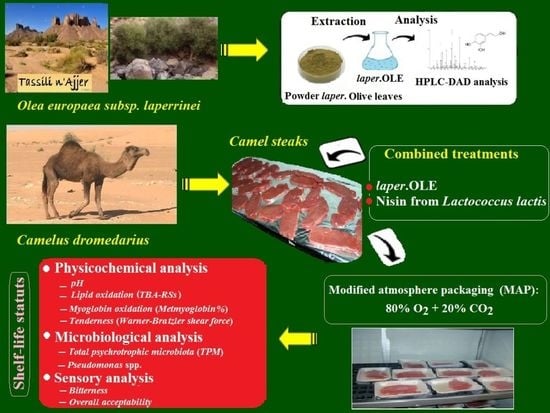
Improvement of the Shelf-Life Status of Modified Atmosphere Packaged Camel Meat Using Nisin and Olea europaea Subsp. laperrinei Leaf Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Powder and Laperrine Olive Leaf Extract and Nisin
2.2. Estimation of the Total Phenolic Compounds in the Laperrine Olive Leaf Extract
2.3. Analysis of Phenolic Constituents in laper.OLE
2.4. Camel Meat Treatment and Packaging under MA
2.5. pH of Camel Meat
2.6. Lipid Oxidation of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin: Thiobarbituric Acid-Reactive Substances (TBA-RSs)
2.7. Pigment Oxidation Analysis (MetMb%) of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
2.8. Warner-Bratzler Shear Force of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
2.9. Determination of Total Psychrotrophic and Pseudomonas spp. Counts in Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
2.10. Sensory Analysis of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
2.11. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Compounds and HPLC-DAD Analyses of laper.OLE
3.2. pH of Camel Steaks
3.3. Lipid Oxidation of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin: Thiobarbituric Acid-Reactive Substances (TBA-RSs)
3.4. Pigment Oxidation of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin: Metmyoglobin Percentage Analysis
3.5. Warner-Bratzler Shear Force of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
3.6. Microbiological Counts in Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
3.7. Bitterness of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
3.8. Correlations and Shelf-Life Status of Packaged Fresh Camel Meat Treated with laper.OLE and Nisin
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization (2005–2017). 2018. Available online: www.fao.org (accessed on 12 September 2020).
- Sahraoui, N.; Dotreppe, O.; Errahmani, M.B.; Boudjenah, S.; Baaissad, B.; Guetarni, D.; Hornick, J.-L. Caractérisation des acides gras de la viande cameline en Algérie. Cah. Nutr. Diét. 2014, 49, 231–234. [Google Scholar] [CrossRef]
- Maqsood, S.; Abushelaibi, A.; Manheem, K.; Al Rashedi, A.; Kadim, I.T. Lipid oxidation, protein degradation, microbial and sensorial quality of camel meat as influenced by phenolic compounds. LWT-Food Sci. Technol. 2015, 63, 953–959. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.; Mohamed, H.M. Improving the physico- chemical and sensory characteristics of camel meat burger patties using ginger extract and papain. Meat Sci. 2016, 118, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Beltrán, J.A.; Camo, J.; Roncalés, P. Influence of vacuum at different ageing times and subsequent retail display on shelf-life of beef cuts packaged with active film under high O2. J. Food Sci. Technol. 2016, 53, 4244–4257. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Darwish, W.S.; El-Ghareeb, W.R.; Al-Humam, N.A.; Chen, L.; Zhong, R.M.; Xiao, Z.J.; Ma, J.K. Microbial quality and formation of biogenic amines in the meat and edible offal of Camelus dromedarius with a protection trial using gingerol and nisin. Food Sci. Nutr. 2020, 8, 2094–2101. [Google Scholar] [CrossRef]
- Djenane, D.; Gómez, D.; Yangüela, J.; Roncalés, P.; Ariño, A. Olive leaves extract from algerian oleaster (Olea europaea var. Sylvestris) on microbiological safety and shelf-life stability of raw Halal minced beef during display. Foods 2019, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Lorán, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus, and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Lorán, S.; Arakrak, A.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res. Int. 2012, 45, 313–319. [Google Scholar] [CrossRef]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; O’Brien, N.M.; Kerry, J.P. The effect of lutein, sesamol, ellagic acid and olive leaf extract on lipid oxidation and oxymyoglobin oxidation in bovine and porcine muscle model systems. Meat Sci. 2009, 83, 201–208. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Christaki, E.; Botsoglou, N. Effect of dietary olive leaves and/or α-tocopheryl acetate supplementation on microbial growth and lipid oxidation of turkey breast fillets during refrigerated storage. Food Chem. 2010, 121, 17–22. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Besnard, G.; Christin, P.A.; Baali-Cherif, D.; Bouguedoura, N.; Anthelme, F. Spatial genetic structure in the Laperrine’s olive (Olea europaea subsp. laperrinei), a long-living tree from the central Saharan mountains. Heredity 2007, 99, 649–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, P.; Kasper Machado, I.; Garavaglia, J.; Terezinha Zani, V.; de Souza, D.; Dal Bosco, S.M. Polyphenols benefits of olive leaf (Olea europaea L) to human health. Nutr. Hosp. 2015, 31, 1427–1433. [Google Scholar]
