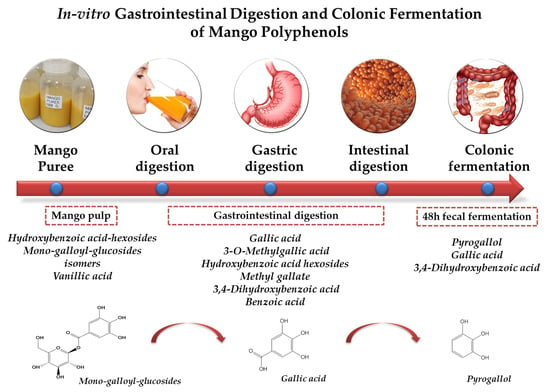
In Vitro Gastrointestinal Digestion and Colonic Catabolism of Mango (Mangifera indica L.) Pulp Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials and Sample Preparation
2.3. In Vitro Gastrointestinal Digestion
2.4. In Vitro Colonic Fermentation
2.5. Extraction of Polyphenols from Digested Mango Samples and from Faecal Incubates
2.6. UHPLC-HRMS Polyphenol Analysis
2.7. Bioaccessibility of (Poly)Phenols
2.8. Statistical Analysis
3. Results and Discussion
3.1. Polyphenols in Mango Pulp Samples
3.2. Stability and Bioaccessibility of Phenolic Compounds in Mango after Simulated Gastrointestinal Digestion
3.3. Degradation of Mango Polyphenols during Fecal Fermentation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UHPLC-HRMS | ultra-high-performance liquid chromatography coupled to high resolution mass spectrometry |
| FA | formic acid |
| FW | fresh Weight |
| BOD | Before Oral Digestion |
| AOD | After Oral Digestion |
| AGD | After Gastric Digestion |
| AID | After Intestinal Digestion |
| RT | retention time |
| [M-H]− Exp | experimental exact mass |
| Δ | mass error |
| OFN | oxygen free nitrogen |
| BMIs | body mass index |
| n.d. | not detected |
References
- Arauz, L. Mango anthracnose: Economic impact and current opinions for integrated management. Plant Dis. 2000, 84, 600–611. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT, 2018. Available online: http://www.fao.org/faostat/es/#data (accessed on 10 November 2020).
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Kim, Y.; Brecht, J.K.; Talcott, S.T. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007, 105, 1327–1334. [Google Scholar] [CrossRef]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. Technol. 2000, 1, 161–166. [Google Scholar] [CrossRef]
- Shabani, Z.; Sayadi, A. The antimicrobial in vitro effects of different concentrations of some plant extracts including tamarisk, march, acetone and mango kernel. J. Appl. Pharma. Sci. 2014, 4, 75. [Google Scholar]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.R.; Parasuraman, S.; Vijaya, C.; Darwhekar, G.; Devika, G.S. Antidiabetic effect of kernel sedes extract of Mangifera indica (anacardiaceae). Int. J. Pharma Bio Sci. 2001, 2, 385–393. [Google Scholar]
- El-Seedi, H.R.; Salem, M.A.; Khattab, O.M.; El-Wahed, A.A.; El-Kersh, D.M.; Khalifa, S.A.; Halabi, M.F. Dietary Xanthones. In Handbook of Dietary Phytochemicals; Springer Nature: Singapore, 2020; pp. 1–22. [Google Scholar]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: Implication as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, A.S.H.; Mohammed, A.S.; Abdullah, R.; Mirghani, M.E.S.; Qubaisi, M.A. Cytotoxic effects of Mangifera indica L. Kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complementary Altern. Med. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and cancer: Mechanisms of action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, S.; Jainu, M.; Sabitha, K.E.; Devi, C.S. Role of mangiferin on biochemical alterations and antioxidant status in isoproterenol-induced myocardial infarction in rats. J. Ethnopharmacol. 2006, 107, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [Green Version]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and their bioactive components: Adding variety to the fruit plate of health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef]
