Enhancement of the Anti-Inflammatory Effect of Mustard Kimchi on RAW 264.7 Macrophages by the Lactobacillus plantarum Fermentation-Mediated Generation of Phenolic Compound Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculation of L. Plantarum
2.2. Preparation of Mustard Leaf Samples
2.3. Determination of the pH, Acidity, and Cell Number of Lactic Acid Bacteria
2.4. Preparation of Extracts
2.5. Determination of Total Phenolic and Total Flavonoid Contents
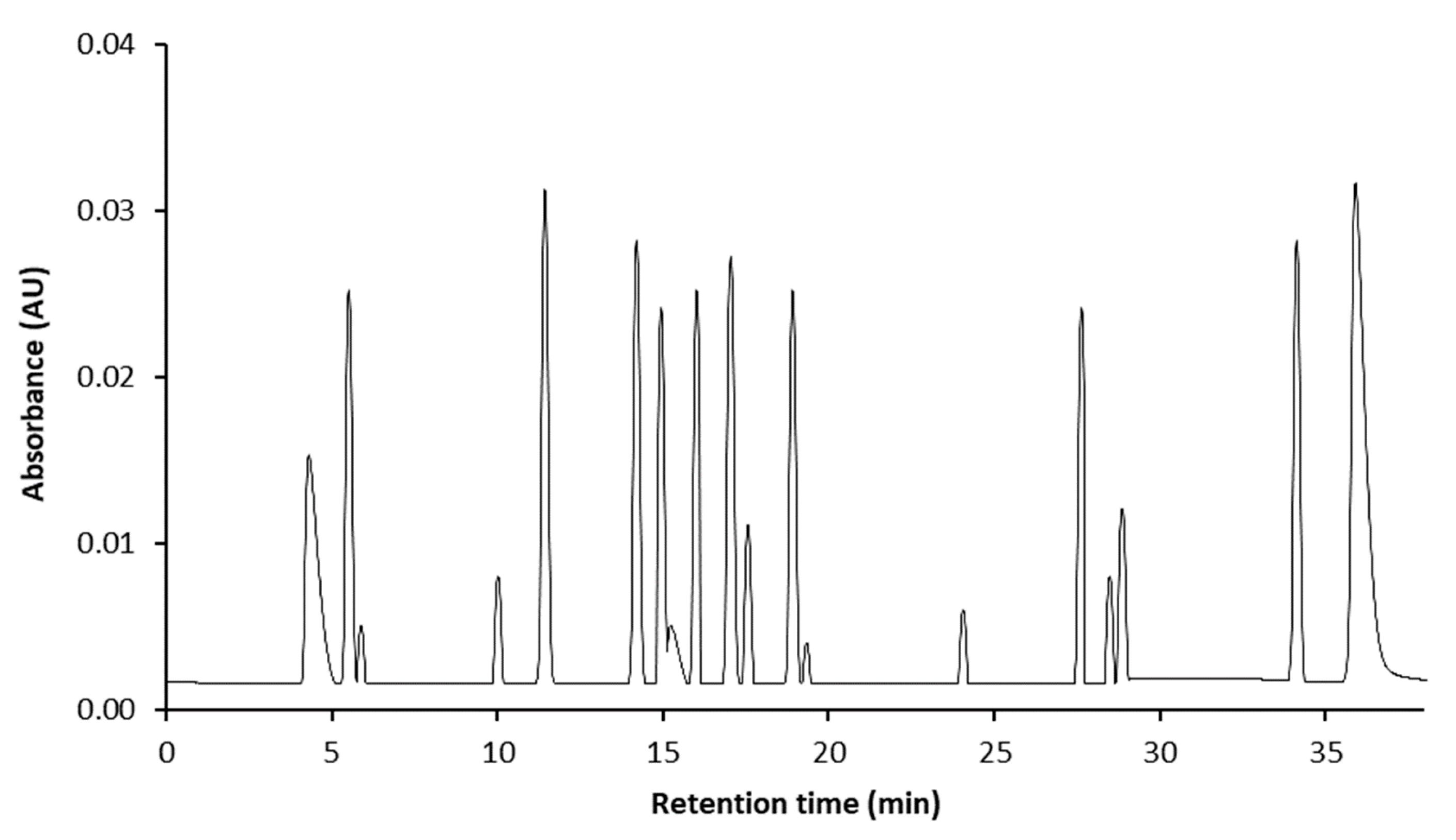
2.6. Liquid Chromatography–Mass Spectrometry/Mass Spectrometry (LC–MS/MS) Analysis
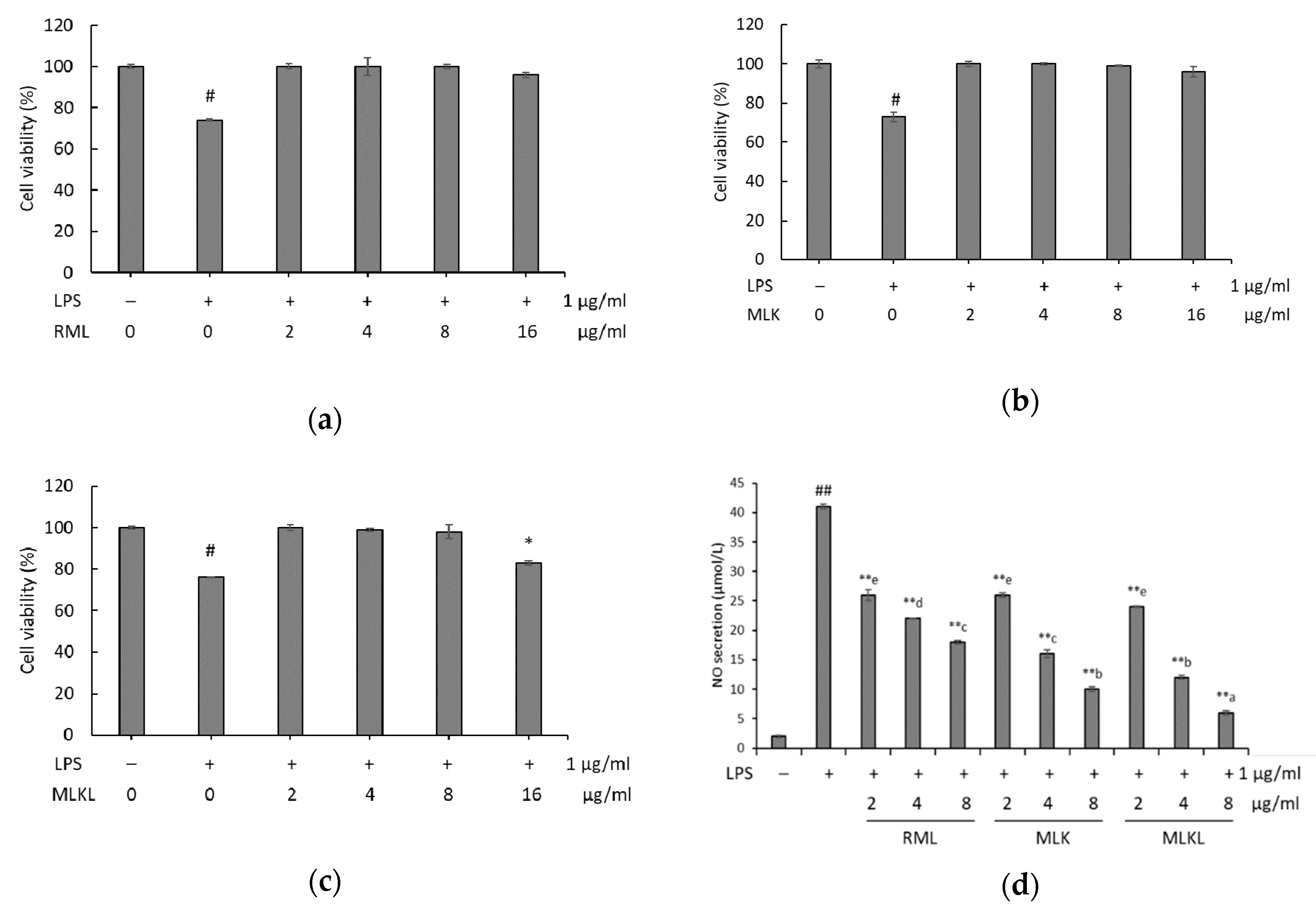
2.7. Cell Culture, Cytotoxicity Assay, and Nitrite Production
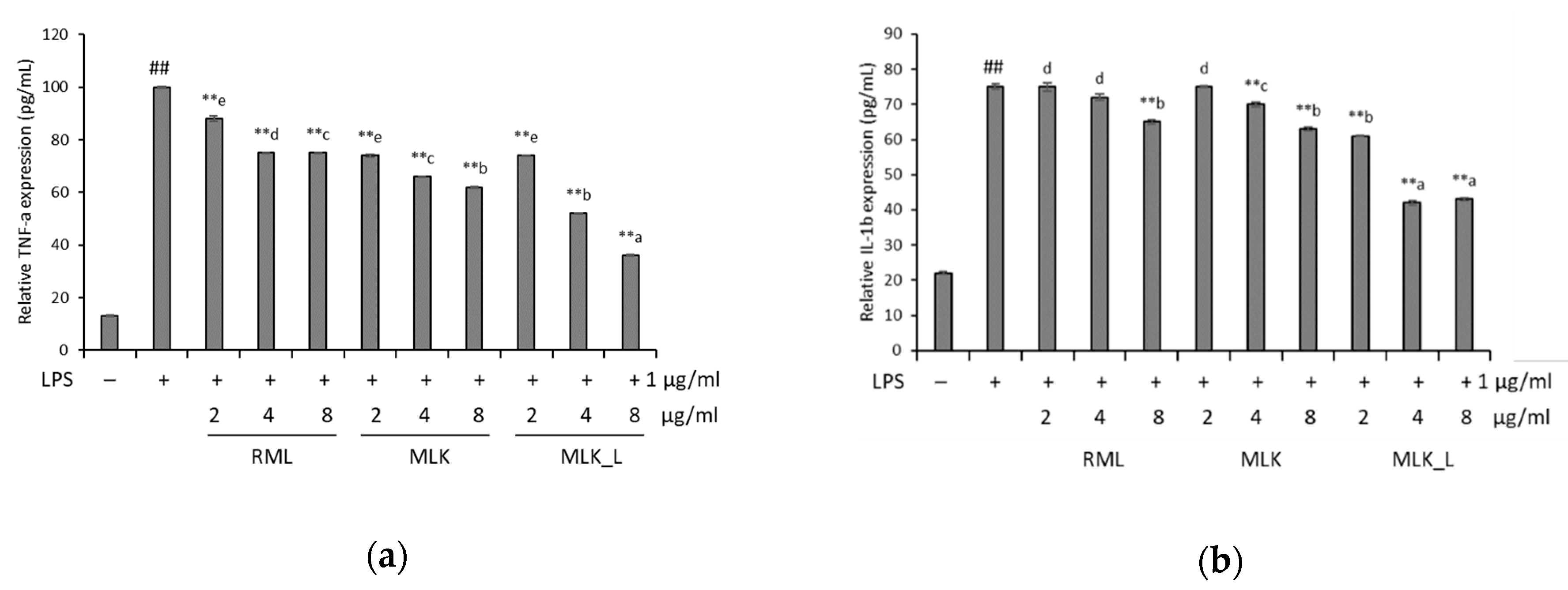
2.8. Cytokine Assay
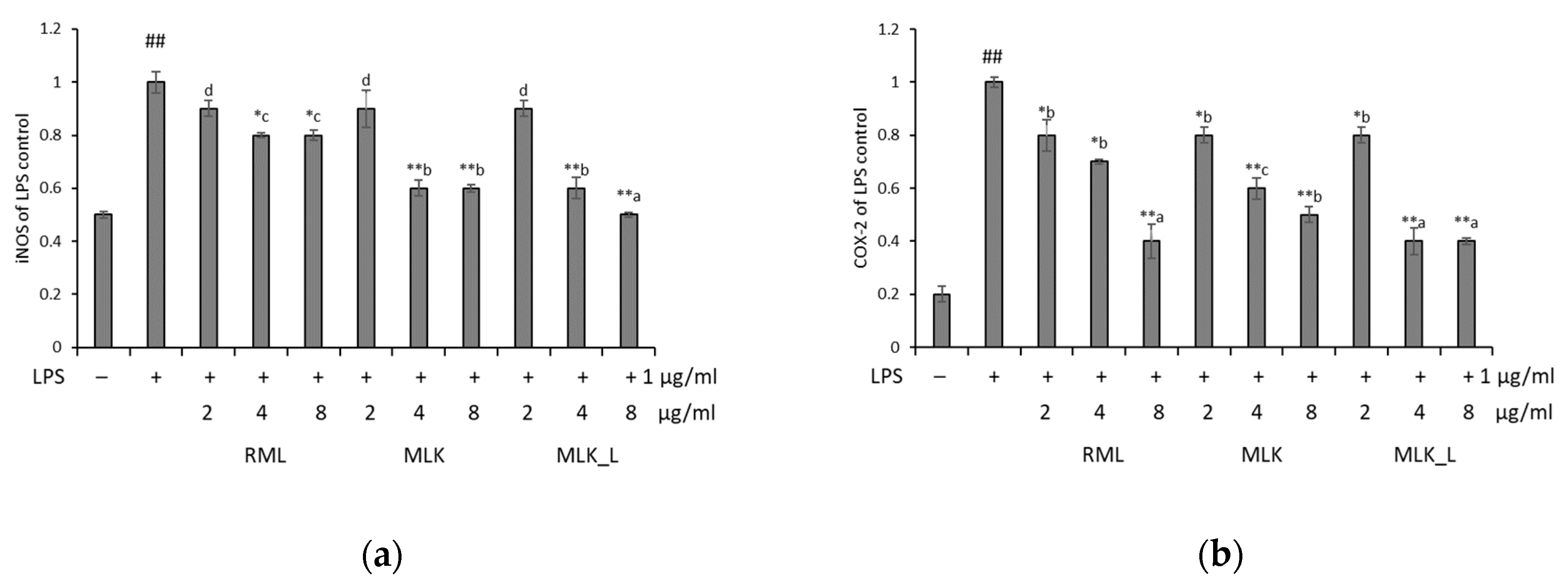
2.9. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.10. Statistical Analysis
3. Results
3.1. Effect of Fermentation on pH, Acidity, Phenolic, and Flavonoid Contents of Mustard Leaf Kimchi
3.2. Effect of Fermentation on Phenolic Profiles
3.3. Effect of Mustard Leaf Kimchi on RAW 264.7 Macrophage Survival and NO Levels
3.4. Effect of Mustard Leaf Kimchi on Pro-Inflammatory Cytokines
3.5. Effect of Mustard Leaf Kimchi on iNOS and COX2 mRNA Expression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Angelo, M.; Castelli, V.; Tupone, M.G.; Catanesi, M.; Antonosante, A.; Dominguez-Benot, R.; Ippoliti, R.; Cimini, A.M.; Benedetti, E. Lifestyle and food habits impact on chronic diseases: Roles of PPARs. Int. J. Mol. Sci. 2019, 20, 5422. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Frazie, M.; Kim, M.; Ku, K.-M. Health-promoting phytochemicals from 11 mustard cultivars at baby leaf and mature stages. Molecules 2017, 22, 1749. