Developing a Commercial Antimicrobial Active Packaging System of Ground Beef Based on “Tsipouro” Alcoholic Distillate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antimicrobial Compound
2.2. Preparation and Storage of Ground Beef
2.3. Headspace Gas Analysis
2.4. Microbiological Analysis
2.5. PH Measurement
2.6. Model Development of B. thermosphacta and LAB Growth
2.7. Colour Measurement
2.8. Model Development of a* Value
2.9. Validation of the Developed Models for Microbial Growth and Colour Deterioration
2.10. Evaluation of Models Performance
2.11. Sensory Evaluation
2.12. SPME/GC-Flame Ionization Detector Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Headspace Gas Analysis
3.2. Kinetics of Microbiological Data and Model Development/Validation
3.3. Colour Measurements and Model Development/Validation of a* Value
3.4. Sensory Evaluation
3.5. SPME/GC-Flame Ionization Detector Analysis
4. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- FAO. World Agriculture: Towards 2015/2030: AN FAO PERSPECTIVE; Bruinsma, J., Ed.; Earthscan Publications Ltd.: London, UK, 2003; pp. 85–98. Available online: http://www.fao.org/3/y4252e/y4252e05b.htm (accessed on 24 August 2020).
- U.S. Department of Agriculture (USDA). Factors Affecting U.S. Beef Consumption. 2005. Available online: https://www.ers.usda.gov/webdocs/outlooks/37388/29633_ldpm13502_002.pdf?v=7581.2 (accessed on 24 August 2020).
- Grand View Research Inc. Beef Market Size, Share & Trends Analysis Report by Cut (Brisket, Shank, Loin), by Slaughter Method (Kosher, Halal), by Region (North America, Europe, APAC, MEA, CSA), And Segment Forecasts, 2019–2025; Report ID: 978-1-68038-175-7; Grand View Research Inc.: San Fransisco, CA, USA, 2019; pp. 1–117. [Google Scholar]
- Speer, N.; Brink, T.; Mc Cully, M. Changes in the Ground Beef Market and What It Means for Cattle Producers; The Angus Foundation: St. Joseph, MO, USA, 2015. [Google Scholar]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Review Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, Κ. Modified atmosphere packaging of meat, poultry and fish. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 475–493. [Google Scholar]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Limbo, S.; Torri, L.; Sinelli, N.; Franzetti, L.; Casiraghi, E. Evaluation and predictive modeling of shelf life of ground beef stored in high-oxygen modified atmosphere packaging at different temperatures. Meat Sci. 2010, 84, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Leistner, L. Principles and applications of hurdle technology. In New Methods of Food Preservation; Gould, G.W., Ed.; Blackie Academic and Professional: London, UK, 1995; pp. 1–21. [Google Scholar]
- Ahvenainen, R. Active and intelligent packaging: An introduction. In Novel Food Packaging Techniques; Ahvenainen, R., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2003; pp. 5–21. [Google Scholar]
- Kapetanakou, A.E.; Skandamis, P.N. Applications of active packaging for increasing microbial stability in foods: Natural volatile antimicrobial compounds. Curr. Opin. Food Sci. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Hashemi, S.M.B.; Es, I.; Fracassetti, D.; Limbo, S. Efficacy of antimicrobial agents for food contact applications: Biological activity, incorporation into packaging, and assessment methods: A review. J. Food Prot. 2018, 81, 1142–1156. [Google Scholar] [CrossRef]
- Regulation (EC) No 10/2011 of the European Parliament and of the Council of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89.
- Kapetanakou, A.E.; Agathaggelou, E.I.; Skandamis, P.N. Storage of pork meat under modified atmospheres containing vapours from commercial alcoholic beverages. Int. J. Food Microbiol. 2014, 178, 65–75. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Karyotis, D.; Skandamis, P.N. Control of Listeria monocytogenes by applying ethanol based antimicrobial edible films on ham slices and microwave-reheated frankfurters. Food Microbiol. 2016, 54, 80–90. [Google Scholar] [CrossRef]
- Mateus, D.; Ferreira, I.; Pinho, O. Headspace SPME–GC/MS evaluation of ethanol retention in cooked meals containing alcoholic drinks. Food Chem. 2011, 126, 1387–1392. [Google Scholar] [CrossRef]
- Soufleros, E.H.; Natskoulis, P.; Mygdalia, A.S. Discrimination and risk assessment due to the volatile compounds and the inorganic elements present in the Greek marc distillates tsipouro and tsikoudia. J. Int. Sci. Vigne Vin 2005, 39, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, description, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No 1576/89. Off. J. Eur. Union 2008, 51, 16–54.
