Physicochemical Properties and Resistant Starch Content of Corn Tortilla Flours Refrigerated at Different Storage Times
Abstract
:1. Introduction
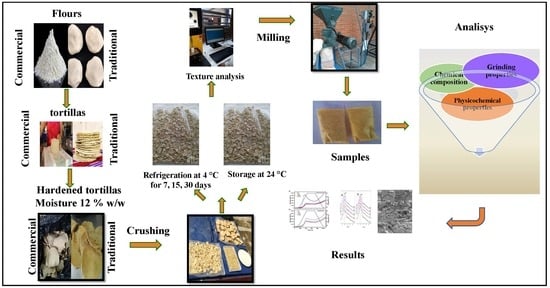
2. Materials and Methods
2.1. Preparation of Experimental Samples
2.2. Determination of the Power and Energy Consumption Average for Grinding
2.3. Texture Analysis of Hardened Tortillas
2.4. Proximal Chemical Analysis
2.5. Resistant Starch Content Analysis
2.6. Calcium Content Analysis
2.7. Color Measurement
2.8. Particle Size Distribution
2.9. Water Absorption Index (WAI) and Water Solubility Index (WSI)
2.10. Determination of Apparent Viscosity Profile
2.11. X-Ray Diffraction Analysis
2.12. Morphological Study
2.13. Differential Scanning Calorimetry (DSC) Thermal Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Power and Energy Consumption for Grinding
3.2. Chemical Composition of Traditional and Industrial Corn Tortilla Flours
3.3. Physicochemical Properties of Traditional and Commercial Tortilla Corn Flours
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kahan, S.; Zvenyach, T. Obesity as a disease: Current policies and implications for the Future. Curr. Obes. Rep. 2016, 5, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Pierlot, R.; Cuevas-Romero, E.; Rodríguez-Antolín, J.; Méndez-Hernández, P.; Martínez-Gómez, M. Prevalencia de síndrome metabólico en niños y adolescentes de América. Rev. Espec. Cienc. Quím.-Biol. 2017, 20, 40–49. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, M.A.; Yáñez-Velasco, L.; Carnevale, A.; Romero-Hidalgo, S.; Bernal, D.; Aguilar-Salinas, C.; Rojas, R.; Villa, A.; Tur, J.A. Prevalence of metabolic syndrome among elderly Mexicans. Arch. Gerontol. Geriat. 2017, 73, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.P. A current look at nutraceuticals—key concepts and future prospects. Trends Food Sci. Technol. 2017, 62, 68–78. [Google Scholar] [CrossRef]
- Fardet, A. Complex foods versus functional foods, nutraceuticals and dietary supplements: Differential health impact (Part 1). Agro. Food Ind. Hi Tech 2015, 26, 20–24. [Google Scholar]
- Mikulíková, D.; Masár, S.; Kraic, J. Biodiversity of legume health-promoting starch. Starch Stärke 2008, 60, 426–432. [Google Scholar] [CrossRef]
- Figueroa-Cárdenas, J.D.; Véles-Medina, J.J.; Esquivel-Martínez, A.M.; Mariscal-Moreno, R.M.; Santiago-Ramos, D.; Hernández-Estrada, Z.J. Effect of processing procedure on the formation of resistant starch in tamales. Starch Staerke 2016, 68, 1121–1128. [Google Scholar] [CrossRef]
- Mariscal-Moreno, R.M.; Figueroa-Cárdenas, J.D.; Santiago-Ramos, D.; Rayas-Duarte, P.; Véles-Medina, J.; Martínez-Flores, H.E. Nixtamalization process affects resistant starch formation and glycemic index of tamales. J. Food Sci. 2017, 82, 1110–1115. [Google Scholar] [CrossRef]
- Islas-Hernández, J.J.; Rendón-Villalobos, R.; Agama-Acevedo, E.; Gutiérrez-Meraz, F.; Tovar, J.; Arámbula-Villa, G.; Bello-Pérez, L.A. In vitro digestion rate and resistant starch content of tortillas stored at two different temperatures. LWT Food Sci. Technol. 2006, 39, 947–951. [Google Scholar] [CrossRef]
- Vargas, S.G. El mercado de harina de maíz en México. Una interpretación microeconómica. Econ. Inf. 2017, 405, 1–26. [Google Scholar]
- Ortega, O. El Financiero. Available online: https://www.elfinanciero.com.mx/economia/mexicanos-desperdician-de-cada-10-tortillas (accessed on 17 March 2020).