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Jeon, Y.; Cheigh, H. Changes in chlorophylls and carotenoids of mustard leaf kimchi during fermentation and their antioxidative activities on the lipid oxidation. J. Korean Soc. Food Sci. Nutr. (Korea Republic) 1997, 26, 563–568. [Google Scholar]
- Kim, Y.-T.; Kim, B.-K.; Park, K.-Y. Antimutagenic and anticancer effects of leaf mustard and leaf mustard kimchi. Prev. Nutr. Food Sci. 2007, 12, 84–88. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kim, H.A.; Lee, J. The effects of Brassica juncea L. leaf extract on obesity and lipid profiles of rats fed a high-fat/high-cholesterol diet. Nutr. Res. Pract. 2018, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.-F.; Hu, Z.; Ip, S.-P.; Chen, J.-N.; Su, Z.-R.; Lai, X.-P.; Lin, Z.-X. Comparison of the anti-inflammatory effects of Sinapis alba and Brassica juncea in mouse models of inflammation. Phytomedicine 2018, 50, 196–204. [Google Scholar] [CrossRef]
- Sarkar, D.; Shetty, K. Metabolic stimulation of plant phenolics for food preservation and health. Annu. Rev. Food Sci. Technol. 2014, 5, 395–413. [Google Scholar] [CrossRef]
- Stich, H.F. The beneficial and hazardous effects of simple phenolic compounds. Mutat. Res. Genet. Toxicol. 1991, 259, 307–324. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef]
- Lee, N.-K.; Paik, H.-D. Bioconversion using lactic acid bacteria: Ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W., III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Yang, S.