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical Properties and anti-proliferative activity of Olea europaea L. leaf extracts against pancreatic cancer cells. Molecules 2015, 20, 12992–13004. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Seasonal variations in the chemical composition of Liangshan olive leaves and their antioxidant and anticancer activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef] [Green Version]
- Abts, A.; Mavaro, A.; Stindt, J.; Bakkes, P.J.; Metzger, S.; Driessen, A.J.M.; Smits, S.H.J.; Schmitt, L. Easy and rapid purification of highly active nisin. Int. J. Pept. 2011, 2011, 157145. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Gidley, M.J.; Dykes, G.A. Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Pack. Shelf Life 2017, 11, 31–33. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Ben Amarab, C.; Oulahalb, N.; Gharsallaoui, A.; Joly, C.; Tongdeesoontorn, W.; Rawdkuen, S.; Degraeve, P. Gelatin films with nisin and catechin for minced pork preservation. Food Pack. Shelf Life 2018, 18, 173–183. [Google Scholar] [CrossRef]
- Batpho, K.; Boonsupthip, W.; Rachtanapun, C. Antimicrobial activity of collagen casing impregnated with nisin against foodborne miroorganisms associated with ready-to-eat Sausage. Food Control 2017, 73, 1342–1352. [Google Scholar] [CrossRef]
- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to the use of nisin (E 234) as a food additive. Question number EFSA-Q-2005-031. EFSA J. 2006, 314, 1–16. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2006.314 (accessed on 26 January 2006).
- Kadim, I.T.; Mahgoub, O.; Purchas, R.W. A review of the growth, and of the carcass and meat quality characteristics of the one-humped camel (Camelus dromedaries). Meat Sci. 2008, 80, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Sanchéz, A.; Beltrán, J.A.; Roncalés, P. Extension of the shelf-life of beef steaks packaged in a modified atmosphere by treatment with rosemary and display under UV-free lighting. Meat Sci. 2003, 64, 417–426. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N. Lipid oxidation and color changes of fresh camel meat stored under different atmosphere packaging systems. J. Food Process. Technol. 2012, 3, 1–11. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-RaventIN, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 52–178. [Google Scholar]
- Kerth, C.R.; Rowe, C.W. Improved sensitivity for determining thiobarbituric acid reactive substances in ground beef. Meat Sci. 2016, 117, 85–88. [Google Scholar] [CrossRef]
- Pfalzgraf, A.; Frigg, M.; Steinhart, H. Alpha tocopherol contents and lipid oxidation in pork muscle and adipose tissue during storage. J. Agric. Food Chem. 1995, 43, 1339–1342. [Google Scholar] [CrossRef]
- Stewart, M.R.; Zipser, M.W.; Watts, B.M. The use of reflectance spectrophotometry for the assay of raw meat pigments. J. Food Sci. 1965, 30, 464–469. [Google Scholar] [CrossRef]
- Caine, W.R.; Aalhus, J.L.; Best, D.R.; Dugan, M.E.; Jeremiah, L.E. Relationship of texture profile analysis and Warner-Bratzler shear force with sensory characteristics of beef rib steaks. Meat Sci. 2003, 64, 333–339. [Google Scholar] [CrossRef]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation and Instrumental Tenderness Measurements of Fresh Meat; American Meat Science Association in cooperation with National Live Stock and Meat Board: Chicago, IL, USA, 1995. [Google Scholar]
- International Commission of Microbiological Specification for Foods (ICMSF). Microorganisms in Food. 1-Their Significance and Methods of Enumeration, 3rd ed.; University of Toronto Press: Toronto, ON, Canada, 1996. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Markin, D.; Duek, L.; Berdicevsky, I. In vitro antimicrobial activity of olive leaves. Mycoses 2003, 46, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M.N.; Kerry, J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Mylonaki, S.; Kiassos, E.; Makris, D.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–998. [Google Scholar] [CrossRef]
