Evaluation of Antioxidant Systems and Ascorbate-Glutathione Cycle in Feijoa Edible Flowers at Different Flowering Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flower Samples and Chemicals
2.2. Physicochemical Properties
2.3. Bioactive Compounds and Antioxidant Activity
2.4. Antioxidant Enzymes
2.4.1. Catalase and Superoxide Dismutase Activity
2.4.2. Ascorbic Acid Metabolism
2.4.3. Polyphenoloxidase Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Traits
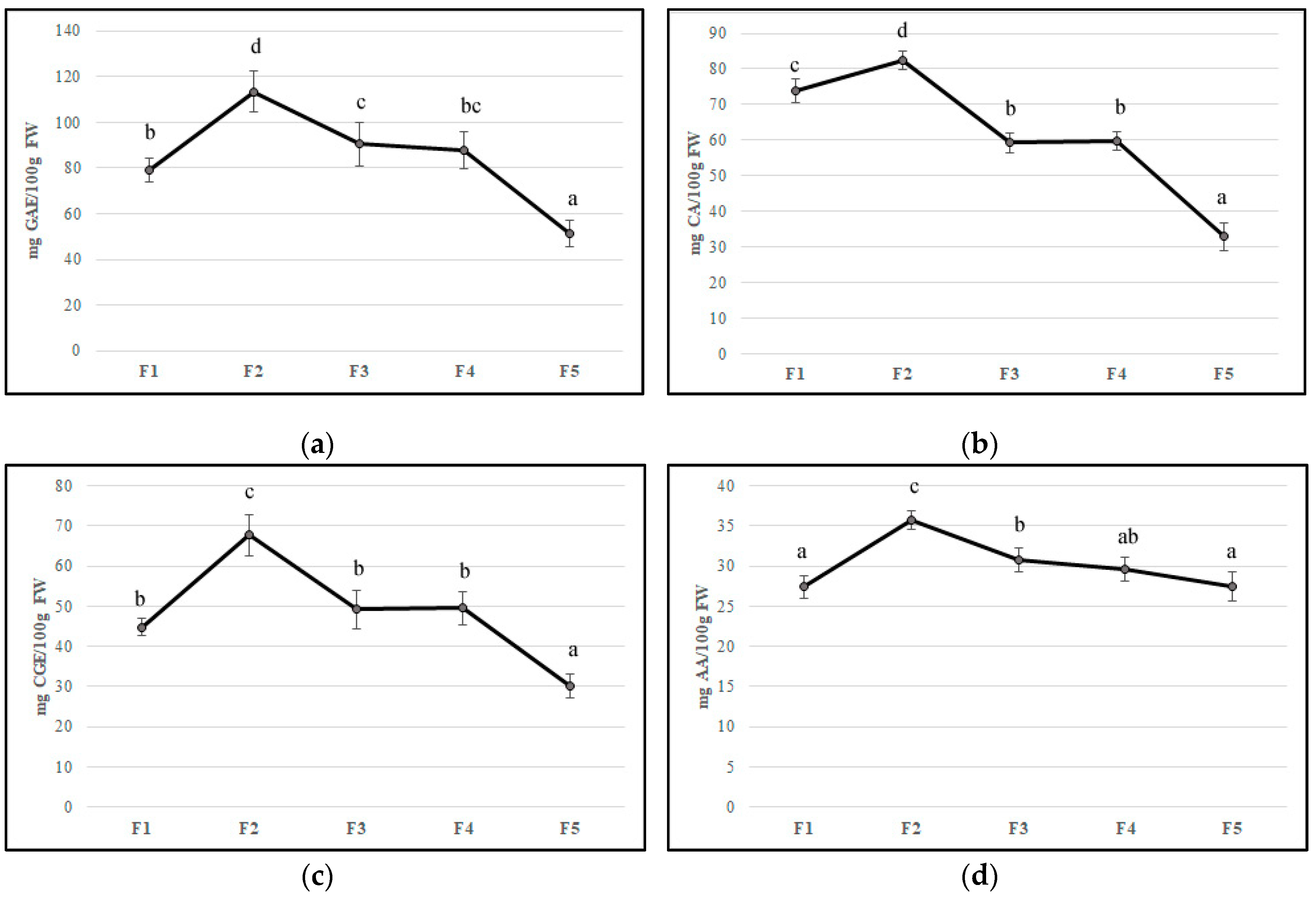
3.2. Bioactive Compounds and Antioxidant Activity
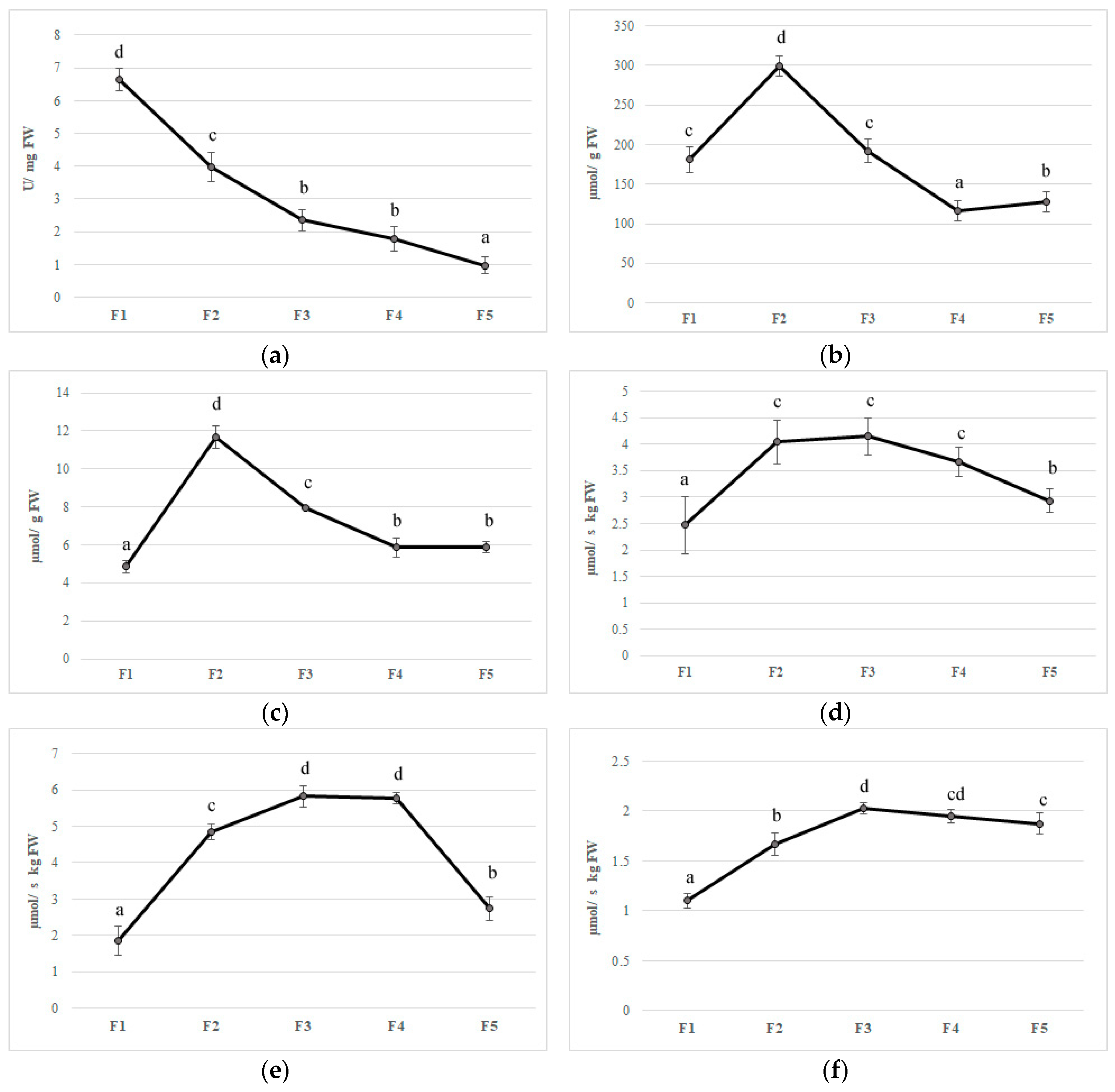
3.3. Antioxidant Enzymes
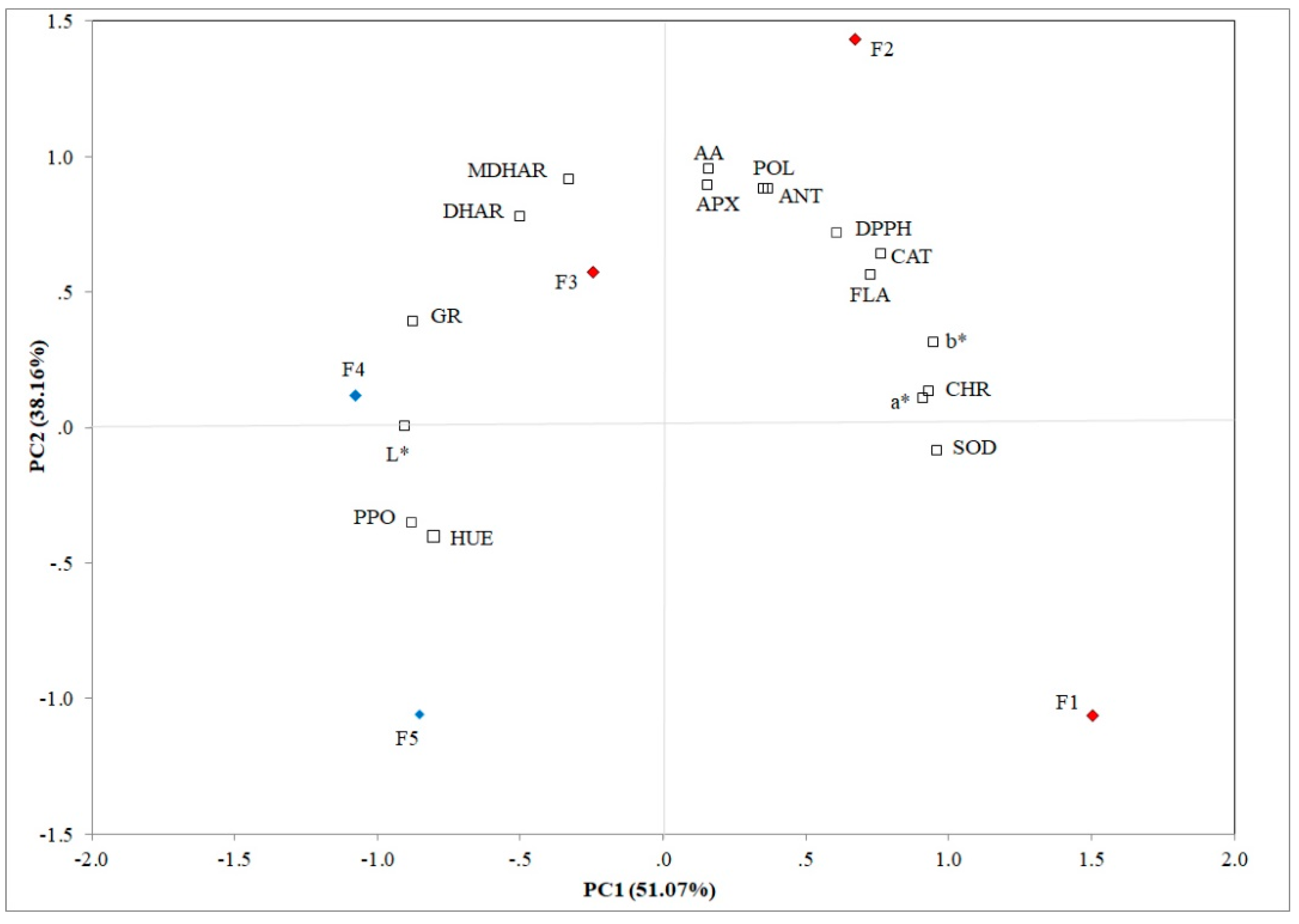
3.4. Evaluation of Different Flowering Stages by PCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, F. Chemical and biological properties of feijoa (Acca sellowiana). Trends Food Sci. Technol. 2018, 81, 121–131. [Google Scholar] [CrossRef]
- Beyhan, Ö.; Elmastaş, M.; Gedikli, F. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae). J. Med. Plants Res. 2010, 4, 1065–1072. [Google Scholar]
- Landrum, L.R. Campomanesia, Pimenta, Blepharocalyx, Legrandia, Acca, Myrrhinium, and Luma (Myrtaceae). In Flora Neotropica Monogr; Flora Neotropica: New York, NY, USA, 1986; ISBN 0893273279. [Google Scholar]
