Discrepancy of Growth Toxicity of Polystyrene Nanoplastics on Soybean (Glycine max) and Mung Bean (Vigna radiata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoplastics and Seeds
2.2. Hydroponic Design
2.3. Measurement Parameters
2.3.1. Growth and Biochemical Indexes of Sprouts
2.3.2. Elemental Content of Sprouts
2.3.3. SEM Observations
2.4. Transcriptome Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of NPs
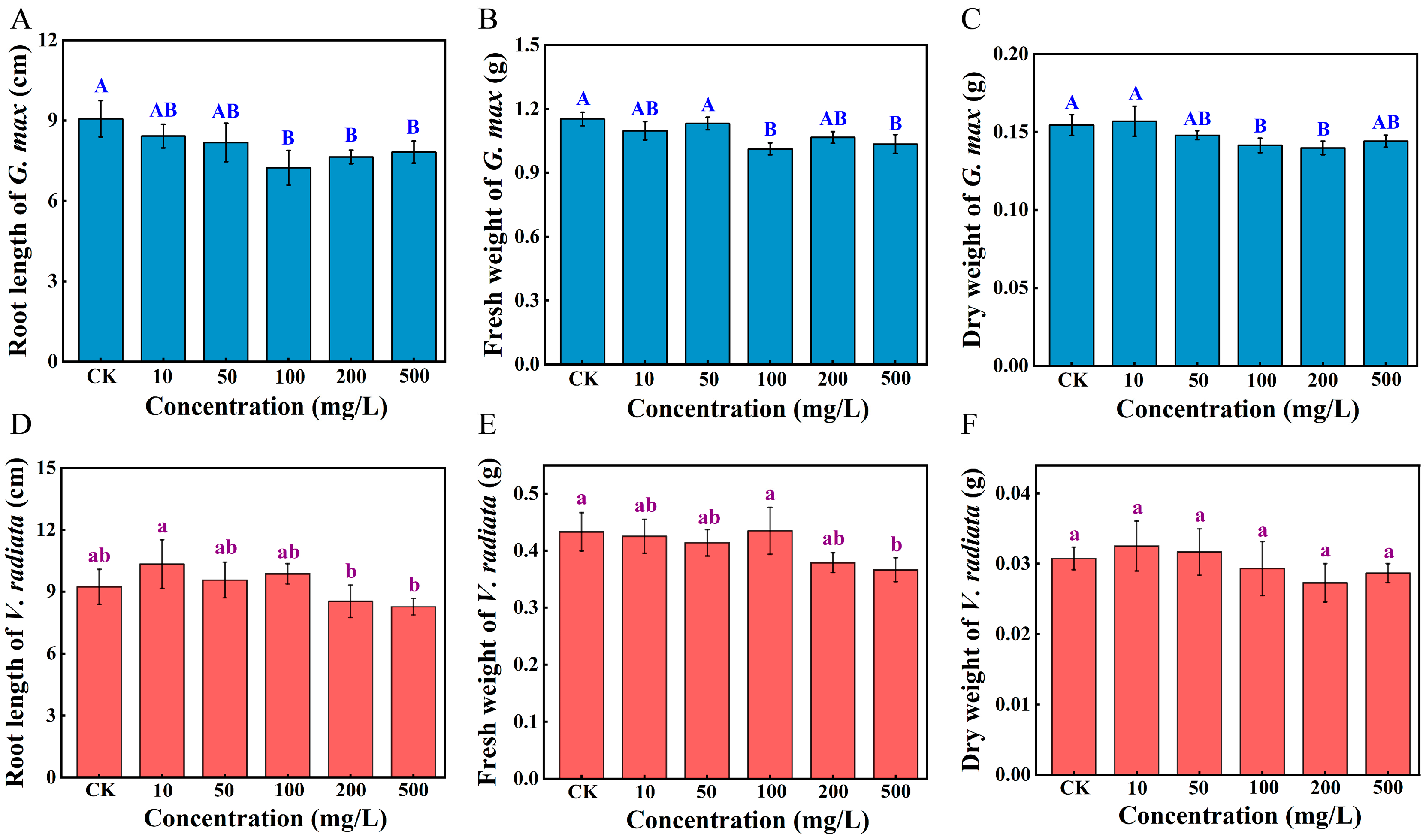
3.2. Alterations in the Phenotypic Parameters of Sprouts
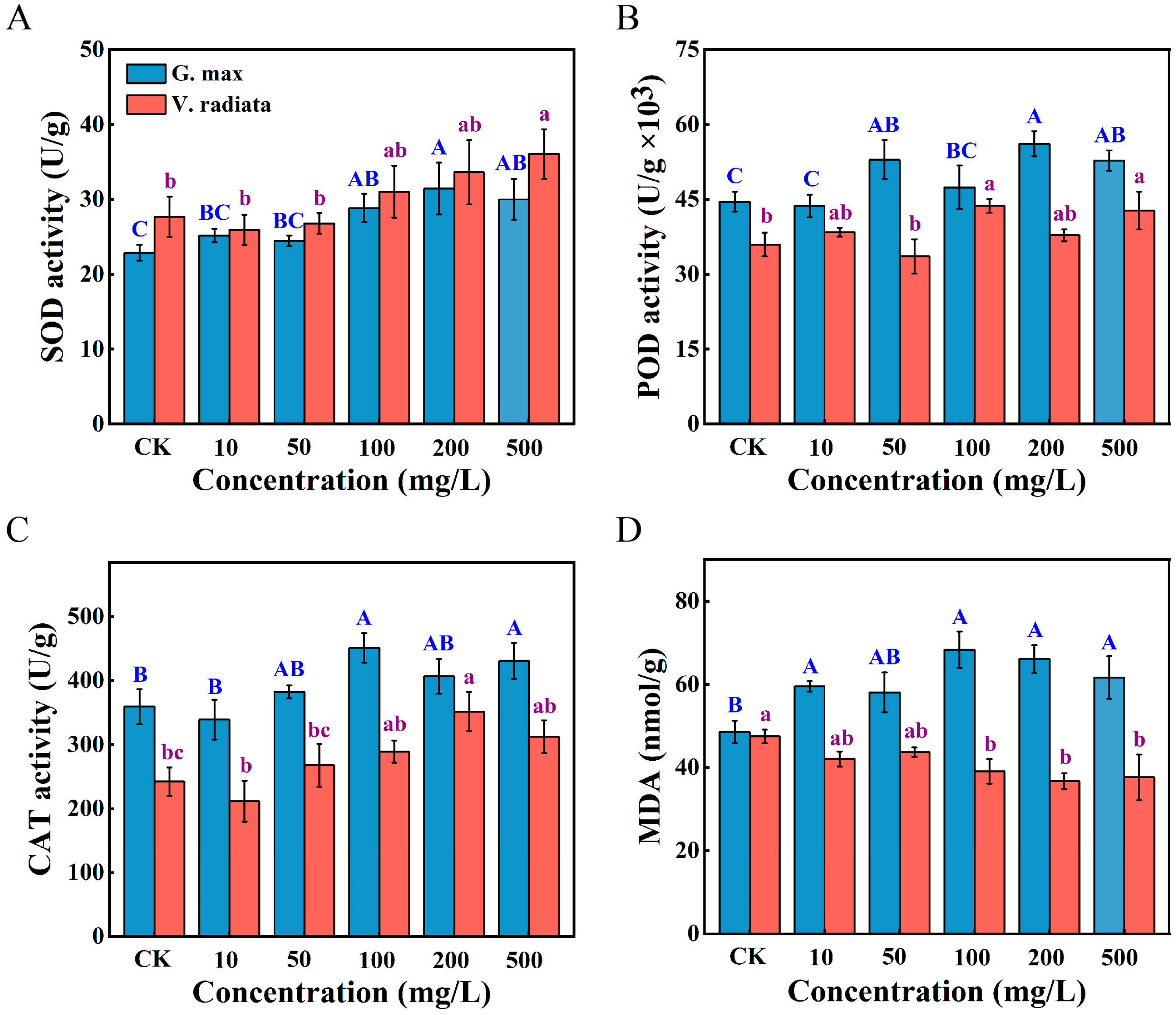
3.3. Alterations in the Oxidative Stress Response of Sprouts
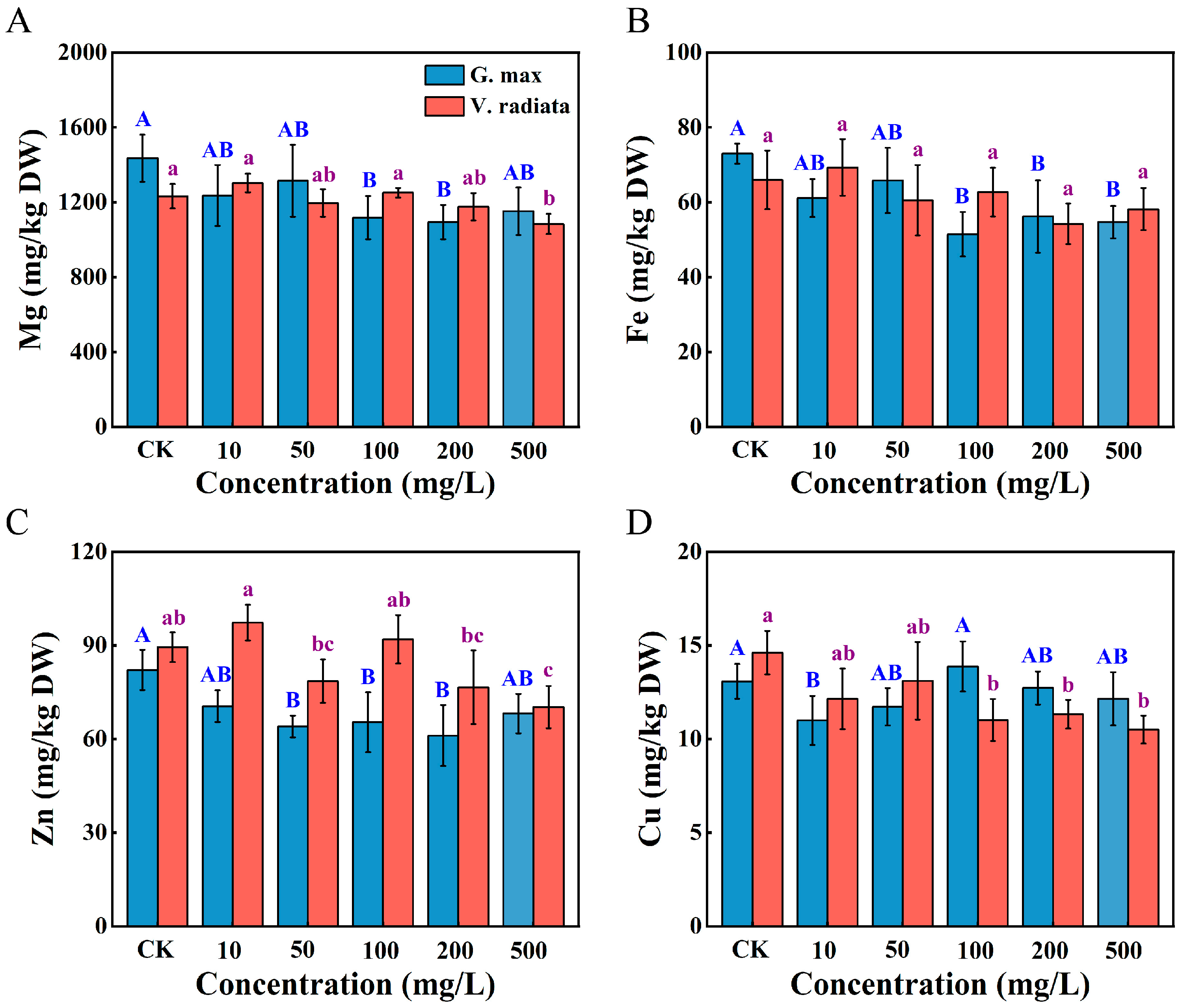
3.4. Alterations in Mineral Elements of Sprouts
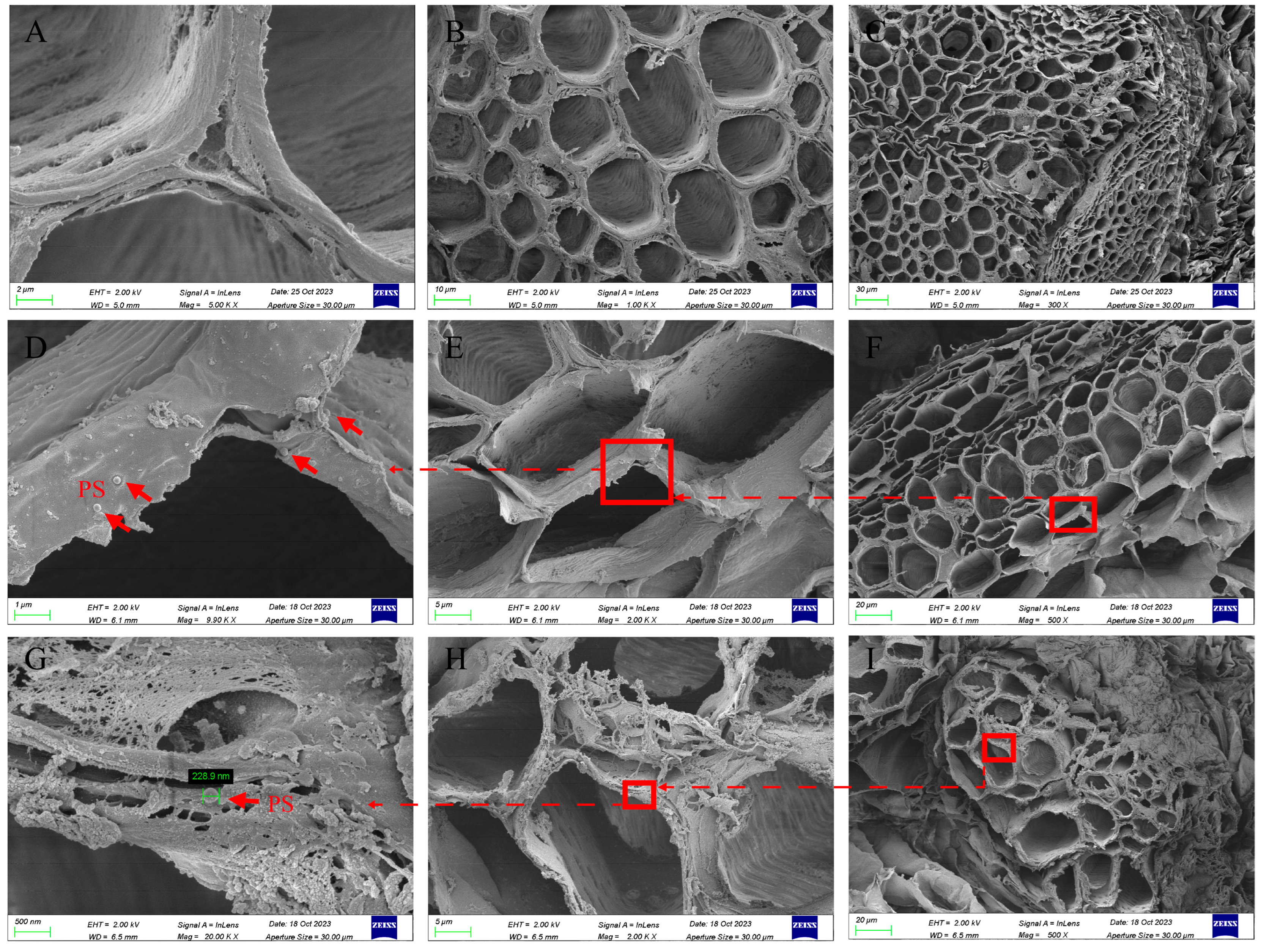
3.5. Scanning Electron Microscope Observation
3.6. Transcriptome Analysis
3.6.1. Identification of Differentially Expressed Genes
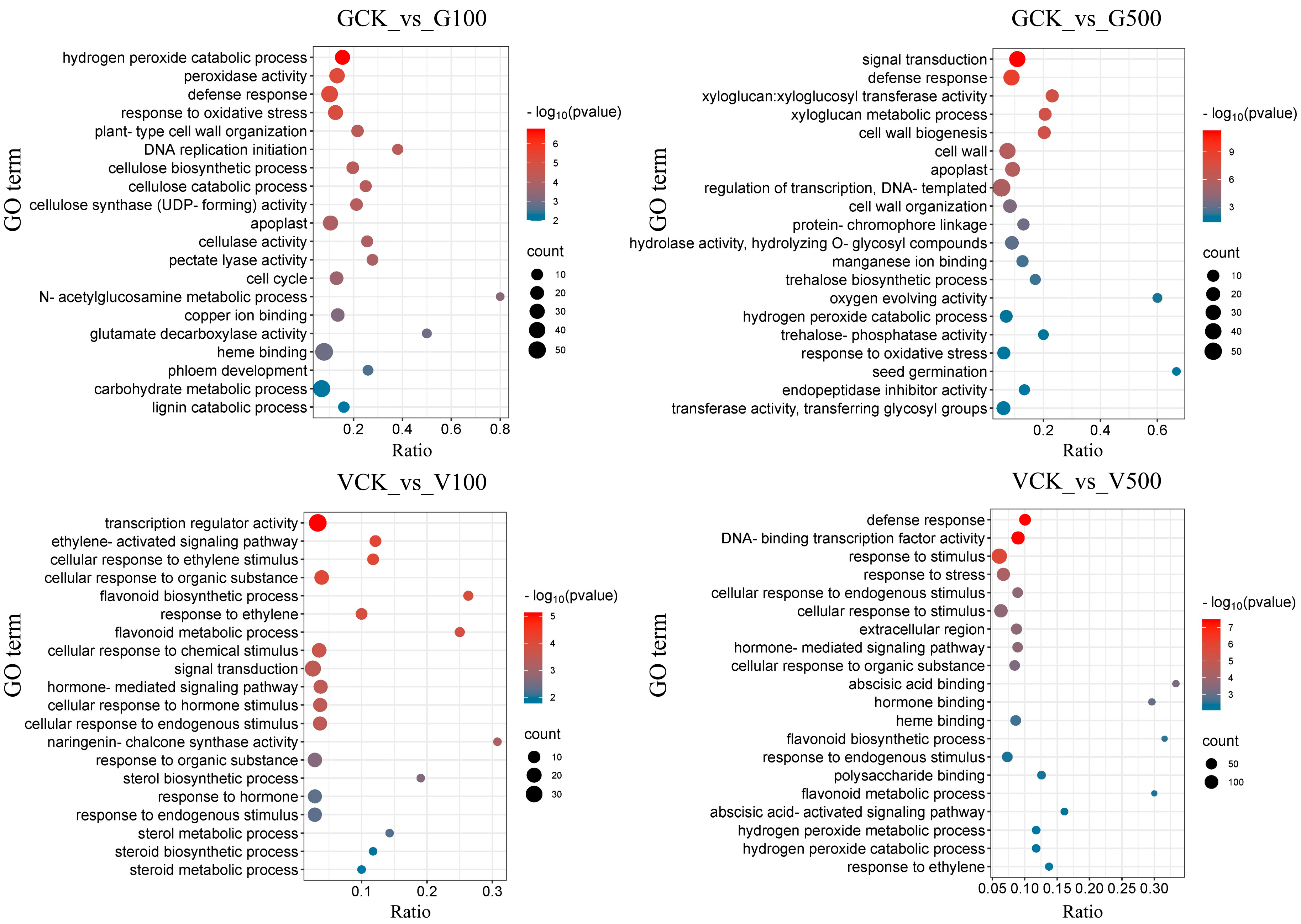
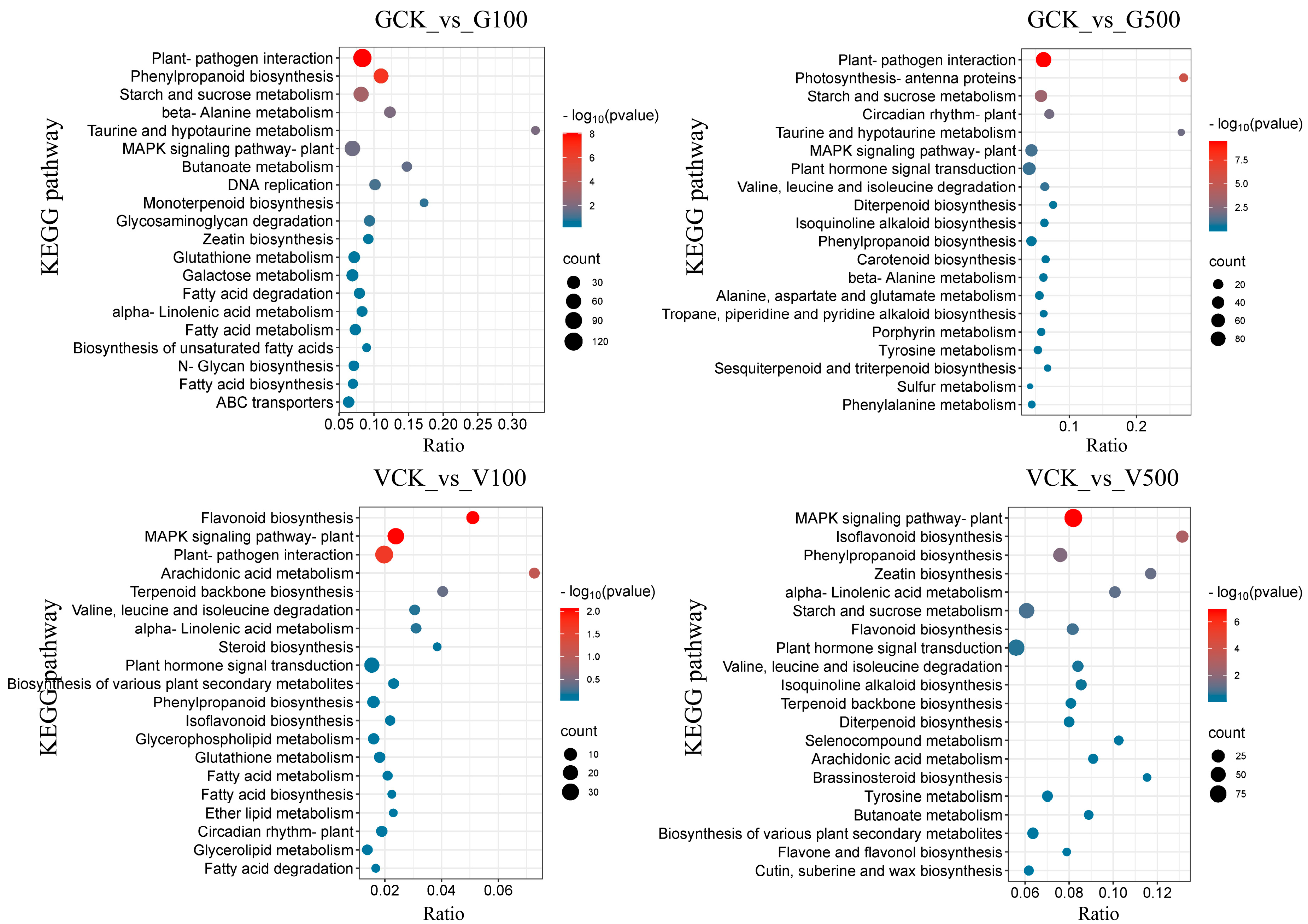
3.6.2. Enrichment Analysis of Differential Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Sid-Cheikh, M.; Rowland, S.J.; Stevenson, K.; Rouleau, C.; Henry, T.B.; Thompson, R.C. Uptake, Whole-Body Distribution, and Depuration of Nanoplastics by the Scallop Pecten maximus at Environmentally Realistic Concentrations. Environ. Sci. Technol. 2018, 52, 14480–14486. [Google Scholar] [CrossRef]