- Fang, C.; Kim, H.; Barnes, R.; Talcott, S.T.; Mertens-Talcott, S.U. Daily mango (Mangifera indica L.) consumption for 42 days differentially modulates metabolism and inflammation in lean and obese individuals. FASEB J. 2017, 31, 431. [Google Scholar]
- O’Hara, C.; Ojo, B.; Emerson, S.R.; Simenson, A.J.; Peterson, S.; Perkins-Veazie, P.; Payton, M.E.; Hermann, J.; Smith, B.J.; Lucas, E.A. Acute Freeze-Dried Mango Consumption With a High-Fat Meal has Minimal Effects on Postprandial Metabolism, Inflammation and Antioxidant Enzymes. Nutr. Metab. Insights 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyado-Ayerdi, S.G. Bioaccesibility of polyphenols associated with dietary fiber and in vitro kinetics release of polylphenols in Mexican “Ataulfo” mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Sañudo-Barajas, J.A.; Vélez-de la Rocha, R.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Effects of ripening on the in vitro antioxidant capacity and bioaccessibility of mango cv.‘Ataulfo’phenolics. J. Food Sci. Technol. 2019, 56, 2073–2082. [Google Scholar] [CrossRef]
- Hernández-Maldonado, L.M.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; Cárdenas-Castro, A.P.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients 2019, 11, 1564. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Cazares, L.A.; Hernández-Navarro, F.; Ramírez-Jiménez, A.K.; Campos-Vega, R.; Reyes-Vega, M.L.; Loarca-Piña, G.; Morales-Sánchez, E.; Wall-Medrano, A.; Gaytán-Martínez, M. Assessment of a functional confectionery added with mango bagasse as vehicle for enhancing bioaccessibility and permeability of phenolic compounds. Food Funct. 2020, 8, 3906–3916. [Google Scholar] [CrossRef]
- Kay, C.D.; Clifford, M.N.; Mena, P.; McDougall, G.J.; Andres-Lacueva, C.; Cassidy, A.; Del Rio, D.; Kuhnert, N.; Manach, C.; Pereira-Caro, G.; et al. Recommendations for standardizing nomenclature for dietary (poly) phenol catabolites. Am. J. Clin. Nutr. 2020, 112, 1051–1068. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordóñez, J.L.; Moreno-Rojas, R.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M. Bioaccessibility of Bioactive Compounds of ‘Fresh Garlic’and ‘Black Garlic’through In Vitro Gastrointestinal Digestion. Foods 2020, 9, 1582. [Google Scholar] [CrossRef] [PubMed]
- De Santiago, E.; Gill, C.I.; Carafa, I.; Tuohy, K.M.; De Peña, M.P.; Cid, C. Digestion and colonic fermentation of raw and cooked Opuntia ficus-indica cladodes impacts bioaccessibility and bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Borges, G.; Ky, I.; Ribas, A.; Calani, L.; Del Rio, D.; Crozier, A. In vitro colonic catabolism of orange juice (poly) phenols. Mol. Nutr. Food Res. 2015, 59, 465–475. [Google Scholar] [CrossRef]
