Mercury in Fish and Human Hair and Estimated Dietary Intake in a Riverside Community of the Madeira River Basin in the Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Preparation
2.3. Total Mercury (THg) Determination
2.4. Methylmercury (MeHg) Determination
2.5. Analytical Quality Control
2.6. Estimating Hg Exposure through Fish Consumption
2.7. Estimating Hg Exposure through Water Ingestion
2.8. Data Analysis
3. Results
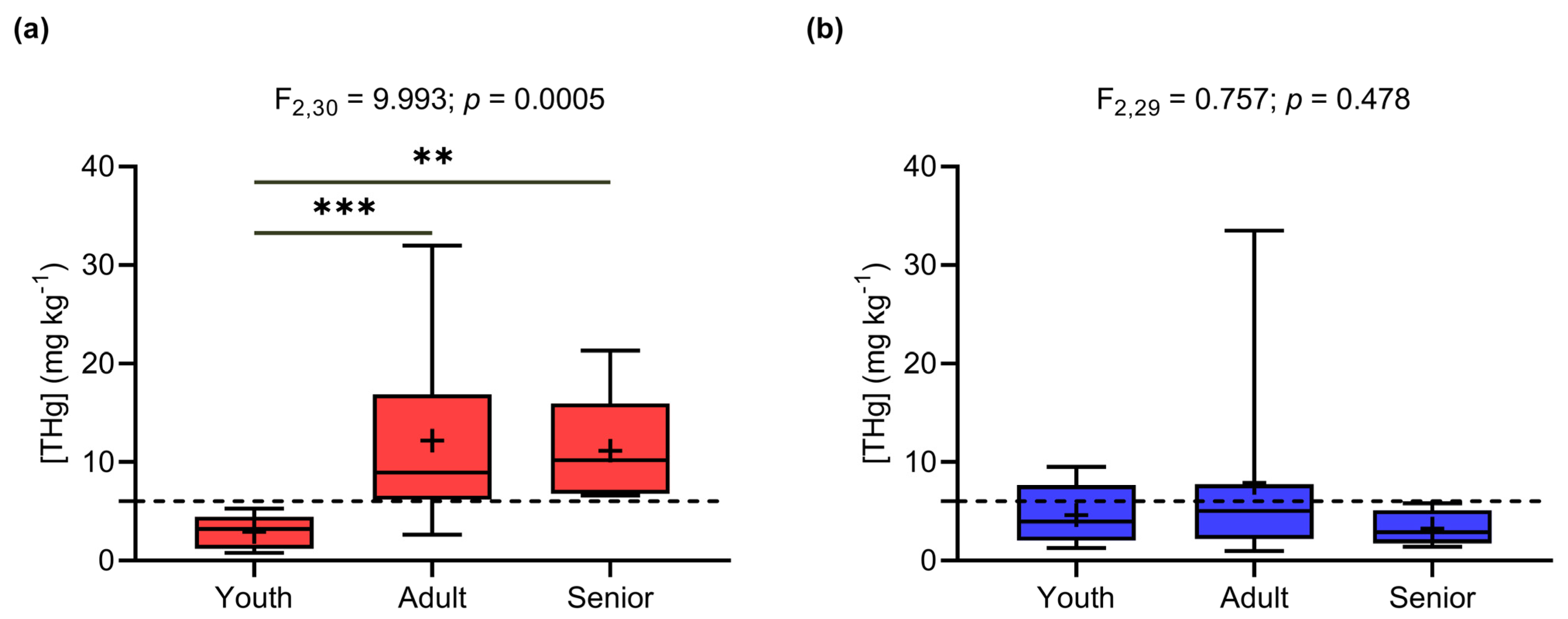
3.1. Effects of Gender and Age on THg Concentrations in Human Hair
3.2. THg Concentrations in Fish Species Consumed by the Population
3.3. Human Exposure through Fish Intake and Maximum Safe Consumption Quantity
3.4. Human Exposure through Water Ingestion
4. Discussion
4.1. Comparison of THg Concentrations in Human Hair with Other Amazonian Communities
| Location | N (Adults) | General | Male | Female | References |
|---|---|---|---|---|---|
| Demarcação (RO) Madeira River | 65 | 7.87 ± 6.78 (0.78–31.97) | 10.62 ± 7.83 (0.79–31.97) | 5.01 ± 3.89 (0.97–18.02) | This study * |
| Demarcação (RO) Madeira River | 4 | 28.01 ± 7.35 (22.07–37.07) | – | – | W. R. Bastos (unpublished data) |
| Calama (RO) Madeira River | 24 a | 9.23 ± 5.78 (1.05–22.48) | 9.61 ± 3.24 (4.34–14.53) | 8.76 ± 6.37 (1.05–22.48) | [47] |
| Cujubim (RO) Madeira River | 12 a | 6.30 ± 4.00 (1.55–14.67) | 7.22 ± 5.18 (1.56–14.67) | 6.76 ± 4.36 (1.55–14.67) | [47] |
| Firmeza (RO) Madeira River | 3 a | 11.80 ± 2.75 (9.40–14.80) | 12.10 ± 3.89 (9.40–14.80) | 11.20 | [47] |
| Itacoã (RO) Madeira River | 6 a | 11.97 ± 4.33 (5.28–16.00) | 11.51 ± 5.57 (5.28–16.00) | 12.42 ± 3.91 (7.90–14.76) | [47] |
| Nazaré (RO) Madeira River | 27 a | 12.14 ± 6.34 (1.08–22.60) | 11.91 ± 7.48 (2.48–22.46) | 11.65 ± 5.95 (1.08–22.60) | [47] |
| Papagaios (RO) Madeira River | 8 a | 12.73 ± 7.37 (4.76–27.22) | 16.14 ± 7.37 (6.66–27.22) | 9.90 ± 4.84 (4.76–17.20) | [47] |
| Santa Rosa (RO) Madeira River | 6 a | 13.30 ± 2.54 (8.56–15.44) | 14.88 ± 0.81 (13.95–15.44) | 11.73 ± 2.84 (8.56–14.08) | [47] |
| São Carlos (RO) Madeira River | 14 a | 8.71 ± 5.77 (1.84–22.83) | 8.94 ± 5.48 (3.30–17.69) | 8.59 ± 6.24 (1.84–22.83) | [47] |
| Terra Caída (RO) Madeira River | 5 a | 10.65 ± 3.61 (5.01–14.61) | - | 14.61 | [47] |
| Puruzinho Lake (AM) Madeira River | 4 a | 20.71 ± 5.10 (14.21–28.27) | 20.22 ± 7.25 (14.21–28.27) | 22.18 | [47] |
| Barreiras (PA) Tapajós River | 89 a | 13.74 ± 4.52 (2.07–20.87) | 13.42 ± 5.12 (2.07–20.87) | 14.03 ± 4.16 (7.16–20.39) | [75] |
| Sawré Muybu Indigenous Land (PA) Tapajós River | 116 a | 8.3 (2.0–22.8) | 8.8 ± 4.6 (2.6–22.8) | 7.8 ± 3.8 (2.0–20.2) | [76] |
| Balbina Village (AM) Uatumã River | 25 a | 6.4 1.2–15.5 | 5.5 ± 3.5 (1.2–12.2) | 7.4 ± 4.6 (2.2–15.5) | [73] |
| Tucuruí Hydropower plant (PA) Tocantins River | 108 | - | 11.5 ± 11.8 | 8.8 ± 8.0 | [89] * |
| Tocantins River (PA) | 37 | 12.0 (7.9–23.8) b (1.1–75.8) | 19.7 (6.2–47.6) b (1.1–75.8) | 11 (8–18) b (3.6–42.9) | [81] |
4.2. Effects of Gender and Age on THg Concentrations in Human Hair
4.3. Human Exposure through Diet and Water
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, L.; Fisher, J.A.; Carmen del Anne Hélène Fostier Rémy, J.; Leaner, J.; Mason, R.P. A synthesis of mercury research in the Southern Hemisphere, part 1: Natural processes. AMBIO A J. Hum. Environ. 2023, 52, 897–917. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; Zhang, P. Mercury Deposition, Climate Change and Anthropogenic Activities: A Review. Front. Earth Sci. 2020, 8, 316. [Google Scholar] [CrossRef]