- Pappas, C.; Basalekou, M.; Konstantinou, E.; Proxenia, N.; Kallithraka, S.; Kotseridis, Y.; Taranilis, P.A. Evaluation of a raman spectroscopic method for the determination of alcohol content in Greek spirit Tsipouro. Cur. Res. Nutr. Food Sci. J. 2016, 4, 01–09. [Google Scholar] [CrossRef]
- Dalgaard, P. Modelling of microbial activity and prediction of shelf life for packed fish. Int. J. Food Microbiol. 1995, 26, 305–307. [Google Scholar] [CrossRef]
- Ercolini, D.; Ferrocino, I.; Nasi, A.; Ndagijimana, M.; Vernocchi, P.; La Storia, A.; Laghi, L.; Gianluigi, M.; Guerzoni, E.M.; Villani, F. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions. Appl. Environ. Microbiol. 2011, 77, 7372–7381. [Google Scholar] [CrossRef] [Green Version]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Lowry, R.K.; McMeekin, T.A.; Stokes, A.N.; Chandler, R.E. Model for bacterial cultures growth rate throughout the entire biokinetic temperature range. J. Bacteriol. 1983, 154, 1222–1226. [Google Scholar] [CrossRef] [Green Version]
- Tsironi, T.N.; Taoukis, P.S. Shelf-life extension of gilthead seabream fillets by osmotic treatment and antimicrobial agents. J. Appl. Microbiol. 2011, 112, 316–328. [Google Scholar] [CrossRef]
- Huang, L. IPMP 2013—A comprehensive data analysis tool for predictive microbiology. Int. J. Food Microbiol. 2014, 171, 100–107. [Google Scholar] [CrossRef]
- Taoukis, P.S.; Labuza, T.P.; Saguy, I.S. Kinetics of food deterioration and shelf-life prediction. In Handbook of Food Engineering Practice; Valentas, K.J., Rotstein, E., Singh, R.P., Eds.; RC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Manios, S.; Skiadaresis, A.; Karavasilis, K.; Drosinos, E.; Skandamis, P. Field