- Alañón, M.E.; Oliver-Simancas, R.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Evolution of bioactive compounds of three mango cultivars (Mangifera indica L.) at different maturation stages analyzed by HPLC-DAD-q-TOF-MS. Food Res. Int. 2019, 125, 108526. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goldagre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemicals analysis chemical analysis Chemical Analylsis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Use of HPLC-and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef]
- Vithana, M.D.K.; Singh, Z.; Johnson, S.K. Cold storage temperatures and durations affect the concentrations of lupeol, mangiferin, phenolic acids and other health-promoting compounds in the pulp and peel of ripe mango fruit. Postharvest Biol. Technol. 2018, 139, 91–98. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Costa, H.B.; Ventura, J.A.; Kondratyuk, T.P.; Barroso, M.E.; Correia, R.M.; Pimentel, E.F.; Pinto, F.E.; Endringer, D.C.; Romão, W. Chemical profile of mango (Mangifera indica L.) using electrospray ionisation mass spectrometry (ESI-MS). Food Chem. 2016, 204, 37–45. [Google Scholar] [CrossRef]
- Meneses, M.A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J. Food Eng. 2015, 163, 45–53. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of majornphenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC-DADMS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Islas-Osuna, M.A.; Astiazarán-García, H.; Vázquez-Ortiz, F.A.; Martín-Belloso, O.; Gorinstein, S. Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh cut “Ataulfo” mangoes (Mangifera indica L.) as affected by low temperature storage. J. Food Sci. 2009, 74, S126–S134. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.A.; Martín-Belloso, O. Effect of minimal processing on bioactive compounds and antioxidant activity of fresh-cut “Kent” mango (Mangifera indica L.). Postharvest Biol. Technol. 2008, 51, 384–390. [Google Scholar]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative assessment of phenolic content and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Álvarez, J.A.P.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Chen, C.Y.O.; Blumberg, J.B.; Astiazaran-Garcia, H.; Wall-Medrano, A.; González-Aguilar, G.A. Processing ‘ataulfo’mango into juice preserves the bioavailability and antioxidant capacity of its phenolic compounds. Nutrients 2017, 9, 1082. [Google Scholar] [CrossRef]
- Sandhu, A.; Fan, J.; Xiao, D.; Edirisinghe, I.; Burton-Freeman, B. Identification of phenolic metabolites in human plasma and urine after mango consumption. Curr. Dev. Nutr. 2019, 3 (Suppl. 1), 021-19. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Lu, Q.; Zhang, L.; Wang, X.; Liu, R. C-ring cleavage metabolites of catechin and epicatechin enhanced antioxidant activities through intestinal microbiota. Food Res. Int. 2020, 135, 109270. [Google Scholar] [CrossRef]