- Outridge, P.M.; Mason, R.P.; Wang, F.; Guerrero, S.; Heimbürger-Boavida, L.E. Updated Global and Oceanic Mercury Budgets for the United Nations Global Mercury Assessment 2018. Environ. Sci. Technol. 2018, 52, 11466–11477. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; Lu, Z.; Levin, L.; Thackray, C.P.; Sunderland, E.M. Five hundred years of anthropogenic mercury: Spatial and temporal release profiles. Environ. Res. Lett. 2019, 14, 084004. [Google Scholar] [CrossRef]
- Fisher, J.A.; Schneider, L.; Anne Hélène Fostier Guerrero, S.; Remy, J.; Leaner, C.L.J.; Martin, L.; Mason, R.P.; Somerset, V.; Walters, C. A synthesis of mercury research in the Southern Hemisphere, part 2: Anthropogenic perturbations. AMBIO A J. Hum. Environ. 2023, 52, 918–937. [Google Scholar] [CrossRef] [PubMed]
- Kocman, D.; Wilson, S.; Amos, H.; Telmer, K.; Steenhuisen, F.; Sunderland, E.; Mason, R.; Outridge, P.; Horvat, M. Toward an Assessment of the Global Inventory of Present-Day Mercury Releases to Freshwater Environments. Int. J. Environ. Res. Public Health 2017, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.C.M.; Dórea, J.G.; Bernardi, J.; Gomes, L.F. Mapping the Evolution of Mercury (Hg) Research in the Amazon (1991–2017): A Scientometric Analysis. Int. J. Environ. Res. Public Health 2019, 16, 1111. [Google Scholar] [CrossRef] [PubMed]
- Boischio, A.A.P.; Barbosa, A. Exposição ao Mercúrio Orgânico em Populações Ribeirinhas do Alto Madeira, Rondônia, 1991: Resultados Preliminares. Cad. Saúde Pública 1993, 9, 155–160. [Google Scholar] [CrossRef]
- Pfeiffer, W.C.; Lacerda, L.D. Mercury inputs into the Amazon Region, Brazil. Environ. Technol. Lett. 1988, 9, 325–330. [Google Scholar] [CrossRef]
- Yoshimura, A.; Suemasu, K.; Veiga, M.M. Estimation of Mercury Losses and Gold Production by Artisanal and Small-Scale Gold Mining (ASGM). J. Sustain. Metall. 2021, 7, 1045–1059. [Google Scholar] [CrossRef]
- Figueiredo, B.; Campos, A.B.D.; Hoffman, N.C. Mercury sink in Amazon rainforest: Soil geochemical data from the Tapajos National Forest, Brazil. Environ. Earth Sci. 2018, 77, 296. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; Macchi, B.M.; Nascimento, J.L.N.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.D.; Cohen, Y.; Winer, A.M. Environmental distribution and transformation of mercury compounds. Crit. Rev. Environ. Sci. Technol. 1996, 26, 1–43. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Q.; Pan, X. Photochemical behaviors of mercury (Hg) species in aquatic systems: A systematic review on reaction process, mechanism, and influencing factor. Sci. Total Environ. 2020, 720, 137540. [Google Scholar] [CrossRef] [PubMed]
- Regnell, O.; Watras, C.J. Microbial Mercury Methylation in Aquatic Environments: A Critical Review of Published Field and Laboratory Studies. Environ. Sci. Technol. 2019, 53, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Ajsuvakova, O.P.O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Hsu-Kim, H.; Kucharzyk, K.H.; Zhang, T.; Deshusses, M.A. Mechanisms Regulating Mercury Bioavailability for Methylating Microorganisms in the Aquatic Environment: A Critical Review. Environ. Sci. Technol. 2013, 47, 2441–2456. [Google Scholar] [CrossRef]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef]
- World Health Organization. Guidance for Identifying Populations at Risk from Mercury Exposure, Switzerland: Geneva. 2008. Available online: https://www.who.int/publications/m/item/guidance-for-identifying-populations-at-risk-from-mercury-exposure (accessed on 6 December 2023).