validation of predictive models for the growth of lactic acid bacteria in acidic cheese- based Greek appetizers. J. Food Prot. 2009, 72, 101–110. [Google Scholar] [CrossRef]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996, 81, 231–245. [Google Scholar]
- Ross, T. Predictive modelling of the growth and survival of Listeria in fishery products. Int. J. Food Microbiol. 2000, 62, 231–245. [Google Scholar] [CrossRef]
- Delignette-Muller, M.L.; Rosso, L.; Flandrois, J.P. Accuracy of microbial growth predictions with square root and polynomial models. Int. J. Food Microbiol. 1995, 27, 139–146. [Google Scholar] [CrossRef]
- Oscar, T.P. Validation of lag time and growth rate models for Salmonella typhimurium: Acceptable prediction zone method. Food Microbiol. Saf. 2005, 70, M129–M137. [Google Scholar]
- Sun, X.D.; Holley, R. Antimicrobial and antioxidative strategies to reduce pathogens and extend the shelf-life of fresh red meats. Compr. Rev. Food Sci. Food Saf. 2012, 11, 340–354. [Google Scholar] [CrossRef]
- Hilgarth, M.; Behr, J.; Vogel, R.F. Monitoring of spoilage-associated microbiota on modified atmosphere packaged beef and differentiation of psychrophilic and psychrotrophic strains. J. Appl. Microbiol. 2017, 124, 740–753. [Google Scholar] [CrossRef]
- Nowak, A.; Kalemba, D.; Krala, L.; Piotrowska, M.; Czyzowska, A. The effects of thyme (Thymus vulgaris) and rosemary (Rosmarinus officinalis) essential oils on Brochothrix thermosphacta and on the shelf life of beef packaged in high-oxygen modified atmosphere. Food Microbiol. 2012, 32, 212–216. [Google Scholar] [CrossRef]