- Li, Y.; Meselhy, M.R.; Wang, L.Q.; Nakamura, N.; Hattori, M. Biotransformation of a C-Glycosylflavone, Abrusin 2”-O-β-D-Apioside, by Human Intestinal Bacteria. Chem. Pharm. Bull. 2000, 48, 1239–1241. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.R.; Trevisan, M.T.S.; Feitosa, J.P.; Ricardo, N.M.; Hull, W.E.; Erben, G.; Wurtele, G.; Breuer, A.; Frei, E.; Ulrich, C.M.; et al. Transformation of Mangiferin to Norathyriol by Human Fecal Matrix in Anaerobic Conditions: Comprehensive NMR of the Xanthone Metabolites, Antioxidant Capacity, and Comparative Cytotoxicity Against Cancer Cell Lines. Nat. Prod. Commun. 2020, 15, 1–9. [Google Scholar]
- Sanugul, K.; Akao, T.; Li, Y.; Kakiuchi, N.; Nakamura, N.; Hattori, M. Isolation of a human intestinal bacterium that transforms mangiferin to norathyriol and inducibility of the enzyme that cleaves a C-glucosyl bond. Biol. Pharm. Bull. 2005, 28, 1672–1678. [Google Scholar] [CrossRef] [Green Version]



| RT (min) | Compound | Chemical Structure | [M-H]− Exp. (m/z)− | Δ (ppm) | MSIMI a |
|---|---|---|---|---|---|
| Phenolic Acid Derivatives | |||||
| 1.3 | Benzene-1,2-diol (pyrogallol) | C6H6O3 | 125.0233 | 0.39 | 1 |
| 3.1 | 3,4,5-Triydroxybenzoic acid (gallic acid) | C7H6O5 | 169.0137 | 0.77 | 1 |
| 6.6 | Gallic acid hexoside | C13H16O11 | 347.0608 | 1.73 | 2 |
| 8.8 | 3-O-Methylgallic acid | C8H7O5 | 183.0287 | 1.8 | 1 |
| 11.9 | 4-O-Methylgallic acid | C8H7O5 | 183.0287 | 1.8 | 1 |
| 8.7 | 3,4-Dihydroxybenzoic acid | C7H6O4 | 153.0182 | 0.23 | 1 |
| 9 | Benzoic acid | C7H6O2 | 121.0284 | 0.74 | 1 |
| 4.7 | Hydroxybenzoic acid hexoside 1 | C13H16O8 | 299.0767 | 3.76 | 2 |
| 7 | Hydroxybenzoic acid hexoside 2 | C13H16O8 | 299.0767 | 3.76 | 2 |
| 7.4 | Syringic acid glucoside 1 | C15H20O10 | 359.0972 | 1.91 | 2 |
| 7.8 | Syringic acid glucoside 2 | C15H20O10 | 359.0972 | 1.91 | 2 |
| 8.8 | Methyl gallate | C8H8O5 | 183.0287 | 1.64 | 2 |
| 11.9 | Methyl digallate ester 1 | C15H21O9 | 335.0397 | 4.3 | 2 |
| 13 | Methyl digallate ester 2 | C15H21O9 | 335.0397 | 4.3 | 2 |
| 7.1 | 4-Hydroxy-3-methoxybenzaldehyde (vanillic acid) | C8H8O4 | 167.0344 | 058 | 1 |
| 3.8 | Galloyl-quinic acid | C14H16O10 | 343.0659 | 1.63 | 2 |
| Flavan-3-ol derivatives | |||||
| 8.4 | Epigallocatechin | C15H14O7 | 305.0655 | 2.39 | 1 |
| 9.8 | Epigallocatechin gallate | C22H18O11 | 457.0765 | 3.87 | 1 |
| 11.1 | Epicatechin gallate | C22H18O11 | 457.0765 | 3.87 | 1 |
| Flavanone Derivatives | |||||
| 8.8 | Eriodyctiol | C15H12O6 | 287.0550 | 3.22 | 1 |
| 9.7 | Eriodyctiol hexoside 1 | C21H22O11 | 449.1078 | 1.59 | 2 |
| 10.4 | Eriodyctiol hexoside 2 | C21H22O11 | 449.1078 | 1.59 | 2 |
| 9.7 | Hesperetin glucoside 1 | C22H24O11 | 463.1234 | 0.18 | 2 |
| 12.3 | Hesperetin glucoside 2 | C22H24O11 | 463.1234 | 0.18 | 2 |
| Flavonol Derivatives | |||||
| 8.3 | Quercetin-hexoside 1 | C21H20O12 | 463.0877 | 2.09 | 2 |
