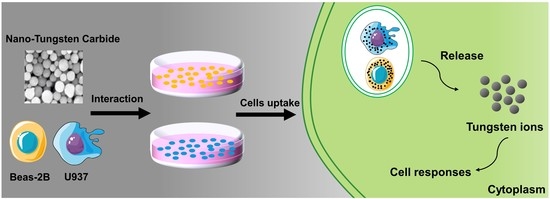
The Cytotoxicity of Tungsten Ions Derived from Nanoparticles Correlates with Pulmonary Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Cell Culture and Measurement of Tungsten Ion Release
2.4. Transmission Electron Microscopy
2.5. Cytotoxicity Analysis
2.6. Statistical Analysis
3. Results
3.1. Decrease in the Viability of Lung Epithelial Cells and Macrophages by Nano-WC
3.2. Release of Tungsten Ions in Cells Exposed to Nano-WC
3.3. Mitigating the Cytotoxicity of Nano-WC by Chelation of W6+
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, S.K.; Panda, P.K.; Kumari, P.; Patel, P.; Arunima, A.; Jha, E.; Husain, S.; Prakash, R.; Hergenröder, R.; Mishra, Y.K.; et al. Determining factors for the nano-biocompatibility of cobalt oxide nanoparticles: Proximal discrepancy in intrinsic atomic interactions at differential vicinage. Green Chem. 2021, 23, 3439–3458. [Google Scholar] [CrossRef]
- Azman, M.N.; Abualroos, N.J.; Yaacob, K.A.; Zainon, R. Feasibility of nanomaterial tungsten carbide as lead-free nanomaterial-based radiation shielding. Radiat. Phys. Chem. 2022, 202, 110492. [Google Scholar] [CrossRef]
- Yao, Z.; Stiglich, J.J.; Sudarshan, T.S. Nanosized WC-Co holds promise for the future. Metal Powder Rep. 1998, 53, 26–33. [Google Scholar] [CrossRef]
- Zhengui, Y.; Stiglich, J.J.; Sudarshan, T.S. Nano-grained Tungsten Carbide Cobalt (WC/Co). Mater Modif. 1998, 2929, 1–27. [Google Scholar]
- Stefaniak, A.B.; Virji, M.A.; Day, G.A. Characterization of exposures among cemented tungsten carbide workers. Part I: Size-fractionated exposures to airborne cobalt and tungsten particles. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Lombaert, N.; De Boeck, M.; Decordier, I.; Cundari, E.; Lison, D.; Kirsch-Volders, M. Evaluation of the apoptogenic potential of hard metal dust (WC–Co), tungsten carbide and metallic cobalt. Toxicol. Lett. 2004, 154, 23–34. [Google Scholar] [CrossRef]
- Rivolta, G.; Nicoli, E.; Ferretti, G.; Tomasini, M. Hard metal lung disorders: Analysis of a group of exposed workers. Sci. Total Environ. 1994, 150, 161–165. [Google Scholar] [CrossRef]
- Forni, A. Bronchoalveolar lavage in the diagnosis of hard metal disease. Sci. Total Environ. 1994, 150, 69–76. [Google Scholar] [CrossRef]
- Kinoshita, M.; Sueyasu, Y.; Watanabe, H.; Tanoue, S.; Okubo, Y.; Koga, T.; Kawahara, M.; Nagata, E.; Oizumi, K. Giant cell interstitial pneumonia in two hard metal workers: The role of bronchoalveolar lavage in diagnosis. Respirology 1999, 4, 263–266. [Google Scholar] [CrossRef]
- Armstead, A.L.; Li, B. Nanotoxicity: Emerging concerns regarding nanomaterial safety and occupational hard metal (WC-Co) nanoparticle exposure. Int. J. Nanomed. 2016, 2016, 6421–6433. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Liu, J.; Wang, Y.; Jin, M.; Ye, Q. Cobalt-related interstitial lung disease or hard metal lung disease: A case series of Chinese workers. Toxicol. Ind. Health 2021, 37, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Kühnel, D.; Busch, W.; Meißner, T.; Springer, A.; Potthoff, A.; Richter, V.; Gelinsky, M.; Scholz, S.; Schirmer, K. Agglomeration of tungsten carbide nanoparticles in exposure medium does not prevent uptake and toxicity toward a rainbow trout gill cell line. Aquat. Toxicol. 2009, 93, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Xie, Y.; Ren, G.; Yang, Z. Attenuated effect of tungsten carbide nanoparticles on voltage-gated sodium current of hippocampal CA1 pyramidal neurons. Toxicol. In Vitro 2013, 27, 299–304. [Google Scholar] [CrossRef]
- Huaux, F.; Lasfargues, G.; Lauwerys, R.; Lison, D. Lung toxicity of hard metal particles and production of interleukin-1, tumor necrosis factor-α, fibronectin, and cystatin-c by lung phagocytes. Toxicol. Appl. Pharmacol. 1995, 132, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lifeng, W.; Fenghua, G.; Zhuo, Y. Effect of tungsten carbide nanoparticles on the development of zebrafish embryos. China Environ. Sci. 2012, 32, 1280–1283. [Google Scholar]