- Moore, E.R.B.; Tindall, B.J.; Dos Santos, V.A.P.; Pieper, D.H.; Ramos, J.-L.; Palleroni, N.J. Nonmedical: Pseudomonas. In The prokaryotes: A Handbook on the Biology of Bacteria; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 646–703. [Google Scholar]
- Koutsoumanis, K.; Nychas, G.E.; Drosinos, E.H.; Nychas, G.E. Control of spoilage microorganisms in minced pork by a self-developed modified atmosphere induced by the respiratory activity of meat microflora. Food Microbiol. 2008, 25, 915–921. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Stamatiou, A.; Skandamis, P.; Nychas, G.E. Development of a microbial model for the combined effect of temperature and pH on spoilage of ground meat, and validation of the model under dynamic temperature conditions. Appl. Environ. Microbiol. 2006, 72, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Vaikousi, H.; Biliaderis, C.G.; Koutsoumanis, K.P. Applicability of a microbial Time Temperature Indicator (TTI) for monitoring spoilage of modified atmosphere packed minced meat. Int. J. Food Microbiol. 2009, 133, 272–278. [Google Scholar] [CrossRef]
- Smith, G.C.; Belk, K.E.; Sofos, J.N.; Tatum, J.D.; Williams, S.N. Economic implications of improved color stability in beef. In Antioxidants in Muscle Foods-Nutritional Strategies to Improve Quality; Decker, E.A., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley and Sons, Inc.: Chichester, UK, 2000; pp. 397–426. [Google Scholar]