- Altiok, E.; Bayçin, D.; Bayraktar, O.; Ülkü, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Separat. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the total phenol contents and the color of fresh and infrared dried olive leaves. Indus. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods 2014, 3, 66–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altemimi, A.B. A study of the protective properties of Iraqi olive leaves against oxidation and pathogenic bacteria in food applications. Antioxidants 2017, 6, 34. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dhibi, M.; Hammami, M. Variations in phenolic compounds and antiradical scavenging activityof Olea europaea leaves and fruits extracts collected in two different seasons. Indus. Crops Prod. 2013, 49, 256–264. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Ahmad-Qasem, B.H.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Drying and storage of olive leaf extracts. Influence on polyphenols stability. Indus. Crops Prod. 2016, 79, 232–239. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013, 445–446, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leonardis, A.; Macciola, V.; Cuomo, F.; Lopez, F. Evidence of oleuropein degradation by olive leaf protein extract. Food Chem. 2015, 175, 568–574. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Conde, E.; Cara, C.; Moure, A.; Ruiz, E.; Castro, E.; Domínguez, H. Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 2008, 114, 806–812. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of freezing and dehydration of olive leaves (var. Serrana) on extract composition and antioxidant potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive tree (Olea europeae L.) leaves: Importance and advances in the analysis of phenolic compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Djenane, D.; Aboudaou, M.; Ferhat, M.A.; Ouelhadj, A.; Ariño, A. Effect of the aromatisation with summer savory (Satureja hortensis L.) essential oil on the oxidative and microbial stabilities of liquid whole eggs during storage. J. Essent. Oil Res. 2019, 31, 444–455. [Google Scholar] [CrossRef]
- Camo, J.; Djenane, D.; Beltrán, J.A.; Roncalés, P. Display life of beef packaged with an antioxidant active film as a function of the concentration of oregano extract. Meat Sci. 2011, 88, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, E.; Govaris, A.; Ambrosiadis, I.; Fletouris, D.; Botsoglou, N. Effect of olive leaf (Olea europea L.) extracts on protein and lipid oxidation of long-term frozen n-3 fatty acids-enriched pork patties. Meat Sci. 2014, 98, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to Substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N’guessan, J.D.; Dinzedi, M.R.; Guessennd, N.; Coulibaly, A.; Dosso, M.; Djaman, A.J.; Guede-Guina, F. Antibacterial activity of the aqueous extract of Thonningia sanguinea against extended-spectrum-β-lactamases (ESBL) producing Escherichia coli and Klebsiella pneumoniae strains. Trop. J. Pharm. Res. 2007, 6, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Erbay, Z.; Icier, F. The Importance and Potential Uses of Olive Leaves. Food Rev. Int. 2010, 26, 319–334. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Biores. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Belasli, A.; Aït Ouahioune, L.; Djenane, D. Utilisation du monoxyde de carbone (CO) pour le conditionnement des viandes rouges au 21ème siècle. Partie II: Un mythe ou une réalité? Viandes Prod. Carnés 2020, 36, 2–4. [Google Scholar]
- Maqsood, S.; Ahmed Al Haddad, N.; Mudgil, P. Vacuum packaging as an effective strategy to retard off-odour development, microbial spoilage, protein degradation and retain sensory quality of camel meat. LWT Food Sci. Technol. 2016, 72, 55–62. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Luño, M.; Roncalés, P.; Djenane, D.; Beltrán, J.A. Beef shelf-life in low O2 and High CO2 atmospheres containing different low CO concentrations. Meat Sci. 2000, 55, 413–419. [Google Scholar] [CrossRef]
- Djenane, D.; Sanchéz, A.; Beltrán, J.A.; Roncalés, P. Extension of the retail display life of fresh beef packaged in modified atmosphere by varying lighting conditions. J. Food Sci. 2001, 66, 181–185. [Google Scholar] [CrossRef]
- Sanchéz, A.; Djenane, D.; Torrescano, G.; Beltrán, J.A.; Roncalés, P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on color and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001, 58, 421–429. [Google Scholar] [CrossRef]
- Martínez, L.; Djenane, D.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of different concentrations of carbon dioxide and low concentration of carbon monoxide on the shelf-life of fresh pork sausages packaged in modified atmosphere. Meat Sci. 2005, 71, 563–570. [Google Scholar] [CrossRef]