| 11.1 | Quercetin-hexoside 2 | C21H20O12 | 463.0877 | 2.09 | 2 |
| 9.5 | Isorhamnetin hexoside 1 | C22H22O12 | 477.1027 | 2.05 | 2 |
| 9.9 | Isorhamnetin hexoside 2 | C22H22O12 | 477.1027 | 2.05 | 2 |
| Hydroxycinnamic acid derivatives | |||||
| 8.6 | Ferulic acid hexoside 1 | C16H20O9 | 355.1023 | 2.31 | 2 |
| 9.5 | Ferulic acid hexoside 2 | C16H20O9 | 355.1023 | 2.31 | 2 |
| 9 | Sinapic acid hexoside 1 | C17H22O10 | 385.1129 | 2.58 | 2 |
| 9.3 | Sinapic acid hexoside 2 | C17H22O10 | 385.1129 | 2.58 | 2 |
| 9.6 | sinapic acid hexoside 3 | C17H22O10 | 385.1129 | 2.58 | 2 |
| 8 | Caffeoyl-hexoside 1 | C15H18O9 | 341.0867 | 2.53 | 2 |
| 8.7 | Caffeoyl-hexoside 2 | C15H18O9 | 341.0867 | 2.53 | 2 |
| 8.9 | Caffeoyl-quinic 1 | C16H18O9 | 353.0867 | 1.96 | 2 |
| 10 | Caffeoyl-quinic 2 | C16H18O9 | 353.0867 | 1.96 | 2 |
| Xanthone | |||||
| 9.4 | Mangiferin | C19H18O11 | 421.0765 | 1.52 | 1 |
| Galloyl derivatives | |||||
| 2.7 | Mono-galloyl-glucose 1 | C13H16O10 | 331.0665 | 3.61 | 2 |
| 6.6 | Mono-galloyl-glucose 2 | C13H16O10 | 331.0665 | 3.61 | 2 |
| 9.9 | Tetra-O-galloyl glucoside 1 | C34H28O19 | 787.0994 | 2.04 | 2 |
| 10.7 | Tetra-O-galloyl glucoside 2 | C34H28O19 | 787.0994 | 2.04 | 2 |
| 11.3 | Penta-O-galloyl glucoside | C41H32O26 | 939.1035 | 1.87 | 2 |
| Compounds | BOD | AOD | % Rem | AGD | % Rem | AID | BI | p-Value |
|---|---|---|---|---|---|---|---|---|
| Phenolic acid derivatives | ||||||||
| Benzene-1,2-diol (pyrogallol) | 27 ± 3 | 18 ± 3 | 66.7 | 21 ± 9 | 77.8 | 17 ± 4 | 63 | ns |
| 3,4,5-Trihydroxybenzoic acid (gallic acid) | 46 ± 4 d | 109 ± 16 c | 237 | 188 ± 26 b | 408.7 | 267 ± 36 a | 580.4 | *** |
| Gallic acid hexoside | 61 ± 3 a | 49 ± 9 b | 80.3 | 42 ± 6 b | 68.9 | 22 ± 3 c | 36.1 | *** |
| 3-O-Methylgallic acid | 47 ± 4 b | 86 ± 8 b | 183 | 85 ± 13 b | 180.9 | 2259 ± 406 a | 4806 | *** |
| 4-O-Methylgallic acid | 69 ± 7 c | 132 ± 28 a | 191.3 | 112 ± 18 b | 162.3 | n.d. d | - | *** |
| 3,4-Dihydroxybenzoic acid | n.d. | n.d. | n.d. | 176 ± 39 a | 100 | *** | ||
| Benzoic acid | n.d. | n.d. | n.d. | 84 ± 15 a | 100 | *** | ||
| Hydroxybenzoic acid hexoside 1 | 111 ± 45 c | 434 ± 88 ab | 391 | 472 ± 141 a | 425.2 | 322 ± 110 b | 290.1 | *** |
| Hydroxybenzoic acid hexoside 2 | 1259 ± 327 d | 5644 ± 571 a | 448.3 | 3985 ± 444 b | 316.5 | 2287 ± 208 c | 181.7 | *** |
| Syringic acid glucoside 1 | 2.7 ± 0.0 a | 2.7 ± 0.3 a | 100 | 1.7 ± 0.3 b | 63 | 0.8 ± 0.1 c | 29.6 | *** |
| Syringic acid glucoside 2 | 1.2 ± 0.1 a | 1.1 ± 0.1 b | 91.7 | 0.5 ± 0.1 c | 41.7 | 0.1 ± 0.0 d | 8.3 | *** |
| Methyl gallate | 1 ± 1 b | 28 ± 3 b | 2800 | 29 ± 4 b | 2900 | 737 ± 132 a | 737 | *** |
| Methyl digallate ester 1 | 16 ± 1 b | 30 ± 6 a | 187.5 | 26 ± 5 ab | 162.5 | 15 ± 13 b | 93.8 | * |
| Methyl digallate ester 2 | 1.6 ± 0.2 b | 2.4 ± 0.7 a | 150 | 2.5 ± 0.5 a | 156.3 | n.d. c | - | *** |
| 4-Hydroxy-3-methoxybenzaldehyde (vanillic acid) | 522 ± 15 a | 486 ± 60 a | 93.1 | 372 ± 71 b | 7.3 | 158 ± 48 c | 30.3 | *** |