- Trinderup, C.H.; Dahl, A.; Jensen, K.; Michael, J.; Conradsen, K. Comparison of a multispectral vision system and a colorimeter for the assessment of meat color. Meat Sci. 2015, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Giannakourou, M.; Strati, I.F.; Manika, E.-M.; Resiti, V.; Tataridis, P.; Zoumpoulakis, P.; Sinanoglou, V. Assessment of phenolic content, antioxidant activity, colour and sensory attributes of wood aged “Tsipouro”. Curr. Res. Nutr. Food Sci. Food Saf. 2018, 6, 318–328. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat colour. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Iulietto, M.; Cenci-Goga, B. Meat spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 2015, 14, 4011. [Google Scholar] [CrossRef]

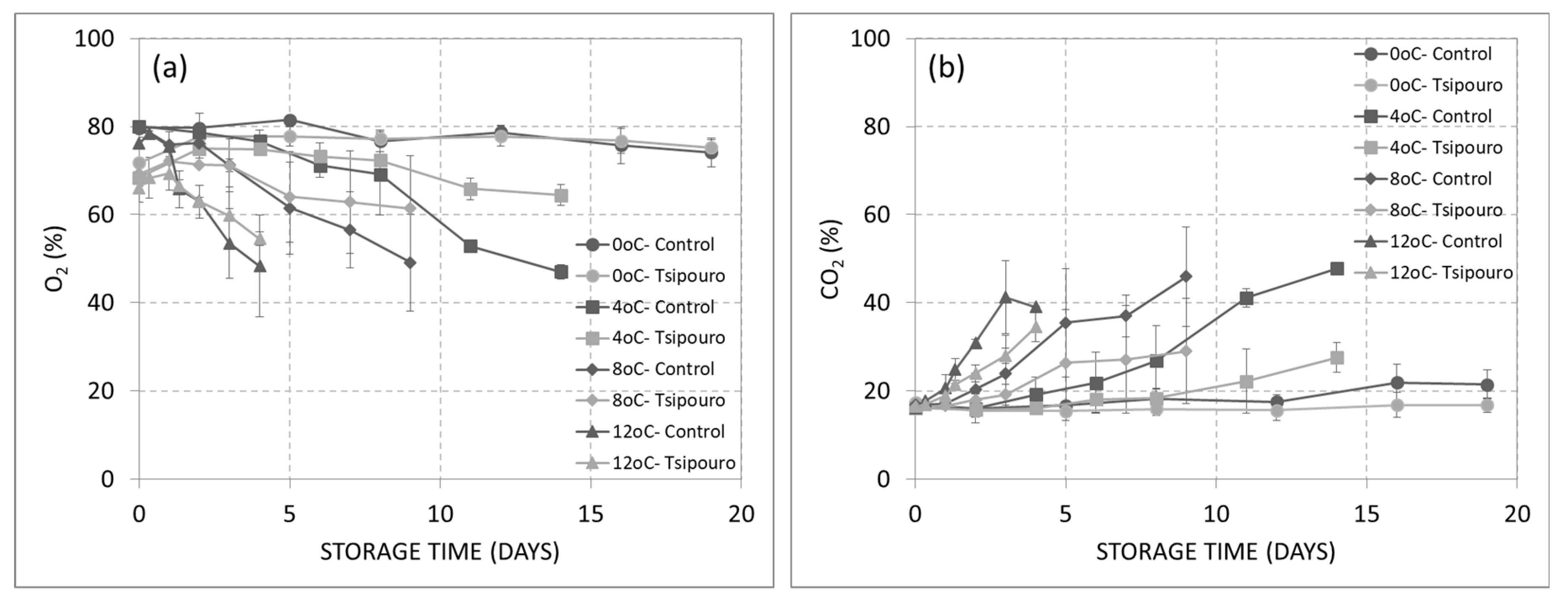

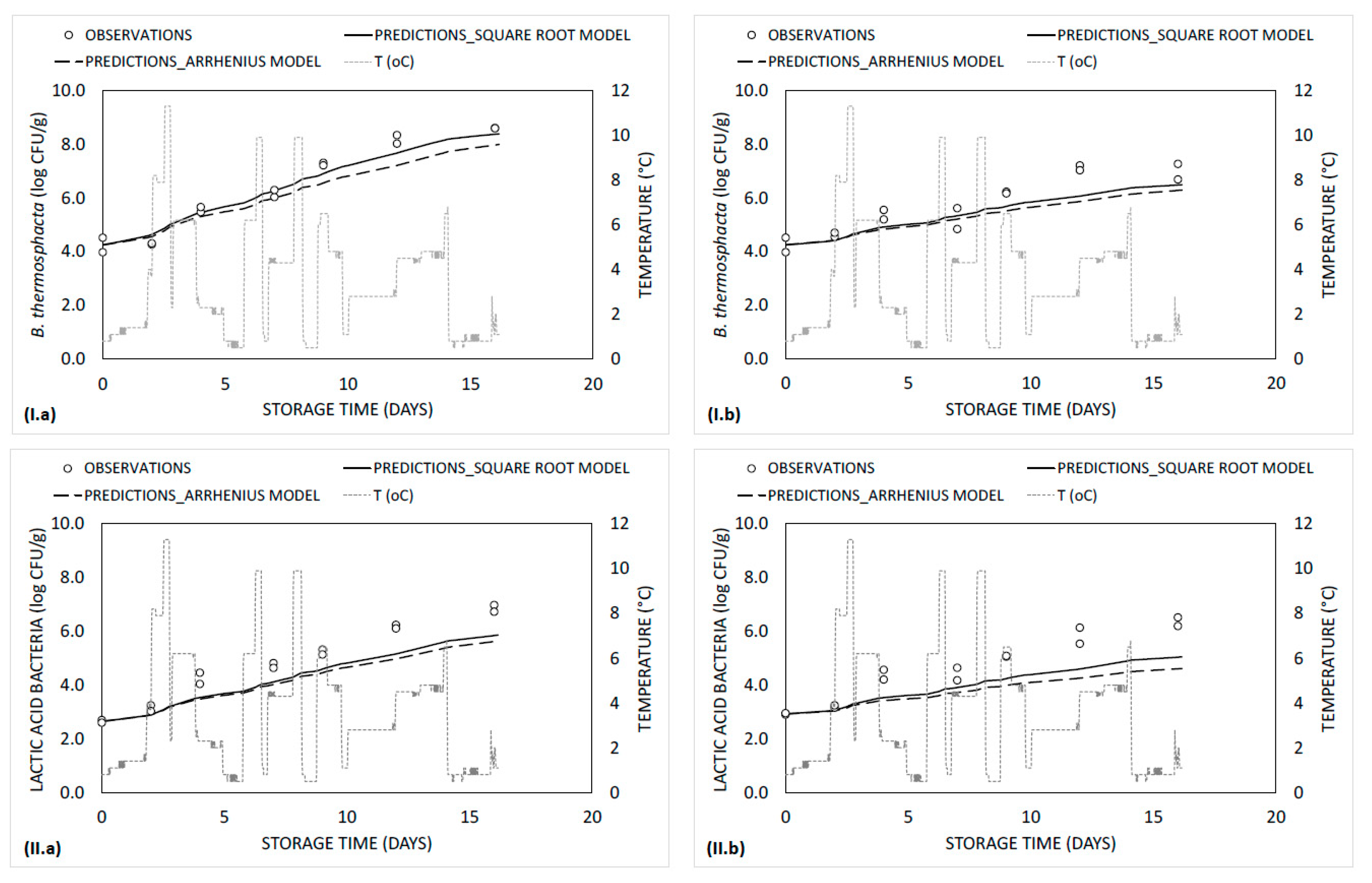


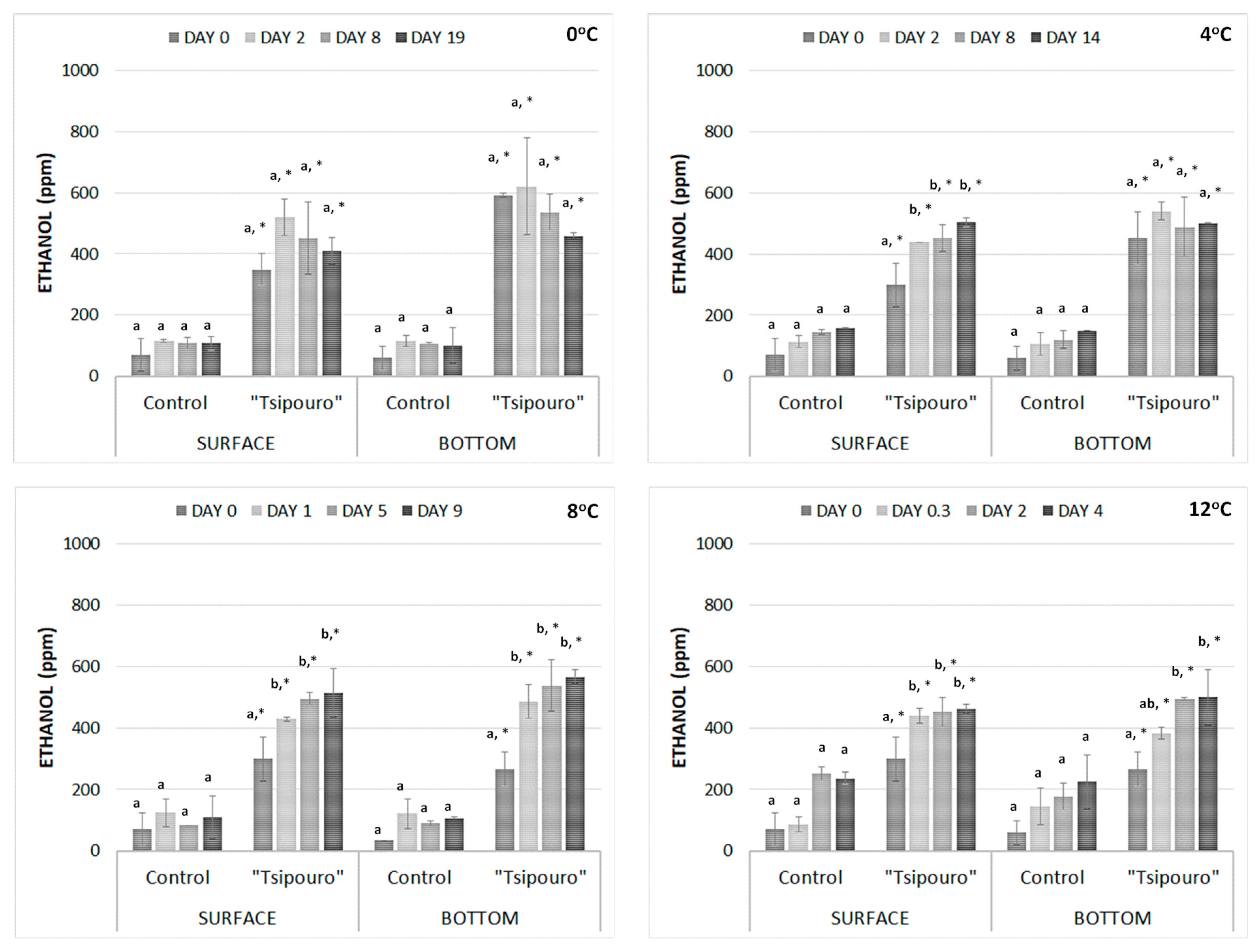
| Microorganism/ Treatment | Temperature (°C) | Ν0 (log CFU/g) 1 | Νmax (log CFU/g) 1 | µmax (day−1) 1 | λ (days) |
|---|---|---|---|---|---|
| B. thermosphacta | |||||
| Control | 0 | 3.91 ± 0.96 A | 7.00 ± 0.56 A | 0.36 ± 0.14 A | N.A. 2 |
| 4 | 4.75 ± 0.75 AB | 8.32 ± 0.31 C | 0.65 ± 0.18 B | N.A. | |
| 8 | 4.18 ± 1.27 AB | 7.55 ± 0.34 AB | 1.26 ± 0.06 C | N.A. | |
| 12 | 5.82 ± 0.21 B | 8.04 ± 0.24 BC | 1.46 ± 0.06 C | N.A. | |
| “Tsipouro” | 0 | 4.10 ± 0.89 A | 6.38 ± 0.45 A | 0.14 ± 0.04 A* | N.A. |
| 4 | 5.06 ± 1.09 A | 7.27 ± 0.55 AB* | 0.37 ± 0.16 A* | N.A. | |
| 8 | 4.14 ± 1.08 A | 6.66 ± 0.50 A* | 0.72 ± 0.26 B* | N.A. | |
| 12 | 5.85 ± 0.17 A | 7.93 ± 0.20 B | 1.15 ± 0.11 C | N.A. | |
| LAB | |||||
| Control | 0 | 2.92 ± 0.31 A | 6.51 ± 0.39 A | 0.28 ± 0.02 A | N.A. |
| 4 | 3.62 ± 0.53 AB | 6.55 ± 0.14 A | 0.45 ± 0.19 A | N.A. | |
| 8 | 4.44 ± 1.47 B | 6.85 ± 0.65 A | 0.87 ± 0.04 B | N.A. | |