- Ramírez, F.; Kallarackal, J. Feijoa [Acca sellowiana (O. Berg) Burret] pollination: A review. Sci. Hortic. 2017, 226, 333–341. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Melillo, L. Diuretic plants in the paintings of Pompeii. Am. J. Nephrol. 1994, 14, 423–425. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Edible flowers with the common name “marigold”: Their therapeutic values and processing. Trends Food Sci. Technol. 2019, 89, 76–87. [Google Scholar] [CrossRef]
- Lu, B.; Li, M.; Yin, R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review (2000–2015). Crit. Rev. Food Sci. Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Meller, E.; Wysocka, G. Mineral composition of some edible flowers. J. Elem. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef]
- Kucekova, Z.; Mlcek, J.; Humpolicek, P.; Rop, O. Edible flowers—Antioxidant activity and impact on cell viability. Cent. Eur. J. Biol. 2013, 8, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, H.; Sakagami, H.; Hatano, T. Three new flavonoids, proanthocyanidin, and accompanying phenolic constituents from Feijoa sellowiana. Biosci. Biotechnol. Biochem. 2018, 82, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosbah, H.; Louati, H.; Boujbiha, M.A.; Chahdoura, H.; Snoussi, M.; Flamini, G.; Ascrizzi, R.; Bouslema, A.; Achour, L.; Selmi, B. Phytochemical characterization, antioxidant, antimicrobial and pharmacological activities of Feijoa sellowiana leaves growing in Tunisia. Ind. Crop. Prod. 2018, 112, 521–531. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wang, W.; Waterhouse, G.I.N.; Wadhwa, S.S. Utilisation Potential of Feijoa Fruit Wastes as Ingredients for Functional Foods. Food Bioprocess. Technol. 2013, 6, 3441–3455. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, F.; Kallarackal, J. Phenological growth stages of Feijoa [Acca sellowiana (O. Berg) Burret] according to the BBCH scale under tropical Andean conditions. Sci. Hortic. 2018, 232, 184–190. [Google Scholar]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Zampella, L.; Mastrobuoni, F.; Di Matteo, M. Overall quality and antioxidant enzymes of ready-to-eat ‘Purple Queen’ pomegranate arils during cold storage. Postharvest Biol. Technol. 2019, 155, 20–28. [Google Scholar] [CrossRef]