- UNEP (United Nations Environment Programme). Global Mercury Assessment. UNEP Chemicals, Switzerland. 2002. Available online: https://wedocs.unep.org/ (accessed on 6 December 2023).
- Dorea, J.G. Fish are central in the diet of Amazonian riparians: Should we worry about their mercury concentrations? Environ. Res. 2003, 92, 232–244. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Dórea, J.G.; Bernardi, J.V.E.; Bastos, W.R.; Almeida, R.; Manzatto, Â.G. Fish consumption by traditional subsistence villagers of the Rio Madeira (Amazon): Impact on hair mercury. Ann. Hum. Biol. 2010, 37, 629–642. [Google Scholar] [CrossRef]
- Hacon, S.; Dórea, J.; Fonseca, M.; Oliveira, B.; Mourão, D.; Ruiz, C.; Gonçalves, R.; Mariani, C.; Bastos, W. The Influence of Changes in Lifestyle and Mercury Exposure in Riverine Populations of the Madeira River (Amazon Basin) near a Hydroelectric Project. Int. J. Environ. Res. Public Health 2014, 11, 2437–2455. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.J.; Mergler, D. Human mercury exposure and adverse health effects in the Amazon: A review. Cad. Saúde Pública 2008, 24, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: An evaluation of human exposure. J. Trace Elem. Med. Biol. 2018, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Feingold, B.J.; Berky, A.; Hsu-Kim, H.; Jurado, E.R.; Pan, W.K. Population-based dietary exposure to mercury through fish consumption in the Southern Peruvian Amazon. Environ. Res. 2020, 183, 108720. [Google Scholar] [CrossRef]
- Basta, P.C.; Vasconcellos, A.C.S.; Hallwass, G.; Yokota, D.; Pinto, D.O.D.; Aguiar, D.S.; Souza, C.C.; Costa, M.O. Risk Assessment of Mercury-Contaminated Fish Consumption in the Brazilian Amazon: An Ecological Study. Toxics 2023, 11, 800. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Vyas, J.B.; Ballatori, Z. Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 2007, 50, 757–764. [Google Scholar] [CrossRef]
- Martinez-Finley, E.J.; Aschner, M. Recent Advances in Mercury Research. Curr. Environ. Health Rep. 2014, 1, 163–171. [Google Scholar] [CrossRef]
- Rand, M.D.; Caito, S.W. Variation in the biological half-life of methylmercury in humans: Methods, measurements and meaning. Biochim. Biophys. Acta BBA Gen. Subj. 2019, 1863, 129301. [Google Scholar] [CrossRef] [PubMed]
- Cernichiari, E.; Myers, G.J.; Ballatori, N.; Zareba, G.; Vyas, J.; Clarkson, T. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology 2007, 28, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Esteban-López, M.; Arrebola, J.P.; Juliá, M.; Pärt, P.; Soto, E.; Cañas, A.; Pedraza-Díaz, S.; González-Rubio, J.; Castaño, A. Selecting the best non-invasive matrix to measure mercury exposure in human biomonitoring surveys. Environ. Res. 2022, 204 Pt D, 112394. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Minamata Convention on Mercury—Text and Annexes. 2019. Available online: https://wedocs.unep.org/20.500.11822/33123 (accessed on 6 December 2023).
- Branco, V.; Aschner, M.; Carvalho, C. Neurotoxicity of mercury: An old issue with contemporary significance. Adv. Neurotoxicology 2021, 5, 239–262. [Google Scholar] [CrossRef]
- Chen, Z.; Myers, R.; Wang, X. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 537–544. Available online: https://www.nature.com/articles/jes201426 (accessed on 10 December 2023). [CrossRef]
- Tong, M.; Yu, J.; Liu, M.; Li, Z.; Wang, L.; Yin, C.; Ren, A.; Chen, L.; Jin, L. Total mercury concentration in placental tissue, a good biomarker of prenatal mercury exposure, is associated with risk for neural tube defects in offspring. Environ. Int. 2021, 150, 106425. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M.; Aaseth, J. Mercury exposure and its effects on fertility and pregnancy outcome. Basic Clin. Pharmacol. Toxicol. 2019, 125, 317–327. [Google Scholar] [CrossRef]
- Vieira, S.M.; Almeida, R.; Holanda, I.B.B.; Mussy, M.H.; Galvão, R.C.F.; Crispim, P.T.B.; Dórea, J.G.; Bastos, W.R. Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. Int. J. Hyg. Environ. Health 2013, 216, 682–689. [Google Scholar] [CrossRef]
- Marques, R.C.; Bernardi, J.V.E.; Dórea, J.G.; Leão, R.S.; Malm, O. Mercury Transfer During Pregnancy and Breastfeeding: Hair Mercury Concentrations as Biomarker. Biol. Trace Elem. Res. 2013, 154, 326–332. [Google Scholar] [CrossRef]
- Marques, R.C.; Abreu, L.; Bernardi, J.V.E.; Dórea, J.G. Traditional living in the Amazon: Extended breastfeeding, fish consumption, mercury exposure and neurodevelopment. Ann. Hum. Biol. 2016, 43, 360–370. [Google Scholar] [CrossRef]
- United Nations. Environment Programm e Guindance for Identifyng Populations at Risc from Mercury Exposure. 2008. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/11786/IdentifyingPopnatRiskExposuretoMercury_2008Web.pdf?sequence=1&isAllowed=y (accessed on 10 December 2023).