| 12 | 3.89 ± 0.34 AB | 6.68 ± 0.06 A | 1.64 ± 0.07 C | N.A. | |
| “Tsipouro” | 0 | 3.11 ± 0.21 A | 6.74 ± 0.77 A | 0.23 ± 0.05 A | N.A. |
| 4 | 3.90 ± 0.95 A | 6.13 ± 1.11 A | 0.17 ± 0.06 A* | N.A. | |
| 8 | 4.31 ± 1.36 A | 6.75 ± 0.67 A | 0.65 ± 0.16 B* | N.A. | |
| 12 | 3.92 ± 0.35 A | 6.61 ± 0.19 A | 1.46 ± 0.06 C* | N.A. | |
| Parameters and Statistical Indices | B. thermosphacta | LAB | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | “Tsipouro” | Control | “Tsipouro” | |||||
| Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | |
| Square Root Model | ||||||||
| b | 0.054 ± 0.005 | 0.000 | 0.059 ± 0.006 | 0.000 | 0.059 ± 0.008 | 0.000 | 0.063 ± 0.009 | 0.000 |
| T0 (°C) | −11.3 ± 1.6 | 0.000 | −6.2 ± 1.2 | 0.000 | −7.8 ± 1.3 | 0.000 | −4.9 ± 1.6 | 0.000 |
| R2 | 0.865 | 0.782 | 0.805 | 0.760 | ||||
| RMSE | 0.017 | 0.016 | 0.019 | 0.029 | ||||
| Arrhenius Model | ||||||||
| Ea (kJ/mol) | 90.2 ± 12.2 | 0.000 | 87.2 ± 17.1 | 0.002 | 92.8 ± 10.4 | 0.000 | 124.8 ± 15.0 | 0.000 |
| ln kref (day−1) | −0.586 ± 0.092 | 0.001 | −1.167 ± 0.129 | 0.000 | −0.833 ± 0.081 | 0.000 | −1.448 ± 0.113 | 0.000 |
| R2 | 0.901 | 0.813 | 0.859 | 0.831 | ||||
| RMSE | 0.057 | 0.111 | 0.082 | 0.173 | ||||
| Sampling Side | Treatment | Temperature (°C) | Kcolour (day−1) | R2 |
|---|---|---|---|---|
| Surface | Control | 0 | −0.852 ± 0.095 A | 0.976 |
| 4 | −1.079 ± 0.028 A | 0.949 | ||
| 8 | −2.060 ± 0.093 B | 0.937 | ||
| 12 | −5.322 ± 0.032 C | 0.971 | ||
| “Tsipouro” | 0 | −0.343 ± 0.004 A* | 0.769 | |
| 4 | −0.737 ± 0.057 AB* | 0.961 | ||
| 8 | −0.838 ± 0.047 AB* | 0.888 | ||
| 12 | −0.953 ± 0.284 B* | 0.887 | ||
