) | Pseudo-Second-Order, qt = k2qe2t/(1 + k2qet) | ||||
|---|---|---|---|---|---|
| qe (mg/g) | k1 (h−1) | R2 | qe (mg/g) | k2 (g/(mg·h) | R2 |
| 2.03 | 7.24 | 0.936 | 2.17 | 4.98 | 0.988 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qmax (mg/g) | b (L/mg) | R2 | KF ((mg/g)(mg/L)n) | 1/n | R2 |
| 4.59 | 18.97 | 0.924 | 5.44 | 0.37 | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, L.; Ma, Y.; Zhang, B.; He, D.; Chen, J.; Li, X.; Wang, S.; Chi, Y. Electrospinning Chitosan/Fe-Mn Nanofibrous Composite for Efficient and Rapid Removal of Arsenite from Water. Toxics 2024, 12, 230. https://doi.org/10.3390/toxics12030230
Min L, Ma Y, Zhang B, He D, Chen J, Li X, Wang S, Chi Y. Electrospinning Chitosan/Fe-Mn Nanofibrous Composite for Efficient and Rapid Removal of Arsenite from Water. Toxics. 2024; 12(3):230. https://doi.org/10.3390/toxics12030230
Chicago/Turabian StyleMin, Lingli, Yahui Ma, Bi Zhang, Dulan He, Jinhua Chen, Xuerong Li, Shuhua Wang, and Yulang Chi. 2024. "Electrospinning Chitosan/Fe-Mn Nanofibrous Composite for Efficient and Rapid Removal of Arsenite from Water" Toxics 12, no. 3: 230. https://doi.org/10.3390/toxics12030230