-H. Identification of a novel potential probiotic Lactobacillus plantarum FB003 isolated from salted-fermented shrimp and its effect on cholesterol absorption by regulation of NPC1L1 and PPARα. Probiot. Antimicrob. Proteins 2019, 11, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Srisook, K.; Kim, C.; Cha, Y.N. Role of NO in enhancing the expression of HO-1 in LPS-stimulated macrophages. Methods Enzymol. 2005, 396, 368–377. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, M.J.; Lim, J.; Hong, J.H. Cross-cultural comparison of consumer acceptability of kimchi with different degree of fermentation. J. Sens. Stud. 2016, 31, 124–134. [Google Scholar] [CrossRef]
- Lim, H.-S.; Park, K.-O.; Nishizawa, N.; Bae, S.-O.; Choi, M.-R. Cytotoxicity of extracts from Dolsan leaf mustard Kimchi treated with lactic acid bacteria on lung and gastric cancer cells. Biotechnol. Bioprocess Eng. 2008, 13, 174. [Google Scholar] [CrossRef]
- Ye, J.-H.; Huang, L.-Y.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Jakubczyk, A.; Bochnak, J.; Sikora, M.; Suliburska, J. Nutritional quality of fresh and stored legumes sprouts–Effect of Lactobacillus plantarum 299v enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Fermented Momordica charantia L. juice modulates hyperglycemia, lipid profile, and gut microbiota in type 2 diabetic rats. Food Res. Int. 2019, 121, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.B.; Roblain, D.; Chammen, N.; Thonart, P.; Hamdi, M. Antioxidant phenolic compounds loss during the fermentation of Chétoui olives. Food Chem. 2009, 116, 662–669. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced antioxidant activity for apple juice fermented with Lactobacillus plantarum ATCC14917. Molecules 2019, 24, 51. [Google Scholar] [CrossRef]