- Ferreira, S.L.; Cerda, V.; Cunha, F.A.; Lemos, V.A.; Teixeira, L.S.; dos Santos, W.N.; Coutinho, J.D.J.; Porto, I.S.d.A.; de Jesus, R.F. Application of human health risk indices in assessing contamination from chemical elements in food samples. TrAC Trends Anal. Chem. 2023, 167, 117281. [Google Scholar] [CrossRef]
- Jiménez-Oyola, S.; Escobar Segovia, K.; García-Martínez, M.-J.; Ortega, M.; Bolonio, D.; García-Garizabal, I.; Salgado, B. Human Health Risk Assessment for Exposure to Potentially Toxic Elements in Polluted Rivers in the Ecuadorian Amazon. Water 2021, 13, 613. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Jiménez-Oyola, S.; Montoya, A.V.G.; Vizuete, D.D.C.; D’Orio, G.; Cedeño-Laje, J.; Straface, S. Assessment of Hg pollution in stream waters and human health risk in areas impacted by mining activities in the Ecuadorian Amazon. Environ. Geochem. Health 2023, 45, 7183–7197. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.S.S.; Lima, M.O. Hair as a Biomarker of Long-Term Mercury Exposure in Brazilian Amazon: A Systematic Review. Environ. Res. Public Health 2018, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Horvat, M.; Evers, D.C.; Zastenskaya, I.; Weihe, P.; Tempowski, J. A State-of-the-Science Review of Mercury Biomarkers in Human Populations Worldwide between 2000 and 2018. Environ. Health Perspect. 2018, 126, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Gomes, J.P.O.; Oliveira, R.C.; Almeida, R.; Nascimento, E.L.; Bernardi, J.V.; Lacerda, L.D.; Silveira, E.G.; Pfeiffer, W.C. Mercury in the environment and riverside population in the Madeira River Basin, Amazon, Brazil. Sci. Total Environ. 2006, 136, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.A.; de Carvalho, D.P.; de Almeida, R.; do NRecktenvald, M.C.N.; Pedrosa, O.P.; de Sousa-Filho, I.F.; Dórea, J.G.; Bastos, W.R. Mercury in blood, hair, and feces from subsistence fish-eating riverines of the Madeira River Basin (Western Amazon). J. Trace Elem. Med. Biol. 2021, 67, 126773. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Rebelo, M.F.; Fonseca, M.F.; Almeida, R.; Malm, O. A description of Mercury in fishes from the Madeira River Basin, Amazon, Brazil. Acta Amaz. 2008, 38, 431–438. [Google Scholar] [CrossRef]
- Bastos, W.R.; Dórea, J.G.; Bernardi, J.V.E.; Lauthartte, L.C.; Mussy, M.H.; Lacerda, L.D.; Malm, O. Mercury in fish of the Madeira River (temporal and spatial assessment), Brazilian Amazon. Environ. Res. 2015, 140, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mussy, M.H.; de Almeida, R.; de Carvalho, D.P.; Lauthartte, L.C.; de Holanda IB, B.; Almeida, M.G.D.; de Sousa-Filho, I.F.; de Rezende, C.E.; Malm, O.; Bastos, W.R. Evaluating total mercury and methylmercury biomagnification using stable isotopes of carbon and nitrogen in fish from the Madeira River basin, Brazilian Amazon. Environ. Sci. Pollut. Res. 2023, 30, 33543–33554. [Google Scholar] [CrossRef]
- Rocha, A.V.; Cardoso, B.R.; Cominetti, C.; Bueno, R.B.; Bortoli, M.C.; Farias, L.A.; Favaro, D.I.T.; Camargo, L.M.A.; Cozzolino, S.M.F. Selenium status and hair mercury levels in riverine children from Rondônia, Amazonia. Nutrition 2014, 30, 1318–1323. [Google Scholar] [CrossRef]
- IBGE|Censo 2010|Resultados. Ibge.gov.br. Available online: https://censo2010.ibge.gov.br/resultados.html (accessed on 10 December 2023).
- Franco, E.C.; Santo, C.d.E.; Arakawa, A.M.; Xavier, A.; França, M.d.L.; Oliveira, A.N.d.; Machado, M.A.M.d.P.; Bastos, R.S.; Bastos, J.R.d.M.; Caldana, M.d.L. Promoção da saúde da população ribeirinha da região amazônica: Relato de experiência. Rev. CEFAC 2015, 17, 1521–1530. [Google Scholar] [CrossRef]
- Lopes, I.d.M.O.; Magalhães, M.T.Q. Hidrovia do Rio Madeira como indutor de desenvolvimento microrregional das comunidades tradicionais do Baixo Madeira em Porto Velho. Paranoá Cad. Arquitetura Urban. 2018, 22, 143–158. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Bastos, W.R.; Almeida, M. The impacts of land use changes in the mercury flux in the Madeira River, Western Amazon. An. Acad. Bras. Cienc. 2012, 84, 69–78. [Google Scholar] [CrossRef]
- Kasper, D.; Forsberg, B.R.; Amaral, J.H.F.; Leitão, R.P.; Py-Daniel, S.S.; Bastos, W.R.; Malm, O. Reservoir Stratification Affects Methylmercury Levels in River Water, Plankton, and Fish Downstream from Balbina Hydroelectric Dam, Amazonas, Brazil. Environ. Sci. Technol. 2014, 48, 1032–1040. [Google Scholar] [CrossRef]
- Bastos, W.R.; Malm, O.; Pfeifer, W.C.; Cleary, D. Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon Brazil. Ciência E Cult. 1998, 50, 255–260. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrom Etry. 2002. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_1631e_2002.pdf (accessed on 10 December 2023).
- Bloom, N.S. On the Chemical Form of Mercury in Edible Fish and Marine Invertebrate Tissue. Can. J. Fish. Aquat. Sci. 1992, 49, 1010–1017. [Google Scholar] [CrossRef]
- Taylor, V.F.; Carter, A.; Davies, C.; Jackson, B.P. Trace-level automated mercury speciation analysis. Anal. Methods 2011, 3, 1143–1148. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Method 1630 Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and CVAFS Draft. 2001. Available online: https://brooksapplied.com/wp-content/uploads/2013/12/1630.pdf (accessed on 23 November 2023).
- Brooks Rand Labs MERX. Automated Methylmercury Analytical System; User’s Guide: Seattle, WA, USA, 2005; 37p. [Google Scholar]
- Dória, C.R.C.; Machado, L.F.; Souza, S.T.B.; Lima, M.A.L. A pesca em comunidades ribeirinhas na região do médio rio Madeira, Rondônia. Novos Cad. NAEA 2016, 19, 163–188. [Google Scholar] [CrossRef]
- IBGE. Tabela 2645: Estimativas Populacionais das Medianas de Altura e peso de Crianças, Adolescentes e Adultos, por Sexo, Situação do Domicílio e Idade—Brasil e Grandes Regiões. (n.d.). 2008. Available online: https://sidra.ibge.gov.br/tabela/2645 (accessed on 6 December 2023).