| Galloyl-quinic acid | 4.4 ± 0.1 a | 3.5 ± 0.4 b | 79.5 | 3.3 ± 0.4 b | 75 | 1.7 ± 0.3 c | 38.6 | *** |
| Total Phenolic Acid Derivatives | 2169 ± 410 c | 7026 ± 793a | 323.9 | 5152 ± 738 b | 246.2 | 6347 ± 1014 a | 292.6 | ** |
| Flavan-3-ol derivatives | ||||||||
| Epigallocatechin | 37 ± 0.5 a | 38 ± 3 a | 102.7 | 24 ± 2 b | 64.9 | 18 ± 2 c | 48.6 | *** |
| Epigallocatechin gallate | 4.3 ± 0.2 a | 4.4 ± 1.4 a | 102.3 | 0.9 ± 0.2 b | 20.9 | 0.00 ± 0.00 b | - | *** |
| Epicatechin gallate | 0.21 ± 0.02 b | 0.27 ± 0.04 a | 128.6 | 0.02 ± 0.01 c | 9.5 | 0.00 ± 0.00 c | - | *** |
| Total Flavan-3-ol derivatives | 41.5 ± 0.7 a | 42.7 ± 4.4 a | 102.8 | 24.9 ± 2.2 b | 60 | 18 ± 2 c | 43.4 | *** |
| Flavanones Derivatives | ||||||||
| Eriodyctiol | 0.66 ± 0.04 a | 0.68 ± 0.09 a | 103 | 0.27 ± 0.05 b | 40.9 | n.d. c | - | *** |
| Eriodyctiol hexoside 1 | 0.11 ± 0.01 a | 0.09 ± 0.03 a | 81.8 | 0.03 ± 0.01 b | 27.3 | n.d. c | - | *** |
| Eriodyctiol hexoside 2 | 0.04 ± 0.02 ab | 0.05 ± 0.02 a | 125 | 0.02 ± 0.01 b | 50 | n.d. c | - | *** |
| Hesperetin glucoside 1 | 0.12 ± 0.05 a | 0.17 ± 0.07 a | 141.7 | n.d. b | 0.0 | n.d. b | - | *** |
| Hesperetin glucoside 2 | 0.03 ± 0.00 a | n.d. b | 0 | n.d.b | 0.0 | n.d. b | - | *** |
| Total Flavanones Derivatives | 0.96 ± 0.12 a | 0.99 ± 0.21 a | 103.1 | 0.32 ± 0.07 b | 33.3 | n.d. c | - | *** |
| Flavonols Derivatives | ||||||||
| Quercetin-hexoside 1 | 0.03 ± 0.01 a | 0.02 ± 0.01 b | 66.7 | 0.02 ± 0.01 b | 66.7 | n.d. c | - | ** |
| Quercetin-hexoside 2 | 0.10 ± 0.00 a | 0.10 ± 0.01 a | 100 | 0.06 ± 0.01 b | 60 | 0.02 ± 0.01 c | 20 | *** |
| Isorhamnetin hexoside 1 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 100 | n.d. b | 0 | n.d. b | - | ** |
| Isorhamnetin hexoside 2 | 0.70 ± 0.01 a | 0.67 ± 0.07 a | 95.7 | 0.50 ± 0.07 b | 71.4 | 0.37 ± 0.07 c | 52.9 | *** |
| Total flavonol derivatives | 0.84 ± 0.02 a | 0.80 ± 0.09 a | 95.2 | 0.58 ± 0.10 b | 69 | 0.39 ± 0.08 c | 46.4 | ** |
| Hydroxycinnamic acid derivatives | ||||||||
| Ferulic acid hexoside 1 | 48 ± 2 a | 48 ± 4 a | 100 | 27 ± 3 b | 56.3 | 20 ± 3 c | 41.7 | *** |
| Ferulic acid hexoside 2 | 63 ± 3 a | 70 ± 8 a | 111.1 | 37 ± 3 b | 58.7 | 13 ± 2 c | 20.6 | *** |
| Sinapic acid hexoside 1 | 19 ± 2 a | 20 ± 1 a | 105.3 | 11 ± 1 b | 57.9 | 7 ± 2 c | 36.8 | *** |
| Sinapic acid hexoside 2 | 28 ± 2 a | 31 ± 4 a | 110.7 | 20 ± 3 b | 71.4 | 11 ± 1 c | 39.3 | *** |
| sinapic acid hexoside 3 | 35 ± 2 a | 35 ± 4 a | 100 | 19 ± 2 b | 54.3 | 8 ± 2 c | 22.0 | *** |
| Caffeoyl-hexoside 1 | 47 ± 2 a | 40 ± 4 b | 85.1 | 17 ± 3 c | 36.2 | 7.9 ± 0.8 d | 16.8 | *** |
| Caffeoyl-hexoside 2 | 3.3 ± 0.6 a | 3.8 ± 0.9 a | 115.2 | 1.6 ± 0.3 b | 48.5 | 0.6 ± 0.3 c | 18.2 | *** |
| Caffeoyl-quinic 1 | 0.5 ± 0.3 a | 0.4 ± 0.1 a | 80 | n.d. b | 0 | n.d. b | - | *** |
| Caffeoyl-quinic 2 | 2.3 ± 0.3 a | 2.5 ± 0.2 a | 108.7 | 1.09 ± 0.19 b | 47.4 | 0.02 ± 0.05 c | - | *** |
| Total Hydroxycinnamic acid derivatives | 246.0 ± 14.2 a | 251 ± 26 a | 101.9 | 133.7 ± 15.5 b | 54.3 | 67.5 ± 11.1 c | 27.4 | *** |