- Gutiérrez, E.; Rojas-Molina, I.; Pons-Hernández, J.L.; Guzmán, H.; Aguas-Angel, B.; Arenas, J.; Fernández, P.; Palacios-Fonseca, A.; Herrera, G.; Rodríguez, M.E. Study of calcium ion diffusion in nixtamalized quality protein maize as a function of cooking temperature. Cereal Chem. 2007, 84, 186–194. [Google Scholar] [CrossRef]
- The Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Gutiérrez-Cortez, E.; Rojas-Molina, J.I.; Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M.; Rojas-Molina, A.; Espinosa-Arbeláez, D.G. Effect of processing conditions on the production of nixtamalized corn flours by the traditional method. CyTA J. Food 2013, 11, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Martínez, L.A.; Castillo-Vázquez, K.; Figueroa-Cárdenas, J.D.; Morales-Castro, J.; Gallegos-Infante, J.A. Quality evaluation of tortilla chips made with corn meal dough and cooked bean flour. Cogent Food Agric. 2016, 2, 1136017. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. Approved Methods; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Tobias, J.R.; Peñarubia, O.R.; Castro, I.J.; Adona, C.E.; Castante, R.B. Characterization of flour made from Philippine-grown sorghum (Sorghum bicolor L. Moench using different pre-processing treatments. Int. Food Res. J. 2018, 25, 2391–2398. [Google Scholar]
- American Society of Agricultural and Biological Engineers. Method of Determining and Expressing Fineness of Feed Materials by Sieving; ASAE: St. Joseph, MI, USA, 2008; p. 1. [Google Scholar]
- Anderson, R.A. Water absorption and solubility and amylograph characteristics of roll-cooked small grain products. Cereal Chem. 1982, 59, 265–269. [Google Scholar]
- Rincón-Londoño, N.; Vega-Rojas, L.J.; Contreras-Padilla, M.; Acosta-Osorio, A.A.; Rodríguez-García, M.E. Analysis of the pasting profile in corn starch: Structural, morphological, and thermal transformations, Part I. Int. J. Biol. Macromol. 2016, 91, 106–114. [Google Scholar] [CrossRef]
- Vega-Rojas, L.J.; Rojas-Molina, I.; Gutiérrez-Cortez, E.; Rincón-Londoño, N.; Acosta-Osorio, A.A.; Del Real-López, A.; Rodríguez-García, M.E. Physicochemical properties of nixtamalized corn flours with and without germ. Food Chem. 2017, 220, 490–497. [Google Scholar] [CrossRef]
- Preciado-Ortiz, R.E.; Vázquez-Carrillo, M.G.; de Dios Figueroa-Cárdenas, J.; Guzmán-Maldonado, S.H.; Santiago-Ramos, D.; Topete-Betancourt, A. Fatty acids and starch properties of high-oil corn hybrids during nixtamalization and tortilla-making process. J. Cereal Sci. 2018, 83, 171–179. [Google Scholar] [CrossRef]
- Amador-Rodríguez, K.Y.; Silos-Espino, H.; Valera-Montero, L.L.; Perales-Segovia, C.; Flores-Benítez, S.; Martínez-Bustos, F. Physico-chemical, thermal, and rheological properties of nixtamalized creole corn flours produced by high-energy milling. Food Chem. 2019, 283, 481–488. [Google Scholar] [CrossRef]