- Ouédraogo, O.; Amyot, M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011, 111, 1064–1069. [Google Scholar] [CrossRef]
- Oliveira, T.A.S.; Dias, R.K.S.; Souza, L.R.R.; Veiga, M.A.M.S. The effect of selenium co-ingestion on mercury bioaccessibility in contaminated fish of the Amazon region. Environ. Adv. 2023, 14, 100450. [Google Scholar] [CrossRef]
- JECFA; World Health Organization (WHO). Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2011. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1806 (accessed on 12 February 2024).
- RAIS. Risk Assessment Guidance for Superfund: Volume III -Part A, Process for Conducting Probabilistic Risk Assessment *134487*. (n.d.). 2023. Available online: https://semspub.epa.gov/work/HQ/134487.pdf (accessed on 12 February 2024).
- USEPA. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A). 1989. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf (accessed on 12 February 2024).
- World Health Organization. Evaluation of Certain Food Additives and Contaminants. Italy, Rome. 2006. Available online: https://iris.who.int/bitstream/handle/10665/43592/WHO_TRS_940_eng.pdf?sequence=1 (accessed on 23 November 2023).
- ANVISA. Ministério da Saúde (BR). Portaria no 685, de 27 de Agosto de 1998. Aprova o Regulamento Técnico: Princípios Gerais para o Estabelecimento de Níveis Máximos de Tolerância para Contaminantes Inorgânicos: Limites Máximos de Tolerância para Contaminantes Inorgânicos; Diário Oficial da União: Brasília, Brazil, 1998; p. 28.
- Kehrig, H.A.; Malm, O.; Akagi, H.; Guimarães, J.R.D.; Torres, J.P.M. Methylmercury in Fish and Hair Samples from the Balbina Reservoir, Brazilian Amazon. Environ. Res. 1998, 77, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, C.; Gallis, J.A.; Ortiz, E.; Berky, A.J.; Morales, A.M.; Diringer, S.E.; Harrington, J.; Bullins, P.; Rogers, L.; Hare-Grogg, J.; et al. A population-based mercury exposure assessment near an artisanal and small-scale gold mining site in the Peruvian Amazon. J. Expo. Sci. Environ. Epidemiol. 2020, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Faial, K.; Deus, R.; Deus, S.; Neves, R.; Jesus, L.; Santos, E.; Alves, C.N.; Brasil, D. Mercury levels assessment in hair of riverside inhabitants of the Tapajós River, Pará State, Amazon, Brazil: Fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 2015, 30, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Basta, P.C.; Viana, P.V.S.; Vasconcellos, A.C.S.; Périssé, A.R.S.; Hofer, C.B.; Paiva, N.S.; Kempton, J.W.; Andrade, D.C.; Oliveira, R.A.A.; Achatz, R.W.; et al. Mercury Exposure in Munduruku Indigenous Communities from Brazilian Amazon: Methodological Background and an Overview of the Principal Results. Int. J. Environ. Res. Public Health 2021, 18, 9222. [Google Scholar] [CrossRef] [PubMed]
- Leino, T.; Lodenius, M. Human hair mercury levels in Tucuruí area, State of Pará, Brazil. Sci. Total Environ. 1995, 175, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Moreno, M.J.; Mateos, V.R.; Silva, N.F.S.; Monteiro, J.R.S.; Oliveira, M.A.; Paraense, R.S.O.; Macchi, B.M.; Nascimento, J.L.M.; et al. Large-scale projects in the amazon and human exposure to mercury: The case-study of the Tucuruí Dam. Ecotoxicol. Environ. Safty 2018, 147, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Lacerda, L.D. A contaminação por mercúrio na bacia do rio madeira: Uma breve revisão. Geochim. Bras. 2004, 18, 99–114. Available online: https://geobrasiliensis.org.br/geobrasiliensis/article/view/215 (accessed on 10 February 2024).
- Pedrosa, O.P.; Barbirato, D.S.; Bastos, W.R.; Ott, A.M.T.; Fogacci, M.F.; Nogueira, L.B. Nutritional Transition of Riverine People from Puruzinho Lake in the Amazon Region. A Qualitative Study. Int. J. Adv. Eng. Res. Sci. 2018, 5, 145–153. [Google Scholar] [CrossRef]
- Brasil. Available online: https://www.planalto.gov.br/ccivil_03/_ato2004-2006/2004/lei/l10.836.htm#:~:text=LEI%20N%C2%BA%2010.836%2C%20DE%209%20DE%20JANEIRO%20DE%202004&text=Cria%20o%20Programa%20Bolsa%20Fam%C3%ADlia,Art (accessed on 21 December 2023).
- Brasil. 2023. Available online: https://www.planalto.gov.br/ccivil_03/_ato2023-2026/2023/Lei/L14601.htm (accessed on 10 February 2024).
- Brasil. 2003. Available online: https://www.gov.br/mme/pt-br/destaques/Programa%20Luz%20para%20Todos/normativos/arquivos/mme_decreto-4-873-de-11-de-novembro-de-2003.pdf (accessed on 12 January 2024).