- Park, S.-Y.; Jang, H.-L.; Lee, J.-H.; Choi, Y.; Kim, H.; Hwang, J.; Seo, D.; Kim, S.; Nam, J.-S. Changes in the phenolic compounds and antioxidant activities of mustard leaf (Brassica juncea) kimchi extracts during different fermentation periods. Food Sci. Biotechnol. 2017, 26, 105–112. [Google Scholar] [CrossRef]
- Sun, G.Y.; Li, R.; Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Lubahn, D.B.; Geng, X.; Lee, J.C.; Greenlief, C.M. Quercetin potentiates docosahexaenoic acid to suppress lipopolysaccharide-induced oxidative/inflammatory responses, alter lipid peroxidation products, and enhance the adaptive stress pathways in BV-2 microglial cells. Int. J. Mol. Sci. 2019, 20, 932. [Google Scholar] [CrossRef]
- Bel-Rhlid, R.; Thapa, D.; Kraehenbuehl, K.; Hansen, C.E.; Fischer, L. Biotransformation of caffeoyl quinic acids from green coffee extracts by Lactobacillus johnsonii NCC 533. AMB Express 2013, 3, 28. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef]
- Chao, P.-C.; Hsu, C.-C.; Yin, M.-C. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr. Metab. 2009, 6, 33. [Google Scholar] [CrossRef]
- Morrison, M.; van der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis 2014, 233, 149–156. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-C.; Cho, W.-K.; Oh, J.H.; Im, G.Y.; Jeong, Y.H.; Yang, M.C.; Ma, J.Y. Fermentation by Lactobacillus enhances anti-inflammatory effect of Oyaksungisan on LPS-stimulated RAW 264.7 mouse macrophage cells. BMC Complement. Altern. Med. 2012, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; De Angelis, M.; Silano, M.; Gobbetti, M.; Di Cagno, R. Lactic acid fermentation of cactus cladodes (Opuntia ficus-indica L.) generates flavonoid derivatives with antioxidant and anti-inflammatory properties. PLoS ONE 2016, 11, e0152575. [Google Scholar]