- Afroz, T.; Hiraku, Y.; Ma, N.; Ahmed, S.; Oikawa, S.; Kawanishi, S.; Murata, M. Nitrative DNA damage in cultured macrophages exposed to indium oxide. J. Occup. Health 2018, 60, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Hiraku, Y.; Nishikawa, Y.; Ma, N.; Afroz, T.; Mizobuchi, K.; Ishiyama, R.; Matsunaga, Y.; Ichinose, T.; Kawanishi, S.; Murata, M. Nitrative DNA damage induced by carbon-black nanoparticles in macrophages and lung epithelial cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2017, 818, 7–16. [Google Scholar] [CrossRef]
- Akiyo, T.; Miyuki, H.; Minoru, O.; Naohide, I.; Takahiro, U.; Toshiaki, H.; Kiyohisa, S. Pulmonary Toxicity of Indium-Tin Oxide and Indium Phosphide after Intratracheal Instillations into the Lung of Hamsters. J. Occup. Health 2002, 44, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Nagano, K.; Nishizawa, T.; Umeda, Y.; Kasai, T.; Noguchi, T.; Gotoh, K.; Ikawa, N.; Eitaki, Y.; Kawasumi, Y.; Yamauchi, T.; et al. Inhalation Carcinogenicity and Chronic Toxicity of Indium-tin Oxide in Rats and Mice. J. Occup. Health 2011, 53, 175–187. [Google Scholar] [CrossRef]
- Oberdörster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 2000, 74, 1–8. [Google Scholar] [CrossRef]
- Lison, D.; Lauwerys, R. Evaluation of the role of reactive oxygen species in the interactive toxicity of carbide-cobalt mixtures on macrophages in culture. Arch. Toxicol. 1993, 67, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.O.; Lejon, C.; Ekstrand-Hammarström, B.; Akfur, C.; Ahlinder, L.; Bucht, A.; Österlund, L. Polymorph-and size-dependent uptake and toxicity of TiO2 nanoparticles in living lung epithelial cells. Small 2011, 7, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Tabei, Y.; Sonoda, A.; Nakajima, Y.; Biju, V.; Makita, Y.; Yoshida, Y.; Horie, M. Intracellular accumulation of indium ions released from nanoparticles induces oxidative stress, proinflammatory response and DNA damage. J. Biochem. 2016, 159, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Lison, D.; Laloy, J.; Corazzari, I.; Muller, J.; Rabolli, V.; Panin, N.; Huaux, F.; Fenoglio, I.; Fubini, B. Sintered indium-tin-oxide (ITO) particles: A new pneumotoxic entity. Toxicol. Sci. 2009, 108, 472–481. [Google Scholar] [CrossRef]
- Badding, M.A.; Schwegler-Berry, D.; Park, J.-H.; Fix, N.R.; Cummings, K.J.; Leonard, S.S. Sintered indium-tin oxide particles induce pro-inflammatory responses in vitro, in part through inflammasome activation. PLoS ONE 2015, 10, e0124368. [Google Scholar] [CrossRef] [Green Version]
- Gwinn, W.M.; Qu, W.; Shines, C.J.; Bousquet, R.W.; Taylor, G.J.; Waalkes, M.P.; Morgan, D.L. Macrophage Solubilization and Cytotoxicity of Indium-Containing Particles In Vitro. Toxicol. Sci. 2013, 135, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Gwinn, W.M.; Qu, W.; Bousquet, R.W.; Price, H.; Shines, C.J.; Taylor, G.J.; Waalkes, M.P.; Morgan, D.L. Macrophage solubilization and cytotoxicity of indium-containing particles as in vitro correlates to pulmonary toxicity in vivo. Toxicol. Sci. 2015, 144, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Bastian, S.; Busch, W.; Kühnel, D.; Springer, A.; Meißner, T.; Holke, R.; Scholz, S.; Iwe, M.; Pompe, W.; Gelinsky, M.; et al. Toxicity of tungsten carbide and cobalt-doped tungsten carbide nanoparticles in mammalian cells in vitro. Environ. Health Perspect. 2009, 117, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Tripathi, A.; Das, M.; Dwivedi, P.D. Cytotoxicity and uptake of zinc oxide nanoparticles leading to enhanced inflammatory cytokines levels in murine macrophages: Comparison with bulk zinc oxide. J. Biomed. Nanotechnol. 2011, 7, 110–111. [Google Scholar] [CrossRef]
- Böhme, S.; Baccaro, M.; Schmidt, M.; Potthoff, A.; Stärk, H.-J.; Reemtsma, T.; Kühnel, D. Metal uptake and distribution in the zebrafish (Danio rerio) embryo: Differences between nanoparticles and metal ions. Environ. Sci. Nano 2017, 4, 1005–1015. [Google Scholar] [CrossRef] [Green Version]