- Ng, E.-L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic diagnostics in humans: “The 3Ps” Progress, problems, and prospects. Sci. Total Environ. 2023, 856, 159164. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Ahmed, A.S.S.; Billah, M.M.; Ali, M.M.; Bhuiyan, M.K.A.; Guo, L.; Mohinuzzaman, M.; Hossain, M.B.; Rahman, M.S.; Islam, M.S.; Yan, M.; et al. Microplastics in aquatic environments: A comprehensive review of toxicity, removal, and remediation strategies. Sci. Total Environ. 2023, 876, 162414. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Pramanick, K. Perspectives and challenges of micro/nanoplastics-induced toxicity with special reference to phytotoxicity. Global Chang. Biol. 2020, 26, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, N.; Li, X.; Shao, H.; Wang, J.; An, L.; Jin, F. Potential translocation process and effects of polystyrene microplastics on strawberry seedlings. J. Hazard. Mater. 2023, 449, 131019. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Zeb, A.; Lian, J.; Sun, Y.; Sun, H. Polystyrene microplastic interaction with Oryza sativa: Toxicity and metabolic mechanism. Environ. Sci. Nano 2021, 8, 3699–3710. [Google Scholar] [CrossRef]
- Sunil, Z.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Microplastics and leachate materials from pharmaceutical bottle: An in vivo study in Donax faba (Marine Clam). Environ. Toxicol. Pharmacol. 2023, 101, 104205. [Google Scholar] [CrossRef]
- De Silva, Y.S.K.; Rajagopalan, U.M.; Kadono, H.; Li, D. Effects of microplastics on lentil (Lens culinaris) seed germination and seedling growth. Chemosphere 2022, 303, 135162. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, T.; Wang, L.; Tang, J. Polystyrene micro and nanoplastics attenuated the bioavailability and toxic effects of Perfluorooctane sulfonate (PFOS) on soybean (Glycine max) sprouts. J. Hazard. Mater. 2023, 448, 130911. [Google Scholar] [CrossRef]
- Ghani, M.; Kulkarni, K.P.; Song, J.T.; Shannon, J.G.; Lee, J.-D. Soybean Sprouts: A Review of Nutrient Composition, Health Benefits and Genetic Variation. Plant Mutat. Breed. Biotechnol. 2016, 4, 398–412. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Kaur, M.; Yao, Z.; Chen, T.; Xu, M. Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata). Int. J. Environ. Res. Public Health 2021, 18, 10629. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Peijnenburg, W.J.G.M.; Li, R.; Yang, J.; Zhou, Q. Confocal measurement of microplastics uptake by plants. MethodsX 2020, 7, 100750. [Google Scholar] [CrossRef]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Huang, P.; Yu, X.; Xiang, Q.; Zhang, L.; Gao, X.; Chen, Q.; Gu, Y. Biochemical and transcriptomic responses of buckwheat to polyethylene microplastics. Sci. Total Environ. 2023, 899, 165587. [Google Scholar] [CrossRef]