- Jiang, M.; Deng, K.; Jiang, C.; Fu, M.; Guo, C.; Wang, X.; Wang, X.; Meng, F.; Yang, S.; Deng, K. Evaluation of the antioxidative, antibacterial, and anti-inflammatory effects of the aloe fermentation supernatant containing Lactobacillus plantarum HM218749. 1. Mediat. Inflamm. 2016, 2016, 2945650. [Google Scholar] [CrossRef]
| Sample | pH | Acidity (%) | LAB Population (log CFU/mL) | Total Polyphenol Content (mg CAE/g Extract Powder) 1 | Total Flavonoid Content (mg GAE/g Extract Powder) | |
|---|---|---|---|---|---|---|
| Initial | Final | |||||
| RML | 5.8 ± 0.2 b | 0.2 ± 0.06 b | 2.7 ± 0.7 b | 2.8 ± 0.4 c | 361.2 ± 2.1 c | 8.0 ± 0.5 b |
| MLK | 4.6 ± 0.6 a | 0.7 ± 0.07 a | 2.5 ± 0.4 b | 6.7 ± 0.5 b | 478.6 ± 2.9 b | 12.2 ± 0.1 a |
| MLKL | 4.2 ± 0.3 a | 0.6 ± 0.04 a | 6.3 ± 0.1 a | 7.8 ± 0.4 a | 512.1 ± 1.8 a | 12.9 ± 0.8 a |
| No | Analytes | RT 1 | Extract (μg/g) | ||
|---|---|---|---|---|---|
| RML | MLK | MLKL | |||
| 1 | catechol | 4.4 | - 2 | - | - |
| 2 | chlorogenic acid | 5.3 | 1630.2 ± 23.1 a,A | 861.2B ± 23.5 b,B | 896.7 ± 29.1 b,B |
| 3 | protocatechuic acid | 5.9 | - | - | - |
| 4 | epicatechin | 10.2 | 263.5 ± 14.2 b,C | 467.4 ± 22.0 a,C | 460.2 ± 6.6 A,c |
| 5 | catechin | 11.1 | - | 324.4 ± 33.3 a,D | 393.7 ± 42.1 a,C |
| 6 | vanillic acid | 14.2 | 123.4 ± 2.3 F | 132.9 ± 3.2 F | 111.9 ± 4.7 G |
| 7 | caffeic acid | 15.1 | 632.9 ± 21.3 c,B | 1213.4 ± 26.4 b,A | 1642 ± 13.2 a,A |
| 8 | syringic acid | 15.3 | 145.7 ± 6.6 E | 111.2 ± 19.4 F | 124 ± 43.2 G |
| 9 | epigallocatechin gallate | 15.8 | 231.1 ± 32.2 a,D | 196.2 ± 24.1 a,b,F | 167.9 ± 23.8 b,G |
| 10 | p-coumaric acid | 16.9 | 150.3 ± 6.4 b,E | 231.2 ± 30.1 a,F | 246.0 ± 26.4 a,F |
| 11 | gallocatechin gallate | 17.3 | - | - | - |