| Xanthone | ||||||||
| Mangiferin | 0.12 ± 0.01 ab | 0.14 ± 0.03 a | 116.7 | 0.09 ± 0.02 b | 75 | 0.10 ± 0.02 b | 83.3 | ** |
| Gallotannins derivatives | ||||||||
| Mono-galloyl-glucose | 481 ± 16 a | 453 ± 30 a | 94.2 | 385 ± 30 b | 80.0 | 516 ± 85 a | 107.3 | ** |
| Mono-galloyl-glucose | 526 ± 34 a | 465 ± 53 b | 88.4 | 373 ± 49 c | 70.9 | 197 ± 21 d | 37.5 | *** |
| Tetra-O-galloyl glucoside | 0.15 ± 0.01 c | 0.33 ± 0.06 b | 220 | 0.5 ± 0.2 a | 333.3 | 0.37 ± 0.09 b | 246.7 | *** |
| Tetra-O-galloyl glucoside | 1.72 ± 0.08 c | 2.3 ± 0.3 b | 133.7 | 2.7 ± 0.4 a | 157 | 2.2 ± 0.4 bc | 127.9 | * |
| Penta-O-galloyl glucoside | 1.9 ± 0.6 b | 2.0 ± 0.7 b | 107.9 | 2.0 ± 0.4 b | 105.3 | 8 ± 2 a | 421.1 | *** |
| Total Galloyl derivatives | 1011 ± 51 a | 923 ± 84 b | 91.3 | 763 ± 80 c | 75.5 | 724 ± 108 c | 71.6 | ** |
| TOTAL POLYPHENOLS | 3469 ± 476 d | 8244 ± 908 ab | 237.6 | 6075 ± 836 c | 180.5 | 7156 ± 1135 bc | 206.3 | ** |
| RT (min) | Catabolites | Chemical Structure | [M-H]− Exp. (m/z) | Δ (ppm) | MSIMI a |
|---|---|---|---|---|---|
| 10.6 | 3-(4-Hydroxyphenyl)propanoic acid | C9H10O3 | 165.0546 | 1.54 | 1 |
| 7.9 | 3-Phenylacetic acid | C8H8O2 | 135.0440 | 0.84 | 1 |
| 8.8 | (-)-Epicatechin | C15H14O6 | 289.0706 | 0.14 | 1 |
| 10.4 | Norathyriol | C13H8O6 | 259.0216 | −2.07 | 2 |
| Compounds | 0 h | 4 h | 8 h | 24 h | 48 h | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Mango | Control | Mango | Control | Mango | Control | Mango | Control | Mango | ||
| Phenolic acid derivatives | |||||||||||
| Benzene-1,2-diol (pyrogallol) | n.d. f | 17 ± 4 e | n.d. f | 40 ± 2 d | n.d. f | 176 ± 5 c | n.d. f | 465 ± 10 b | n.d. f | 691 ± 49 a | *** |
| 3,4,5-Triydroxybenzoic acid (gallic acid) | n.d. e | 268 ± 36 d | n.d. e | 410 ± 11 c | n.d. e | 662 ± 61 b | n.d. e | 1092 ± 61 a | n.d. e | 1033 ± 38 a | *** |
| Gallic acid hexoside | n.d. c | 21.9 ± 0.9 a | n.d. c | 1.8 ± 0.1 b | n.d. c | 1.1 ± 0.1 b | n.d. c | n.d. c | n.d. c | n.d. c | *** |
| 3,4-Dihydroxybenzoic acid | n.d. f | 177 ± 39 e | n.d. f | 949 ± 108 b | n.d. f | 1410 ± 85 a | n.d. f | 659 ± 184 c | n.d. f | 267 ± 28 d | *** |
| Hydroxybenzoic acid hexoside 1 | n.d. c | 323 ± 56 a | n.d. c | 338 ± 5 a | n.d. c | 217 ± 52 b | n.d. c | n.d. c | n.d. c | n.d. c | *** |
| Hydroxybenzoic acid hexoside 2 | n.d. d | 2294 ± 102 a | n.d. d | 1569 ± 36 b | n.d. d | 1080 ± 68 c | n.d. d | 41 ± 13 d | n.d. d | 27 ± 2 d | *** |
| Syringic acid glucoside 1 | n.d. c | 0.9 ± 0.1 a | n.d. c | 0.7 ± 0.0 b | n.d. c | 0.7 ± 0.1 b | n.d. c | n.d. c | n.d. c | n.d. c | *** |
| Syringic acid glucoside 2 | n.d. b | 0.2 ± 0.0 a | n.d. b | 0.1 ± 0.0 a | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | *** |
| 4-Hydroxy-3-methoxybenzaldehyde (vanillic acid) | n.d. c | 162 ± 14 a | n.d. c | 104 ± 44 b | n.d. c | 167 ± 12 a | n.d. c | n.d. c | n.d. c | n.d. c | *** |
| 3-(4-Hydroxyphenyl)propanoic acid | n.d. d | n.d. d | 0.9 ± 0.1 d | 2 ± 1 c | 0.4 ± 0.2 c | 9 ± 1 b | 0.4 ± 0.1 d | 18 ± 3 a | n.d. e | 19 ± 1 a | *** |