- Santiago-Ramos, D.; de Figueroa-Cárdenas, J.D.; Véles-Medina, J.J.; Mariscal-Moreno, R.M.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Gaytán-Martínez, M.; Morales-Sánchez, E. Resistant starch formation in tortillas from an ecological nixtamalization process. Cereal Chem. 2015, 92, 185–192. [Google Scholar] [CrossRef]
- Di Monaco, R.; Cavella, S.; Masi, P. Predicting sensory cohesiveness, hardness and springiness of solid foods from instrumental measurements. J. Texture Stud. 2008, 39, 129–149. [Google Scholar] [CrossRef]
- Matiacevich, S.B.; Mery, D.; Pedreschi, F. Prediction of mechanical properties of corn and tortilla chips by using computer vision. Food Bioproc. Tech. 2012, 5, 2025–2030. [Google Scholar] [CrossRef]
- Yang, Z.; Swedlund, P.; Gu, Q.; Hemar, Y.; Chaieb, S. Retrogradation of maize starch after high hydrostatic pressure gelation: Effect of amylose content and depressurization rate. PLoS ONE 2016, 11, e0156061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NMX-F-046-S-1980. Norma Oficial Mexicana. Harina de maíz nixtamalizado. Dirección General de Normas. Secretaría de Comercio y Fomento Industrial de los Estados Unidos Mexicanos.
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Figueroa-Cardenas, J.D.; Lopez-Valenzuela, J.A.; Campanella, O.H. Effect of the nixtamalization with calcium carbonate on the indigestible carbohydrate content and starch digestibility of corn tortilla. J. Cereal Sci. 2014, 60, 421–425. [Google Scholar] [CrossRef]
- Morales, J.C.; Zepeda, R.A.G. Effect of different corn processing techniques in the nutritional composition of nixtamalized corn tortillas. J. Nutr. Food Sci. 2017, 7. [Google Scholar] [CrossRef]
- Rojas-Molina, I.; Gutiérrez, E.; Rojas, A.; Cortés-Álvarez, M.; Campos-Solís, L.; Hernández-Urbiola, M.; Arjona, J.L.; Cornejo, A.; Rodríguez-García, M.E. Effect of temperature and steeping time on calcium and phosphorus. Cereal Chem. 2009, 516–521. [Google Scholar] [CrossRef]
- Palacios-Fonseca, A.J.; Vazquez-Ramos, C.; Rodríguez-García, M.E. Physicochemical characterizing of industrial and traditional nixtamalized corn flours. J. Food Eng. 2009, 93, 45–51. [Google Scholar] [CrossRef]
- González, R.; Reguera, E.; Mendoza, L.; Figueroa, J.M.; Sánchez-Sinencio, F. Physicochemical changes in the hull of corn grains during their alkaline cooking. J. Agric. Food Chem. 2004, 52, 3831–3837. [Google Scholar] [CrossRef]
- Martínez-Bustos, F.; Martínez-Flores, H.E.; Sanmartín-Martínez, E.; Sánchez-Sinencio, F.; Chang, Y.K.; Barrera-Arellano, D.; Rios, E. Effect of the components of maize on the quality of masa and tortillas during the traditional nixtamalisation process. J. Sci. Food Agric. 2001, 81, 1455–1462. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Rendón-Villalobos, R.; Tovar, J.; Paredes-López, O.; Islas-Hernández, J.J.; Bello-Pérez, L.A. In vitro starch digestibility changes during storage of maize flour tortillas. Nahrung Food 2004, 48, 38–42. [Google Scholar] [CrossRef]