- Zantis, L.J.; Rombach, A.; Adamczyk, S.; Velmala, S.M.; Adamczyk, B.; Vijver, M.G.; Peijnenburg, W.; Bosker, T. Species-dependent responses of crop plants to polystyrene microplastics. Environ. Pollut. 2023, 335, 122243. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Hu, X.; Xiang, H.; Chen, S.; Li, L.; Qi, B.; Li, C.; Liu, S.; Yang, X. Structural characterization and hypolipidemic activity of Gracilaria lemaneiformis polysaccharide and its degradation products. Food Chem. X 2022, 14, 100314. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, W.; Jiang, M.; Li, S.; Liang, G.; Bu, Q.; Xu, L.; Zhu, H.; Lu, A. Species-dependent response of food crops to polystyrene nanoplastics and microplastics. Sci. Total Environ. 2021, 796, 148750. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Canas, J.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Ambikapathi, R.; Lee, E.; Olszyk, D. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tu, C.; Li, L.; Wang, X.; Yang, J.; Feng, Y.; Zhu, X.; Fan, Q.; Luo, Y. Visual tracking of label-free microplastics in wheat seedlings and their effects on crop growth and physiology. J. Hazard. Mater. 2023, 456, 131675. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kurzeja, E.; Synowiec-Wojtarowicz, A.; Stec, M.; Glinka, M.; Gawron, S.; Pawłowska-Góral, K. Effect of a Static Magnetic Fields and Fluoride Ions on the Antioxidant Defense System of Mice Fibroblasts. Int. J. Mol. Sci. 2013, 14, 15017–15028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-Q.; Wang, Y.-S.; Lou, Z.-P.; Dong, J.-D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.-C.; Bhindi, R.; Figuretree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.; Guo, L.; Wang, P.; He, C.; Liu, D.; Bian, H.; Sheng, L. Effects of polystyrene nanoplastics with different functional groups on rice (Oryza sativa L.) seedlings: Combined transcriptome, enzymology, and physiology. Sci. Total Environ. 2022, 834, 155092. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-Mediated Lipid Peroxidation and RES-Activated Signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Yin, S.; Xiao, K.; Xiong, Q.; Bian, S.; Liang, S.; Hou, H.; Hu, J.; Yang, J. Metabolomics revealing the response of rice (Oryza sativa L.) exposed to polystyrene microplastics. Environ. Pollut. 2020, 266, 115159. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-Talks Between Macro- and Micronutrient Uptake and Signaling in Plants. Front. Plant Sci. 2021, 12, 663477. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-applied polystyrene nanoplastics (PSNPs) reduce the growth and nutritional quality of lettuce (Lactuca sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef]
- Eskandari, S.; Höfte, H.; Zhang, T. Foliar manganese spray induces the resistance of cucumber to Colletotrichum lagenarium. J. Plant Physiol. 2020, 246–247, 153129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Z.; Tang, S.; Li, D.; Jiang, Q.; Zhang, T. Transcriptional response provides insights into the effect of chronic polystyrene nanoplastic exposure on Daphnia pulex. Chemosphere 2020, 238, 124563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-Q.; Lu, C.-H.; Mai, L.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Williams, K.M.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J. Nanobiotechnol. 2016, 14, 62. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Li, R.; Zhou, J.; Wang, G. The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ. Sci. Pollut. Res. 2020, 28, 16042–16053. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Li, J.; Li, G.; Zahid, M.S.; Li, D.; Ma, C.; Xu, W.; Song, S.; Li, X.; et al. Transcriptome analysis revealed the expression levels of genes related to abscisic acid and auxin biosynthesis in grapevine (Vitis vinifera L.) under root restriction. Front. Plant Sci. 2022, 13, 959693. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Meng, F.; Xiao, Y.; Dai, W.; Luan, Y. Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity. Toxics 2021, 9, 179. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, G.; Huang, X.; Pan, T.; Chen, W.; Qu, M.; Ouyang, B.; Yu, M.; Shabala, S. Candidate regulators of drought stress in tomato revealed by comparative transcriptomic and proteomic analyses. Front. Plant Sci. 2023, 14, 1282718. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. The ethylene signaling pathway: New insights. Curr. Opin. Plant Biol. 2004, 7, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I. Nutrient cycling is an important mechanism for homeostasis in plant cells. Plant Physiol. 2021, 187, 2246–2261. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Rasheed, R.; Ashraf, M.A.; Rizwan, M.; Ali, S. Effects of exogenous taurine on growth, photosynthesis, oxidative stress, antioxidant enzymes and nutrient accumulation by Trifolium alexandrinum plants under manganese stress. Chemosphere 2022, 308, 136523. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Meng, Y.; Bai, Y.; Yu, H.; Qian, Y.; Zhang, D.; Zhou, Y. Starch and Sucrose Metabolism and Plant Hormone Signaling Pathways Play Crucial Roles in Aquilegia Salt Stress Adaption. Int. J. Mol. Sci. 2023, 24, 3948. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, H.; Sagurthi, S.; Paris, V.; Zhuo, C.; Dixon, R.A. The protein conformational basis of isoflavone biosynthesis. Commun. Biol. 2022, 5, 1249. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, X.; Han, R.; Wang, X.; Wang, K.; Qi, G.; Ma, P.; Komatsuda, T.; Liu, C. Integrated transcriptome and metabolome analysis reveals that flavonoids function in wheat resistance to powdery mildew. Front. Plant Sci. 2023, 14, 1125194. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Li, W.; Zhang, Z.; Cai, H.; Zhang, L.; Sun, Y.; Liu, X.; Tian, Z. Discrepancy of Growth Toxicity of Polystyrene Nanoplastics on Soybean (Glycine max) and Mung Bean (Vigna radiata). Toxics 2024, 12, 155. https://doi.org/10.3390/toxics12020155
Su D, Li W, Zhang Z, Cai H, Zhang L, Sun Y, Liu X, Tian Z. Discrepancy of Growth Toxicity of Polystyrene Nanoplastics on Soybean (Glycine max) and Mung Bean (Vigna radiata). Toxics. 2024; 12(2):155. https://doi.org/10.3390/toxics12020155
Chicago/Turabian StyleSu, Dan, Wangwang Li, Zhaowei Zhang, Hui Cai, Le Zhang, Yuanlong Sun, Xiaoning Liu, and Zhiquan Tian. 2024. "Discrepancy of Growth Toxicity of Polystyrene Nanoplastics on Soybean (Glycine max) and Mung Bean (Vigna radiata)" Toxics 12, no. 2: 155. https://doi.org/10.3390/toxics12020155