| 3-Phenylacetic acid | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | 26 ± 11 a | n.d. b | 41 ± 8 a | *** |
| Flavan-3-ol derivatives | |||||||||||
| Epigallocatechin | n.d. f | 21 ± 1 a | n.d. f | 13 ± 1 b | n.d. f | 5.8 ± 0.8 c | n.d. f | 1.3 ± 0.1 e | n.d. f | 1.5 ± 0.1 e | *** |
| (-)-Epicatechin | n.d. d | n.d. d | n.d. d | 8.2 ± 0.3 a | n.d. d | 7.5 ± 0.0 a | n.d. d | 5.7 ± 0.0 b | n.d. d | 2.7 ± 0.2 c | *** |
| Flavanone derivatives | |||||||||||
| Eriodyctiol | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | 0.4 ± 0.1 c | n.d. c | 1.6 ± 0.4 b | n.d. c | 2.3 ± 0.3 a | *** |
| Hydroxycinnamic acid derivatives | |||||||||||
| Ferulic acid hexoside 1 | n.d. c | 18 ± 2 a | n.d. c | 10.9 ± 0.8 b | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | *** |
| Sinapic acid hexoside 1 | n.d. d | 7 ± 1 c | n.d. d | 19 ± 1 b | n.d. d | 35 ± 3 a | n.d. d | n.d. d | n.d. d | n.d. d | *** |
| Caffeoyl-hexoside 1 | n.d. c | 8 ± 1 a | n.d. c | 2.8 ± 0.4 b | n.d. c | 3.1 ± 0.3 b | n.d. c | 1.4 ± 0.1 b | n.d. c | 1.4 ± 0.2 b | *** |
| Caffeoyl-hexoside 2 | n.d. c | 0.6 ± 0.1 b | n.d. c | 2.0 ± 0.0 a | n.d. c | 2.6 ± 0.1 a | n.d. c | n.d. c | n.d. c | n.d. c | *** |
| Xanthone derivatives | |||||||||||
| Mangiferin | n.d. b | 0.10 ± 0.01 a | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | n.d. b | *** |
| Norathyriol | n.d. c | n.d. c | n.d. c | 0.07 ± 0.02 a | n.d. c | 0.02 ± 0.01 b | n.d. c | 0.02 ± 0.01 b | n.d. c | n.d. c | *** |
| Galloyl derivatives | |||||||||||
| Mono-galloyl-glucose 1 | n.d. e | 518 ± 16 a | n.d. e | 384 ± 25 b | n.d. e | 266 ± 42 c | n.d. e | 19 ± 2 d | n.d. e | 17 ± 5 d | *** |
| Mono-galloyl-glucose 2 | n.d. c | 220 ± 34 a | n.d. c | 242.5 ± 0.5 a | n.d. c | 173 ± 32 b | n.d. c | n.d. c | n.d. c | n.d. c | *** |
| Total phenolic compounds | n.d. c | 4057 ± 310 a | 0.9 ± 0.1 g | 4095 ± 239 a | 0.4 ± 0.1 f | 4202 ± 364 a | 0.4 ± 0.1 f | 2286 ± 288 b | n.d. c | 2043 ± 135 b | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordoñez-Díaz, J.L.; Moreno-Ortega, A.; Roldán-Guerra, F.J.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Pereira-Caro, G. In Vitro Gastrointestinal Digestion and Colonic Catabolism of Mango (Mangifera indica L.) Pulp Polyphenols. Foods 2020, 9, 1836. https://doi.org/10.3390/foods9121836
Ordoñez-Díaz JL, Moreno-Ortega A, Roldán-Guerra FJ, Ortíz-Somovilla V, Moreno-Rojas JM, Pereira-Caro G. In Vitro Gastrointestinal Digestion and Colonic Catabolism of Mango (Mangifera indica L.) Pulp Polyphenols. Foods. 2020; 9(12):1836. https://doi.org/10.3390/foods9121836
Chicago/Turabian StyleOrdoñez-Díaz, José Luis, Alicia Moreno-Ortega, Francisco Javier Roldán-Guerra, Victor Ortíz-Somovilla, José Manuel Moreno-Rojas, and Gema Pereira-Caro. 2020. "In Vitro Gastrointestinal Digestion and Colonic Catabolism of Mango (Mangifera indica L.) Pulp Polyphenols" Foods 9, no. 12: 1836. https://doi.org/10.3390/foods9121836