- Rendón-Villalobos, R.; Agama-Acevedo, E.; Islas-Hernández, J.J.; Sánchez-Muñoz, J.; Bello-Pérez, L.A. In vitro starch bioavailability of corn tortillas with hydrocolloids. Food Chem. 2006, 97, 631–636. [Google Scholar] [CrossRef]
- Enriquez-Castro, C.M.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Quintero-Ramos, A.; Ledesma-Osuna, A.I.; López-Cervantes, J.; Gerardo-Rodríguez, J.E. Physicochemical, rheological, and morphological characteristics of products from traditional and extrusión nixtamalization processes and their relation to starch. Int. J. Food Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Gurkin, S. Hydrocolloids—Ingredients that add flexibility to tortilla processing. Cereal Food World 2002, 47, 41–43. [Google Scholar]
- Platt-Lucero, L.C.; Ramirez-Wong, B.; Torres-Chávez, P.I.; López-Cervantes, J.; Sánchez-Machado, D.I.; Reyes-Moreno, C.; Milán-Carrillo, J.; Morales-Rosas, I. Improving textural characteristics of tortillas by adding gums during extrusion to obtain nixtamalized corn flour. J. Texture Stud. 2010, 41, 736–755. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Gao, Q. Effect of resistant starch as dietary fiber substitute on cookies quality evaluation. Food Sci. Technol. Res. 2014, 20, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Winger, M.; Khouryieh, H.; Aramouni, F.; Herald, T. Sorghum flour characterization and evaluation in gluten-free flour tortilla. J. Food Qual. 2014, 37, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Salazar, R.; Arámbula-Villa, G.; Luna-Bárcenas, G.; Figueroa-Cárdenas, J.D.; Azuara, E.; Vázquez-Landaverde, P.A. Effect of added calcium hydroxide during corn nixtamalization on acrylamide content in tortilla chips. LWT Food Sci. Technol. 2014, 56, 87–92. [Google Scholar] [CrossRef]
- Liu, K.S. Effects of particle size distribution, compositional and color properties of ground corn on quality of distillers dried grains with solubles (DDGS). Bioresour. Technol. 2009, 100, 4433–4440. [Google Scholar] [CrossRef]
- Sahai, D.; Buendia, M.O.; Jackson, D.S. Analytical techniques for understanding nixtamalized corn flour: Particle size and functionality relationships in a masa flour sample. Cereal Chem. 2001, 78, 14–18. [Google Scholar] [CrossRef]
- Carvalho, C.W.P.; Takeiti, C.Y.; Onwulata, C.I.; Pordesimo, L.O. Relative effect of particle size on the physical properties of corn meal extrudates: Effect of particle size on the extrusion of corn meal. J. Food Eng. 2010, 98, 103–109. [Google Scholar] [CrossRef]
- Liu, T.Y.; Ma, Y.; Yu, S.F.; Shi, J.; Xue, S. The effect of ball milling treatment on structure and porosity of maize starch granule. Innov. Food Sci. Emerg. 2011, 12, 586–593. [Google Scholar] [CrossRef]