| 12 | epicatechin gallate | 18.7 | 267.6 ± 17.8 b,C | 133.8 ± 3.5 c,F | 362.6 ± 11.8 a,D |
| 13 | rutin | 19.1 | 160.1 ± 42.5 b,E | 228.7 ± 19.9 b,D | 305.3 ± 18.2 a,E |
| 14 | naringin | 24.2 | 532.1 ± 22.5 a,B | 296.7 ± 24.1 b,D | 144.0 ± 30.9 c,G |
| 15 | ferulic acid | 27.5 | 112.3 ± 17.2 b,F | 293.4 ± 43.1 a,D | 234.6 ± 18.7 a,b,F |
| 16 | sinapic acid | 28.6 | - | - | - |
| 17 | quercetin | 29.1 | - | - | - |
| 18 | kaempferol | 34.4 | 351.4 ± 23.7 B | 364.6 ± 53.5 C | 359.0 ± 12.8 D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, B.; Anh, P.T.N.; Yang, S.H. Enhancement of the Anti-Inflammatory Effect of Mustard Kimchi on RAW 264.7 Macrophages by the Lactobacillus plantarum Fermentation-Mediated Generation of Phenolic Compound Derivatives. Foods 2020, 9, 181. https://doi.org/10.3390/foods9020181
Le B, Anh PTN, Yang SH. Enhancement of the Anti-Inflammatory Effect of Mustard Kimchi on RAW 264.7 Macrophages by the Lactobacillus plantarum Fermentation-Mediated Generation of Phenolic Compound Derivatives. Foods. 2020; 9(2):181. https://doi.org/10.3390/foods9020181
Chicago/Turabian StyleLe, Bao, Pham Thi Ngoc Anh, and Seung Hwan Yang. 2020. "Enhancement of the Anti-Inflammatory Effect of Mustard Kimchi on RAW 264.7 Macrophages by the Lactobacillus plantarum Fermentation-Mediated Generation of Phenolic Compound Derivatives" Foods 9, no. 2: 181. https://doi.org/10.3390/foods9020181
APA StyleLe, B., Anh, P. T. N., & Yang, S. H. (2020). Enhancement of the Anti-Inflammatory Effect of Mustard Kimchi on RAW 264.7 Macrophages by the Lactobacillus plantarum Fermentation-Mediated Generation of Phenolic Compound Derivatives. Foods, 9(2), 181. https://doi.org/10.3390/foods9020181