- Nardoto, G.B.; Rui Gainette, E.; Adams, C.; Elisa, M.; Schor, T.; De Moraes, A.; Rinaldi, F.D.; Gragnani, J.G.; Arnaud, E.; Neto, P.J.D.; et al. Frozen chicken for wild fish: Nutritional transition in the Brazilian Amazon region determined by carbon and nitrogen stable isotope ratios in fingernails. Am. J. Hum. Biol. 2011, 23, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Piperata, B.A.; Spence, J.E.; Da-Gloria, P.; Hubbe, M. The nutrition transition in amazonia: Rapid economic change and its impact on growth and development in Ribeirinhos. Am. J. Phys. Anthropol. 2011, 146, 1–13. [Google Scholar] [CrossRef]
- Silva, R.J.; Garavello, E.M.E.P.; Martinelli, L.A. Factors influencing the food transition in riverine communities in the Brazilian Amazon. Environ. Dev. Sustain. 2016, 19, 1087–1102. [Google Scholar] [CrossRef]
- Nascimento, L.M.; Cristina Grazielle, B.; Campos, F.; Cristina Lucarini, M.; Durazzo, A.; Dias, D.; Maria, K. Association between Consumption of Ultra-Processed Food and Body Composition of Adults in a Capital City of a Brazilian Region. Nutrients 2023, 15, 3157. [Google Scholar] [CrossRef]
- Nouri, M.; Eskandarzadeh, S.; Makhtoomi, M.; Rajabzadeh-Dehkordi, M.; Najafi, N.O.M.; Faghih, S. Association between ultra-processed foods intake with lipid profile: A cross-sectional study. Sci. Rep. 2023, 13, 7258. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Valentini, J.; Fillion, M.; Passos, C.J.S.; Garcia, S.C.; Mergler, D.; Barbosa, F. Mercury exposure and oxidative stress in communities of the Brazilian Amazon. Sci. Total Environ. 2010, 408, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Crompton, P.; Ventura, A.M.; Souza, J.M.; Santos, E.; Strickland, G.T.; Silbergeld, E. Assessment of Mercury Exposure and Malaria in a Brazilian Amazon Riverine Community. Environ. Res. 2002, 90, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Mosquera, H.; SBSujitha Jonathan, M.P.; Sarkar, S.K.; Medina-Mosquera, F.; Ayala-Mosquera, H.; Morales-Mira, G.; Arreola-Mendoza, L. Mercury levels in human population from a mining district in Western Colombia. J. Environ. Sci. 2018, 68, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Knobeloch, L.; Gliori, G.; Anderson, H. Assessment of methylmercury exposure in Wisconsin. Environ. Res. 2007, 103, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.; James, K.A.F.; Levy, L.S. Is low-level environmental mercury exposure of concern to human health? Sci. Total Environ. 2009, 408, 171–182. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Garcia, A.M.; de Souza, J.R. Mercury contamination in hair of riverine populations of Apiacás reserve in the Brazilian Amazon. Water Air Soil Pollut. 1997, 97, 1–8. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, T.; Iwata, H.; Monirith, I.; Tana, T.S.; Subramanian, A.; Tanabe, S. Mercury contamination in human hair and fish from Cambodia: Levels, specific accumulation and risk assessment. Environ. Pollut. 2005, 134, 79–86. [Google Scholar] [CrossRef]
- Janicka, M.; Binkowski, Ł.J.; Błaszczyk, M.; Paluch, J.; Wojtaś, W.; Massanyi, P.; Stawarz, R. Cadmium, lead and mercury concentrations and their influence on morphological parameters in blood donors from different age groups from southern Poland. J. Trace Elem. Med. Biol. 2015, 29, 342–346. [Google Scholar] [CrossRef]
- Axelweiner, J.A.; Nylander, M. The relationship between mercury concentration in human organs and different predictor variables. Sci. Total Environ. 1993, 138, 101–115. [Google Scholar] [CrossRef]
- Dórea, J.G.; Barbosa, A.C.; Silva, G.S. Fish mercury bioaccumulation as a function of feeding behavior and hydrological cycles of the Rio Negro, Amazon. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, T.C.; Penteado, J.O.; dos Santos, M.; da Silva Júnior, F.M.R. Methylmercury in Fish from the Amazon Region—A Review Focused on Eating Habits. Water Air Soil Pollut. 2021, 232, 199. [Google Scholar] [CrossRef]
- Sastraprawira, S.M.; Abd Razak, I.H.; Shahimi, S.; Pati, S.; Edinur, H.A.; John, A.B.; Ahmad, A.; Kumaran, J.V.; Martin, M.B.; Chong, J.L.; et al. A review on introduced Cichla spp. and emerging concerns. Heliyon 2020, 6, e05370. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.D.; Almeida, R.; Bastos, W.R. A 35-Year Record (1987–2022) of Hg Concentrations in Two of the Fish Species Most Consumed by People Living in the Upper Madeira River Basin, Brazilian Amazon Region. Toxics 2024, 12, 144. [Google Scholar] [CrossRef]
- Silva, S.F.; Lima, M.O. Mercury in fish marketed in the Amazon Triple Frontier and Health Risk Assessment. Chemosphere 2020, 248, 125989. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.S. Selênio e Mercúrio em Guildas Tróficas de Peixes em um Lago da Amazônia Ocidental (Puruzinho, Humaitá-AM). Programa de Pós-Graduação em Desenvolvimento Regional e Meio Ambiente. Master’s Thesis, Universidade Federal de Rondônia, Porto Velho, Brazil, 2023. Available online: https://biogeoquimica.unir.br/uploads/42029128/arquivos/Dissertacao_Cassio_PGDRA_Verso_Final_2023_619363477.pdf (accessed on 10 December 2023).
- Ralston, N.V.C.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pestana, I.A.; Nascimento, L.; Oliveira, R.C.; Bastos, W.R.; Beneditto, A.P.M. Risk of exposure to Hg and pesticides residues in a traditional fishing community in the Amazon: A probabilistic approach based on dietary pattern. Environ. Sci. Pollut. Res. 2022, 29, 34164–34173. [Google Scholar] [CrossRef]
- Isaac, V.J.; Almeida, M.C.; Giarrizzo, T.; Deus, C.P.; Vale, R.; Klein, G.; Begossi, A. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. An. Acad. Bras. Ciências 2015, 87, 2229–2242. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Pfeiffer, W.C.; Malm, O.; De Souza, C.M.M.; Mierle, G. Mercury pollution in Brazil. Nature 1992, 356, 389. [Google Scholar] [CrossRef]
- Bastos, W.R.; Dórea, J.G.; Lacerda, L.D.; Almeida, R.; Costa-Junior, W.A.; Baía, C.C.; Sousa-Filho, I.F.; Sousa, E.A.; Oliveira, I.A.S.; Cabral, C.S.; et al. Dynamics of Hg and MeHg in the Madeira River basin (Western Amazon) before and after impoundment of a run-of-river hydroelectric dam. Environ. Res. 2020, 189, 109896. [Google Scholar] [CrossRef]
- Brasil. Resolução Conama N° 357, De 17 De Março De 2005* (RETIFICADA—AO FINAL). 2005. Available online: https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Resolucao/2005/res_conama_357_2005_classificacao_corpos_agua_rtfcda_altrd_res_393_2007_397_2008_410_2009_430_2011.pdf (accessed on 12 February 2024).