- Olguín-Arteaga, G.M.; Amador-Hernández, M.; Quintanar-Guzmán, A.; Díaz-Sánchez, F.; Sánchez-Ortega, I.; Castañeda-Ovando, A.; Ávila-Pozos, R.; Santos-López, E.M. Correlation between gelatinization enthalpies, water absorption index and water soluble index in grits, corn meals and nixtamalized corn flours. Rev. Mex. Ing. Quim. 2015, 4, 303–310. [Google Scholar]
- Yousf, N.; Nazir, F.; Salim, R.; Ahsan, H.; Sirwal, A. Water solubility index and water absorption index of extruded product from rice and carrot blend. J. Pharmacogn. Phytochem. 2017, 6, 2165–2168. [Google Scholar]
- Campus-Baypoli, O.N.; Rosas-Burgos, E.C.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Serna-Saldívar, S.O. Physiochemical changes of starch during maize tortilla production. Starch Stärke 1999, 51, 173–177. [Google Scholar] [CrossRef]
- Neder-Suárez, D.; Amaya-Guerra, C.; Quintero-Ramos, A.; Pérez-Carrillo, E.; Alanís-Guzmán, M.; Báez-González, J.; García-Díaz, C.; Núñez-González, M.; Lardizábal-Gutiérrez, D.; Jiménez-Castro, J. Physicochemical changes and resistant-starch content of extruded cornstarch with and without storage at refrigerator temperatures. Molecules 2016, 21, 1064. [Google Scholar] [CrossRef] [Green Version]
- Yadav, B.S.; Sharma, A.; Yadav, R.B. Resistant starch content of conventionally boiled and pressure-cooked cereals, legumes and tubers. J. Food Sci. Tech. 2010, 47, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, S.; Koksel, H.; Ng, P.K.W. Production of resistant starch from acid-modified amylotype starches with enhanced functional properties. J. Food Eng. 2011, 103, 156–164. [Google Scholar] [CrossRef]
- Yau, J.C.; Waniska, R.D.; Rooney, L.W. Effects of food additives on storage stability of corn tortillas. Cereal Chem. 1994, 39, 396–402. [Google Scholar]
- Flores, F.R.; Martínez, B.F.; Salinas, M.Y.; Ríos, E. Caracterización de harinas comerciales de maíz nixtamalizado. Agrociencia 2002, 36, 557–567. [Google Scholar]
- Calderón-Peralta, C.V.; Jiménez-Hernández, J.; Maldonado-Astudillo, Y.I.; Flores-Casamayor, V.; Arámbula-Villa, G.; Salazar, R. Influence of Hymenaea courbaril gum as a new additive on nixtamalized flour properties and quality of tortilla. J. Food Process Eng. 2017, 40, e12525. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Rosas-Burgos, E.C.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Serna-Saldívar, S.O. Physicochemical changes of starch in maize tortillas during storage at room and refrigeration temperatures. Starch Stärke 2002, 54, 358–363. [Google Scholar] [CrossRef]
- Shi, L.; Li, W.; Sun, J.; Qiu, Y.; Wei, X.; Luan, G.; Hu, Y.; Tatsumi, E. Grinding of maize: The effects of fine grinding on compositional, functional and physicochemical properties of maize flour. J. Cereal Sci. 2016, 68, 25–30. [Google Scholar] [CrossRef]