- Suliman, G.M.; Alowaimer, A.N.; Hussein, E.O.S.; Ali, H.S.; Abdelnour, S.A.; Abd El-Hack, M.E.; Swelum, A.A. Chemical composition and quality characteristics of meat in three one-humped camel (Camelus dromedarius) breeds as affected by muscle type and post-mortem storage period. Animals 2019, 9, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garmyn, A.; Hardcastle, N.; Polkinghorne, R.; Lucherk, L.; Miller, M. Extending aging of beef Longissimus lumborum from 21 to 84 days post-mortem influences consumer eating quality. Foods 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.T.; Dal molin, E. Relationship between beef consumer tenderness perception and Warner–Bratzler shear force. Meat Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef]
- Vitale, M.; Pérez-Juan, M.; Lloret, E.; Arnau, J.; Realini, C.E. Effect of aging time in vacuum on tenderness, and color and lipid stability of beef from mature cows during display in high oxygen atmosphere Packaged. Meat Sci. 2014, 96, 270–277. [Google Scholar] [CrossRef]
- Zahedi, Y.; Varidi, M.-J.; Varidi, M. Proteome changes in biceps femoris muscle of Iranian one-humped camel and their effect on meat quality traits. Food Technol. Biotechnol. 2016, 54, 324–334. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.-J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.-J.E.; Skandamis, P. Fresh meat spoilage and modified atmosphere packaging (MAP). In Improving the Safety of Fresh Meat; Sofos, J.N., Ed.; CRC/Woodhead Publishing Limited: Cambridge, UK, 2005; pp. 461–502. [Google Scholar]
- Russo, F.; Ercolini, D.; Mauriello, G.; Villani, F. Behaviour of Brochotrix thermosphacta in the presence of other meat spoilage microbial group. Food Microbiol. 2006, 23, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Bhunia, A. Fundamental Food Microbiology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Fontana, C.; Cocconcelli, P.S.; Vignolo, G. Direct molecular approach to monitoring bacterial colonization on vacuum-packaged beef. Appl. Environ. Microbiol. 2006, 72, 5618–5622. [Google Scholar] [CrossRef] [Green Version]
- Ercolini, D.; Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Ferranti, P.; Mauriello, G.; Villani, F. Different molecular types of Pseudomonas fragi have the overall behavior as meat spoilers. Int. J. Food Microbiol. 2010, 142, 120–131. [Google Scholar] [CrossRef]
- Lahcene, S.; Taibi, F.; Mestar, N.; Ali Ahmed, S.; Boumendjel, M.; Ouafi, S.; Houali, K. Insecticidal effects of the Olea europaea subsp. laperrinei extracts on the flour Pyralid Ephestia kuehniella. Cell. Molec. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N.; Saija, A. On the invitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef]
- Chen, H.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of nisin after glycation with lactose, maltodextrin and dextran and the thyme oil emulsions prepared thereof. J. Food Microbiol. 2014, 191, 75–81. [Google Scholar] [CrossRef]
- Kakatkar, A.S.; Gautam, R.K.; Shashidhar, R. Combination of glazing, nisin treatment and radiation processing for shelf-life extension of seer fish (Scomberomorous guttatus) steaks. Rad. Phys. Chem. 2017, 130, 303–305. [Google Scholar] [CrossRef]
- Hasper, H.E.; de Kruijff, B.; Breukink, E. Assembly and stability of nisin-lipid II pores. Biochemistry 2004, 43, 11567–11575. [Google Scholar] [CrossRef]
- Frank, O.; Ottinger, H.; Hofmann, T. Characterization of an intense bitter-tasting 1H,4H-Quinolizinium-7-olate by application of the taste dilution analysis, a novel bioassay for the screening and identification of taste-active compounds in foods. J. Agric. Food Chem. 2001, 49, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Aouidi, F.; Ayari, S.; Ferhi, H.; Roussos, S.; Hamdi, M. Gamma irradiation of air-dried olive leaves: Effective decontamination and impact on the antioxidative properties and on phenolic compounds. Food Chem. 2011, 127, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. World Health Organization 2007. Available online: https://apps.who.int/iris/handle/10665/43510 (accessed on 1 June 2012).
- World Health Organization. WHO Guidelines on Good Herbal Processing Practices for Herbal Medicines. Annex 1: WHO Technical Report Series, No. 1010, Fifty-Second Report, 2018. Available online: https://www.who.int/traditional-complementary-integrative-medicine/publications/trs1010_annex1.pdf?ua=1 (accessed on 12 September 2020).