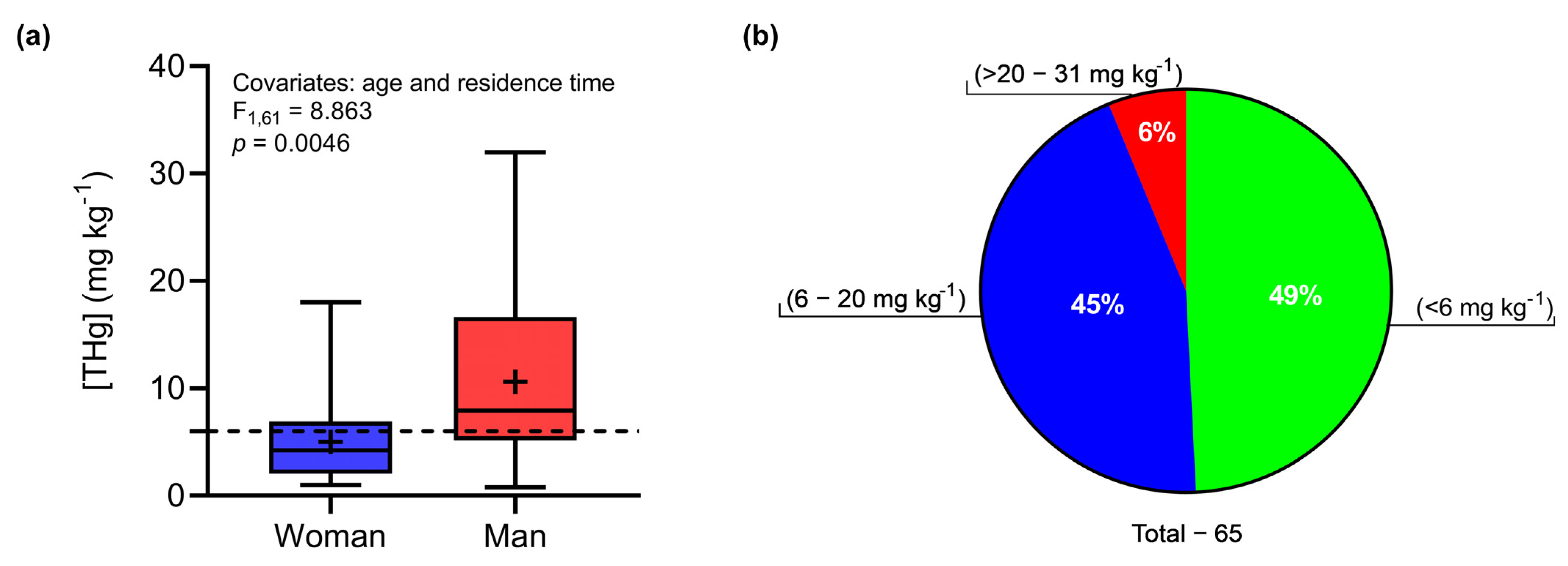

| Total | Men | Women | |
|---|---|---|---|
| Gender | 65 (100%) | 33 (51%) | 32 (49%) |
| Age (years) | 41.5 ± 17.6 (18–84) | 42.3 ± 18.5 (19–79) | 40.6 ± 16.8 (18–84) |
| Age Classes | |||
| Young | 13 (20%) | 5 (15%) | 8 (25%) |
| Adult | 43 (66%) | 23 (70%) | 20 (62.5%) |
| Elderly | 9 (14%) | 5 (15%) | 4 (12.5%) |
| Residence Time (years) | 19.01 ± 16.1 (2–71) | 19.7 ± 17.7 (2–71) | 18.2 ± 14.6 (2–56) |
| Fish Consumption (meals per week) | 3.6 ± 0.7 (2–6) | 3.5 ± 0.6 (2–4) | 3.75 ± 0.7 (3–6) |
| Pregnant | - | - | 2 (6%) |
| Lactating | - | - | 1 (3%) |
| Matrices | LOD | LOQ | Reference Material | Recovery (%) |
|---|---|---|---|---|
| THg | ||||
| Hair | 0.0008 | 0.0024 | BCR-463 | 119 |
| Fish | 0.0085 | 0.0255 | DORM-2 | 100 |
| Water | 0.0002 | 0.0006 | Spikes | 104 |
| MeHg | ||||
| Hair | 0.004 | 0.012 | BCR-463 | 108 |
| Fish | 0.000016 | 0.000048 | DORM-2 | 96 |
| Water | 0.000014 | 0.000042 | Spikes | 95 |
| Scientific Name (Common Name) | Frequency (%) | Feeding Habit | THg (mg kg−1) | Reference | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | N | ||||
| Mylossoma spp. (Pacú) | 37 | Herbivore | 0.06 ± 0.08 | 0.02–0.82 | 50 | This study |
| Semaprochilodus sp. (Jaraqui) | 24 | Detritivore | 0.17 ± 0.05 | 0.10–0.24 | 8 | [50] |
| Pseudoplatystoma puntictifer (Pintado) | 8 | Carnivore | 0.31 ± 0.08 | 0.21–0.41 | 4 | This study |
| Triportheus spp. (Sardinha) | 7 | Omnivore | 0.24 ± 0.14 | 0.06–0.79 | 70 | This study |
| Psectrogaster rutiloides (Branquinha) | 4 | Detritivore | 0.08 ± 0.05 | 0.02–0.42 | 135 | This study |
| Prochilodus nigricans (Curimatá) | 3 | Detritivore | 0.12 ± 0.07 | 0.01–0.24 | 44 | This study |
| Schizodon fasciatum (Piau) | 3 | Herbivore | 0.11 ± 0.16 | 0.01–0.85 | 51 | This study |
| Hemiodus microlepis (Jatuarana) | 2.5 | Detritivore | 0.08 ± 0.09 | 0.01–0.53 | 61 | [50] |
| Cichla spp. (Tucunaré) | 2.5 | Carnivore | 0.91 ± 0.40 | 0.40–1.92 | 23 | This study |
| Geophagus spp. (Cará) | 1.5 | Omnivore | 0.14 ± 0.07 | 0.06–0.23 | 8 | [50] |
| Pseudoplatismtoma tigrinum (Surubim) | 1.5 | Piscivore | 0.48 ± 0.23 | 0.24–1.20 | 16 | [50] |
| Brachyplatystoma rousseauxii (Dourada) | 1 | Piscivore | 0.89 ± 0.89 | 0.01–6.54 | 96 | [50] |
| Piaractus brachypomus (Pirapitinga) | 1 | Omnivore | 0.07 ± 0.05 | 0.01–0.22 | 20 | [50] |
| Colossoma macropomum (Tambaqui) | 1 | Omnivore | 0.15 ± 0.13 | 0.02–0.55 | 42 | [50] |
| Potamorhina spp. (Chora) | 1 | Detritivore | 0.11 ± 0.06 | 0.02–0.31 | 123 | This study |
| Brachyplastystoma filamentosum (Piraíba) | 1 | Piscivore | 1.48 ± 0.88 | 0.28–3.80 | 30 | [50] |
| Hypophthalmus marginatus (Mapará) | 1 | Planktivore | 0.29 ± 0.21 | 0.06–0.76 | 13 | This study |
| Scientific Name (Common Name) | N | EDI | >PTWI/7 | RR | RR ≥ 1 | MSCQ | <Daily Consumption |