- Rojas-Molina, I.; Gutierrez-Cortez, E.; Palacios-Fonseca, A.; Baños, L.; Pons-Hernandez, J.L.; Guzmán-Maldonado, S.H.; Pineda-Gomez, P.; Rodríguez, M.E. Study of structural and thermal changes in endosperm of quality protein maize during traditional nixtamalization process. Cereal Chem. 2007, 84, 304–312. [Google Scholar] [CrossRef]
- Shi, X.; BeMiller, J.N. Effects of food gums on viscosities of starch suspensions during pasting. Carbohydr. Polym. 2002, 50, 7–18. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications-A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, W.R.; Hughes, J.G.; Cockman, R.W.; Small, D.M. The effects of temperature on the crystalline properties and resistant starch during storage of white bread. Food Chem. 2017, 228, 57–61. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Ponce-García, N.; Ramírez-Wong, B.; Santiago-Ramos, D.; Véles-Medina, J.J.; de Dios Figueroa Cárdenas, J. Effect of extrusion factors and particle size on starch properties of nixtamalized whole blue corn snacks. Starch Staerke 2016, 68, 1111–1120. [Google Scholar] [CrossRef]
- Zobel, H.F. Starch crystal transformations and their industrial importance. Starch Stärke 1988, 40, 1–7. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Hanna, M.A. Amylose-lipid complex formation during single screw extrusion of various corn starches. Cereal Chem. 1994, 71, 582–587. [Google Scholar]
- Santiago-Ramos, D.; Figueroa-Cárdena, J.D.; Véles-Medina, J.J.; Mariscal-Moreno, R.M. Changes in the termal and structural properties of maize starch during nixtamalization and tortilla-making processes as affected by grain hardness. J. Cereal Chem. 2017, 74, 72–78. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, T.; Wang, R.; Liu, Y.; Fang, G. The properties and tortilla making of corn flour from enzymatic wet-milling. Molecules 2019, 24, 2137. [Google Scholar] [CrossRef] [Green Version]
- Villada, J.A.; Sánchez-Sinencio, F.; Zelaya-Ángel, O.; Gutiérrez-Cortez, E.; Rodríguez-García, M.E. Study of the morphological, structural, thermal, and pasting corn transformation during the traditional nixtamalization process: From corn to tortilla. J. Food Eng. 2017, 212, 242–251. [Google Scholar] [CrossRef]
- Estrada-Girón, Y.; Aguilar, J.; Morales del Rio, J.A.; Valencia-Botin, A.J.; Guerrero-Beltrán, J.A.; Martínez-Preciado, A.H.; Macía, E.R.; Soltero, J.F.A.; Solorza-Feria, J.; Fernández, V.V.A. Effect of moisture content and temperature, on the rheological, microstructural and thermal properties of masa (dough) from a hybrid corn (Zea mays sp.) variety. Rev. Mex. Ing. Quim. 2014, 13, 429–446. [Google Scholar]
- Altay, F.; Gunasekaran, S. Influence of drying temperature, water content, and heating rate on gelatinization of corn starches. J. Agric. Food Chem. 2006, 54, 4235–4245. [Google Scholar] [CrossRef]