| Compounds | mg/g | % |
|---|---|---|
| Hydroxytyrosol | 1.63 | 5.93 |
| Tyrosol | 0.07 | 0.25 |
| Catechin | 0.10 | 0.36 |
| Caffeic acid | 0.20 | 0.72 |
| Rutin | 0.37 | 1.35 |
| Luteolin-7-glucoside | 3.11 | 11.28 |
| Verbascoside | 0.79 | 2.85 |
| Apigenin-7-glucoside | 2.24 | 8.15 |
| Diosmetin-7-glucoside | 0.21 | 0.77 |
| Oleuropein | 17.36 | 63.03 |
| Luteolin-4-glucoside | 1.46 | 5.30 |
| Total | 27.54 |
| Higher O2/CO2 Packaging Storage Period | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | 0 | 5 | 11 | 16 | 20 | 25 | 30 |
| Control | 8.75 ± 0.11 aW | 8.60 ± 0.13 aW | 8.50 ± 0.12 aW | 8.09 ± 0.18 abW | 7.40 ± 0.16 bW | 6.85 ± 0.11 bW | 5.85 ± 0.17 cW |
| Nisin ∗ | 8.75 ± 0.16 aW | 8.73 ± 0.10 aW | 8.45 ± 0.14 aW | 8.30 ± 0.17 aW | 7.13 ± 0.16 abW | 6.50 ± 0.12 bW | 5.79 ± 0.12 cW |
| laper.OLE ∗∗ | 8.75 ± 0.14 aW | 8.65 ± 0.14 aW | 8.50 ± 0.17 aW | 8.45 ± 0.10 aW | 7.53 ± 0.11 abW | 6.41 ± 0.11 bW | 5.89 ± 0.12 bcW |
| Nisin/laper.OLE | 8.75 ± 0.12 aW | 8.76 ± 0.14 aW | 8.35 ± 0.16 aW | 8.41 ± 0.15 aW | 7.04 ± 0.12 bW | 6.72 ± 0.15 bW | 5.68 ± 0.16 cW |
| Nisin/2 × laper.OLE | 8.75 ± 0.14 aW | 8.64 ± 0.13 aW | 8.55 ± 0.14 aW | 8.45 ± 0.14 aW | 7.80 ± 0.13 abW | 6.46 ± 0.10 cW | 5.65 ± 0.14 cW |
| Storage Period | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | 0 | 5 | 11 | 16 | 20 | 25 | 30 |
| Bitterness | |||||||
| Control | 1 a | 1 a | 1 a | 1 | 1 | nd | nd |
| Nisin ∗ | 1 a | 1 a | 1 a | 1 | 1 | nd | nd |
| laper.OLE ∗∗ | 1.25 ± 0.45 ab | 1.19 ± 0.40 a | 1.13 ± 0.34 a | 1 | 1 | 1 | 1 |
| Nisin/laper.OLE | 1.31 ± 0.48 ab | 1.25 ± 0.45 ab | 1.19 ± 0.40 a | 1 | 1 | 1 | 1 |
| Nisin/2 × laper.OLE | 2.31 ± 0.40 c | 1.69 ± 0.48 b | 1.50 ± 0.52 b | 1 | 1 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djenane, D.; Aboudaou, M.; Djenane, F.; García-Gonzalo, D.; Pagán, R. Improvement of the Shelf-Life Status of Modified Atmosphere Packaged Camel Meat Using Nisin and Olea europaea Subsp. laperrinei Leaf Extract. Foods 2020, 9, 1336. https://doi.org/10.3390/foods9091336
Djenane D, Aboudaou M, Djenane F, García-Gonzalo D, Pagán R. Improvement of the Shelf-Life Status of Modified Atmosphere Packaged Camel Meat Using Nisin and Olea europaea Subsp. laperrinei Leaf Extract. Foods. 2020; 9(9):1336. https://doi.org/10.3390/foods9091336
Chicago/Turabian StyleDjenane, Djamel, Malek Aboudaou, Fatiha Djenane, Diego García-Gonzalo, and Rafael Pagán. 2020. "Improvement of the Shelf-Life Status of Modified Atmosphere Packaged Camel Meat Using Nisin and Olea europaea Subsp. laperrinei Leaf Extract" Foods 9, no. 9: 1336. https://doi.org/10.3390/foods9091336