- Fielding, J.; Hall, J. A biolchemical and cytochemical study of peroxidase activity in roots of Pisum sativum: I. a comparison of DAB-peroxidase and guaiacol-peroxidase with particular emphasis on the properties of cell wall activity. J. Exp. Bot. 1978, 29, 969–981. [Google Scholar] [CrossRef]
- Melanie, M.; Jan, L. The Driving Force: Nuclear Mechanotransduction in Cellular Function, Fate, and Disease. HHS Public Access 2019, 4, 443–468. [Google Scholar] [CrossRef]
- Li, L. Ginkgo Biloba Conducting Tissue Development, Transcellular Transport and Effects of Water Logging Stress. Master’s Thesis, Yangzhou University, Yangzhou, China, 2007. [Google Scholar]
- Roberg, K.; Kågedal, K.; Öllinger, K. Microinjection of cathepsin d induces caspase-dependent apoptosis in fibroblasts. Am. J. Pathol. 2002, 161, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Leinardi, R.; Pavan, C.; Yedavally, H.; Tomatis, M.; Salvati, A.; Turci, F. Cytotoxicity of fractured quartz on THP-1 human macrophages: Role of the membranolytic activity of quartz and phagolysosome destabilization. Arch. Toxicol. 2020, 94, 2981–2995. [Google Scholar] [CrossRef]
- Singh, R.P.; Ramarao, P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol. Lett. 2012, 213, 249–259. [Google Scholar] [CrossRef]
- Bastani, A.; Asghary, A.; Karimi-Busheri, F. Evaluation of the sensitivity and specificity of serum level of prostasin, CA125, LDH, AFP, and hCG+β in epithelial ovarian cancer patients. Eur. J. Gynaecol. Oncol. 2017, 38, 418–424. [Google Scholar] [CrossRef]
- Repo, E.; Warchol, J.K.; Kurniawan, T.A.; Sillanpää, M.E. Adsorption of Co (II) and Ni (II) by EDTA-and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 161, 73–82. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, M.-H.; Peng, H.; Huang, K.-X. Removal of heavy metals from extract of Angelica sinensis by EDTA-modified chitosan magnetic adsorbent. China J. Chin. Mater. Med. 2013, 38, 3709–3712. [Google Scholar] [CrossRef]
- Monu, V.; Waseem, A.; Ju-Hyun, P.; Vinod, K.; Mikhail, S.V.; Dipti, V.; Hyunook, K. One-step functionalization of chitosan using EDTA: Kinetics and isotherms modeling for multiple heavy metals adsorption and their mechanism. J. Water Process Eng. 2022, 49, 102989. [Google Scholar] [CrossRef]
- Chibli, H.; Carlini, L.; Park, S.; Dimitrijevic, N.M.; Nadeau, J.L. Cytotoxicity of InP/ZnS quantum dots related to reactive oxygen species generation. Nanoscale 2011, 3, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, J.C.Ø.; Cropp, A.; Paradise, D.C. Solubility of indium-tin oxide in simulated lung and gastric fluids: Pathways for human intake. Sci. Total Environ. 2017, 579, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Guan, J.; Song, R.; Zhang, X.; Mao, S. Physicochemical properties of nanoparticles affecting their fate and the physiological function of pulmonary surfactants. Acta Biomater. 2022, 140, 76–87. [Google Scholar] [CrossRef]
- Olga, B.; Manon, F.; Shirin, B.; Abdullah, K.; Jennifer, C.; Christine, D.; Antonella, B. Silica Nanoparticle-Induced Structural Reorganizations in Pulmonary Surfactant Films: What Monolayer Compression Isotherms Do Not Say. ACS Appl. Nano Mater. 2018, 1, 5268–5278. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Zhou, P.; Zhang, X.; Yuan, B.; Pan, Y.; Jiang, J. The Cytotoxicity of Tungsten Ions Derived from Nanoparticles Correlates with Pulmonary Toxicity. Toxics 2023, 11, 528. https://doi.org/10.3390/toxics11060528
Yao J, Zhou P, Zhang X, Yuan B, Pan Y, Jiang J. The Cytotoxicity of Tungsten Ions Derived from Nanoparticles Correlates with Pulmonary Toxicity. Toxics. 2023; 11(6):528. https://doi.org/10.3390/toxics11060528
Chicago/Turabian StyleYao, Jun, Pengfei Zhou, Xin Zhang, Beilei Yuan, Yong Pan, and Juncheng Jiang. 2023. "The Cytotoxicity of Tungsten Ions Derived from Nanoparticles Correlates with Pulmonary Toxicity" Toxics 11, no. 6: 528. https://doi.org/10.3390/toxics11060528