- Aguirre-Cruz, A.; Méndez-Montealvo, G.; Solorza-Feria, J.; Bello-Pérez, L.A. Effect of carboxymethylcellulose and xanthan gum on the thermal, functional and rheological properties of dried nixtamalised maize masa. Carbohydr. Polym. 2005, 62, 222–231. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Singh, N.; Singh, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Siswoyo, A.T.; Morita, N. Thermal dissociation of amylose-lipid complex influenced by acyl chain lengths in monoacyl-sn-glycerophosphatidylcholine. Anal. Sci. 2002, 17, i1053–i1055. [Google Scholar]






| Sample | Power (W) | Energy (W.h/kg) | Hardness Kgforce | Flour Yield (g) |
|---|---|---|---|---|
| TF | 1031.86 ± 27.54 f | 15.78 ± 2.21 e | NE | 58.76 ± 1.39 c |
| CF | NE | NE | NE | 66.04 ± 1.62 a |
| TF0 | 1512.58 ± 15.19 a | 36.58 ± 3.19 a | 4.73± 0.47 a | 56.59 ± 0.15 cd |
| CF0 | 1441.96 ± 27.48 b | 33.40 ± 2.74 b | 3.66 ± 0.15 c | 54.49 ± 0.98 d |
| TFR7 | 1402.59 ± 24.05 c | 33.78 ±2.15 ab | 4.04 ± 0.71 b | 61.74 ± 0.79 b |
| CFR7 | 1382.67 ± 17.60 c | 29.17 ± 1.51 c | 3.33 ± 0.07 d | 56.63 ± 0.95 cd |
| TFR15 | 1205.08 ± 25.35 d | 23.32 ± 0.02 d | 3.87 ± 0.21 bc | 50.94 ± 1.018 e |
| CFR15 | 1115.03 ± 18.79 e | 17.67 ± 1.71 e | 3.23 ± 0.06 d | 51.76 ± 1.59 e |
| TFR30 | 931.15 ± 16.01 g | 10.54 ± 0.97 f | 3.21 ± 0.14 d | 45.35 ± 1.67 f |
| CFR30 | 928.11 ±19.55 g | 9.87 ± 0.30 f | 3.06 ± 0.06 d | 46.41 ± 1.40 f |
| Samples. | Moisture | Protein | Fat | Crude Fiber | Ashes | NFE | Calcium |
|---|---|---|---|---|---|---|---|
| TF | 8.77 ± 0.05 a | 7.40 ± 0.10 bcd | 4.10 ± 0.05 a | 2.60 ± 0.10 a | 2.32 ± 0.10 a | 74.81 | 0.21 ± 0.04 a |
| CF | 8.53 ± 0.05 bc | 7.70 ± 0.10 a | 3.12 ± 0.07 b | 1.86 ± 0.15 b | 1.51 ± 0.12 b | 77.28 | 0.08 ± 0.02 b |
| TF0 | 8.64 ± 0.10 ab | 7.36 ± 0.05 cd | 3.97 ± 0.11 a | 2.65 ± 0.10 a | 2.42 ± 0.17 a | 74.96 | 0.23 ± 0.03 a |
| CF0 | 8.64 ± 0.12 ab | 7.64 ± 0.20 a | 3.17 ± 0.07 b | 1.86 ± 0.15 b | 1.61 ± 0.11 b | 77.08 | 0.10 ± 0.01 b |
| TFR7 | 8.58 ± 0.01 abc | 7.16 ± 0.25 d | 3.92 ± 0.11 a | 2.53 ± 0.20 a | 2.32 ± 0.10 a | 75.49 | 0.19 ± 0.01 a |
| CFR7 | 8.54 ± 0.08 bc | 7.60 ± 0.10 abc | 3.09 ± 0.07 b | 1.96 ± 0.15 b | 1.61 ± 0.12 b | 77.20 | 0.09 ± 0.01 b |
| TFR15 | 8.44 ± 0.22 c | 7.30 ± 0.10 d | 4.20 ± 0.20 a | 2.63 ± 0.10 a | 2.25 ± 0.05 a | 75.18 | 0.20 ± 0.01 a |
| CFR15 | 8.57 ± 0.11 bc | 7.63 ± 0.06 ab | 3.13 ± 0.22 b | 1.83 ± 0.25 b | 1.64 ± 0.13 b | 77.2 | 0.08 ± 0.01 b |
| TFR30 | 8.66 ± 0.10 ab | 7.33 ± 0.21 d | 3.95 ± 0.07 a | 2.70 ± 0.17 a | 2.28 ± 0.03 a | 75.08 | 0.21 ± 0.02 a |
| CFR30 | 8.70 ± 0.10 ab | 7.66 ± 0.15 a | 3.20 ± 0.13 b | 1.80 ± 0.17 b | 1.67 ± 0.07 b | 76.97 | 0.08 ± 0.02 b |
| Sample | L | a | b |
|---|---|---|---|
| TF | 64.01 ± 0.16 b | 0.10 ± 0.01 c | 9.04 ± 0.05 d |
| CF | 68.86 ± 0.46 a | −1.04 ± 0.02 d | 10.73 ± 0.07 d |
| TFR0 | 55.62 ± 0.18 c | 0.31 ± 0.01 b | 12.12 ± 0.03 c |
| CFR0 | 64.60 ± 1.06 b | 0.35 ± 0.03 a | 13.85 ± 0.08 b |
| TFR7 | 56.42 ± 0.65 c | 0.32 ± 0.03 b | 13.75 ± 0.11 b |
| CFR7 | 66.74 ± 0.18 b | 0.35 ± 0.05 a | 14.21 ± 0.04 b |