|---|---|---|---|---|---|---|---|
| Mylossoma spp. (Pacú) | 50 | 0.28 ± 0.41 (0.07–3.05) | 64 | 2.3 ± 3.3 (0.6–24.4) | 84 | 175 ± 93 (11–464) | 92 |
| Pseudoplatystoma puntictifer (Pintado) | 4 | 1.54 ± 0.41 (1.05–2.01) | 100 | 12.4 ± 3.3 (8.4–16.1) | 100 | 22 ± 6 (16–30) | 100 |
| Triportheus spp. (Sardinha) | 70 | 1.18 ± 0.71 (0.32–3.89) | 100 | 9.4 ± 5.7 (2.6–31.1) | 100 | 36 ± 19 (8–100) | 100 |
| Psectrogaster rutiloides (Branquinha) | 135 | 0.39 ± 0.27 (0.11–2.07) | 15 | 3.1 ± 2.1 (0.9–16.5) | 99 | 105 ± 47 15–283) | 100 |
| Prochilodus nigricans (Curimatá) | 44 | 0.61 ± 0.33 (0.06–1.20) | 23 | 4.5 ± 6.1 (0.3–33.5) | 93 | 101 ± 121 (27–542) | 93 |
| Schizodon fasciatus (Piau) | 51 | 0.56 ± 0.77 (0.03–4.18) | 43 | 4.9 ± 2.6 (0.5–9.6) | 80 | 174 ± 186 (8–929) | 84 |
| Cichla spp. (Tucunaré) | 23 | 4.46 ± 1.96 (1.97–9.45) | 100 | 35.7 ± 15.7 (15.8–75.6) | 100 | 9 ± 4 (3–16) | 100 |
| Potamorhina spp. (Chora) | 123 | 0.52 ± 0.32 (0.09–1.51) | 17 | 4.2 ± 2.5 (0.7–12.1) | 97 | 91 ± 64 (21–342) | 98 |
| Hypophthalmus marginatus (Mapará) | 13 | 1.44 ± 1.04 (0.29–3.75) | 100 | 11.5 ± 8.4 (2.3–30.0) | 100 | 41 ± 35 (9–112) | 100 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| [THg] (ng L−1) | 7.04 | 6.42 | 0.87 | 25.10 |
| [MeHg] (ng L−1) | 0.15 | 0.11 | 0.04 | 0.66 |
| MeHg:THg (%) | 4 | 5 | 0.5 | 24.5 |
| Ingestion | ||||
| ADD–THg (µg kg−1) | 1.66 × 10−2 | 1.52 × 10−2 | 2.06 × 10−3 | 5.93 × 10−2 |
| ADD–MeHg (µg kg−1) | 3.65 × 10−4 | 2.52 × 10−4 | 9.68 × 10−5 | 1.55 × 10−3 |
| HQ–THg | 4.16 × 10−2 | 3.79 × 10−2 | 5.16 × 10−3 | 1.48 × 10−1 |
| HQ–MeHg | 3.65 × 10−3 | 2.52 × 10−3 | 9.68 × 10−4 | 1.55 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canela, T.A.; Monteiro, L.C.; Cabral, C.d.S.; Ximenes, F.d.S.; Oliveira, I.A.d.S.; Bernardi, J.V.E.; Almeida, R.d.; Bastos, W.R. Mercury in Fish and Human Hair and Estimated Dietary Intake in a Riverside Community of the Madeira River Basin in the Brazilian Amazon. Toxics 2024, 12, 208. https://doi.org/10.3390/toxics12030208
Canela TA, Monteiro LC, Cabral CdS, Ximenes FdS, Oliveira IAdS, Bernardi JVE, Almeida Rd, Bastos WR. Mercury in Fish and Human Hair and Estimated Dietary Intake in a Riverside Community of the Madeira River Basin in the Brazilian Amazon. Toxics. 2024; 12(3):208. https://doi.org/10.3390/toxics12030208
Chicago/Turabian StyleCanela, Thayson Araujo, Lucas Cabrera Monteiro, Cássio da Silva Cabral, Fábio da Silva Ximenes, Iuri Aparecida da Silva Oliveira, José Vicente Elias Bernardi, Ronaldo de Almeida, and Wanderley Rodrigues Bastos. 2024. "Mercury in Fish and Human Hair and Estimated Dietary Intake in a Riverside Community of the Madeira River Basin in the Brazilian Amazon" Toxics 12, no. 3: 208. https://doi.org/10.3390/toxics12030208