| Bottom | Control | 0 | −0.472 ± 0.074 A | 0.846 |
| 4 | −1.027 ± 0.021 A | 0.972 | ||
| 8 | −1.039 ± 0.153 A | 0.869 | ||
| 12 | −2.966 ± 0.532 B | 0.906 | ||
| “Tsipouro” | 0 | −0.242 ± 0.031 A* | 0.775 | |
| 4 | −0.578 ± 0.028 B* | 0.901 | ||
| 8 | −0.823 ± 0.045 C* | 0.913 | ||
| 12 | −1.261 ± 0.088 D* | 0.837 |
| Parameters and Statistical Indices | Control | “Tsipouro” | ||
|---|---|---|---|---|
| Estimated Value | p-Value | Estimated Value | p-Value | |
| Surface | ||||
| Ea (kJ/mol) | 99.2 ± 11.3 | 0.000 | 63.6 ± 10.6 | 0.002 |
| ln kref (day−1) | 0.279 ± 0.086 | 0.017 | −0.532 ± 0.070 | 0.001 |
| R2 | 0.927 | 0.878 | ||
| RMSE | 0.049 | 0.032 | ||
| Bottom | ||||
| Ea (kJ/mol) | 89.0 ± 14.1 | 0.001 | 86.1 ± 8.0 | 0.000 |
| ln kref (day−1) | −0.172 ± 0.106 | 0.156 | −0.743 ± 0.061 | 0.000 |
| R2 | 0.870 | 0.950 | ||
| RMSE | 0.076 | 0.025 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapetanakou, A.E.; Pateraki, G.-L.; Skandamis, P.N. Developing a Commercial Antimicrobial Active Packaging System of Ground Beef Based on “Tsipouro” Alcoholic Distillate. Foods 2020, 9, 1171. https://doi.org/10.3390/foods9091171
Kapetanakou AE, Pateraki G-L, Skandamis PN. Developing a Commercial Antimicrobial Active Packaging System of Ground Beef Based on “Tsipouro” Alcoholic Distillate. Foods. 2020; 9(9):1171. https://doi.org/10.3390/foods9091171
Chicago/Turabian StyleKapetanakou, Anastasia E., Georgia-Lito Pateraki, and Panagiotis N. Skandamis. 2020. "Developing a Commercial Antimicrobial Active Packaging System of Ground Beef Based on “Tsipouro” Alcoholic Distillate" Foods 9, no. 9: 1171. https://doi.org/10.3390/foods9091171