| TFR15 | 54.04 ± 0.55 c | 0.30 ± 0.03 b | 14.43 ± 0.06 b |
| CFR15 | 65.24 ± 0.11 b | 0.36 ± 0.02 a | 14.89 ± 0.06 b |
| TFR30 | 55.39 ± 0.15 c | 0.31 ± 0.01 b | 15.42 ± 0.09 a |
| CFR30 | 66.66 ± 0.12 b | 0.36 ± 0.02 a | 16.25 ± 0.11 a |
| Sample | WAI (%) | WSI (%) |
|---|---|---|
| TF | 3.71 ± 0.17 d | 4.69 ± 0.20 a |
| CF | 4.86 ± 0.15 c | 4.62 ± 0.17 ab |
| TF0 | 5.26 ± 0.22 b | 3.71 ± 0.15 bc |
| CF0 | 5.72 ± 0.10 a | 3.60 ± 0.13 c |
| TFR7 | 5.12 ± 0.10 bc | 3.67 ± 0.17 c |
| CFR7 | 5.44 ± 0.20 a | 3.50 ± 0.15 c |
| TFR15 | 4.96 ± 0.25 c | 3.51 ± 0.12 c |
| CFR15 | 5.31 ± 0.20 b | 3.41 ± 0.12 c |
| TFR30 | 4.91 ± 0.20 c | 3.40 ± 0.10 c |
| CFR30 | 5.21 ± 0.27 b | 2.30 ± 0.13 d |
| Sample | Endotherm 1 (Gelatinization) | Endotherm 2 | Endotherm 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Togel (°C) | Tpgel (°C) | Tfgel (°C) | ΔHgel (J/g) | ToRS5I (°C) | ΔHRS5I (J/g) | ToRS5II (°C) | ΔHRS5II (J/g) | |
| TF | 70.23 a | 78.15 a | 80.17 a | 5.30 a | 102.20 f | 6.31 d | 112.42 e | 10.07 g |
| CF | 65.19 b | 67.30 b | 69.28 b | 4.04 b | 100.20 f | 5.19 e | 107.18 f | 8.69 h |
| TF0 | 64.14 b | 65.98 c | 69.28 b | 4.19 b | 103.66 e | 7.50 c | 124.17 d | 12.63 f |
| CF0 | 63.22 b | 64.64 c | 67.02 c | 3.19 c | 102.30 f | 5.84 d | 126.47 d | 12.87 f |
| TFR7 | 62.21 c | 65.15 c | 68.83 b | 2.76 d | 107.54 d | 8.45 c | 130.83 c | 16.25 d |
| CFR7 | 58.89 d | 60.19 e | 63.08 c | 2.14 d | 104.65 e | 6.20 d | 128.34 d | 14.64 e |
| TFR15 | 60.98 c | 62.99 d | 66.27 c | 1.70 e | 111.33 c | 10.24 b | 133.81 b | 19.92 c |
| CFR15 | 57.68 d | 59.15 e | 61.18 d | 1.38 e | 110.79 c | 7.66 c | 130.19 c | 17.43 d |
| TFR30 | 60.36 c | 62.75d | 64.20 c | 0.84 f | 116.04 a | 11.32 a | 136.97 a | 23.87 a |
| CFR30 | 56.64 d | 58.61e | 60.34 d | 0.73 f | 114.25 b | 9.34 b | 135.91 a | 21.84 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Molina, I.; Mendoza-Avila, M.; Cornejo-Villegas, M.d.l.Á.; Real-López, A.D.; Rivera-Muñoz, E.; Rodríguez-García, M.; Gutiérrez-Cortez, E. Physicochemical Properties and Resistant Starch Content of Corn Tortilla Flours Refrigerated at Different Storage Times. Foods 2020, 9, 469. https://doi.org/10.3390/foods9040469
Rojas-Molina I, Mendoza-Avila M, Cornejo-Villegas MdlÁ, Real-López AD, Rivera-Muñoz E, Rodríguez-García M, Gutiérrez-Cortez E. Physicochemical Properties and Resistant Starch Content of Corn Tortilla Flours Refrigerated at Different Storage Times. Foods. 2020; 9(4):469. https://doi.org/10.3390/foods9040469
Chicago/Turabian StyleRojas-Molina, Isela, Monsserrat Mendoza-Avila, María de los Ángeles Cornejo-Villegas, Alicia Del Real-López, Eric Rivera-Muñoz, Mario Rodríguez-García, and Elsa Gutiérrez-Cortez. 2020. "Physicochemical Properties and Resistant Starch Content of Corn Tortilla Flours Refrigerated at Different Storage Times" Foods 9, no. 4: 469. https://doi.org/10.3390/foods9040469