- Goffi, V.; Zampella, L.; Forniti, R.; Petriccione, M.; Botondi, R. Effects of ozone postharvest treatment on physicochemical and qualitative traits of Actinidia chinensis ‘Soreli’ during cold storage. J. Sci. Food Agric. 2019, 99, 5654–5661. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Goffi, V.; Magri, A.; Botondi, R.; Petriccione, M. Response of antioxidant system to postharvest ozone treatment in ‘Soreli’ kiwifruit. J. Sci. Food Agric. 2020, 100, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Pagano, L.; Forniti, R.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Mencarelli, F. Postharvest treatment with chitosan affects the antioxidant metabolism and quality of wine grape during partial dehydration. Postharvest Biol. Technol. 2018, 137, 38–45. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Arrigoni, O.; Dipierro, S.; Borraccino, G. Ascorbate free radical reductase, a key enzyme of the ascorbic acid system. FEBS Lett. 1981, 125, 242–244. [Google Scholar] [CrossRef] [Green Version]
- Nakano, J.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant. Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp.ciceris. Physiol. Mol. Plant. Pathol. 2002, 61, 325–337. [Google Scholar]
- Modesti, M.; Petriccione, M.; Forniti, R.; Zampella, L.; Scortichini, M.; Mencarelli, F. Methyl jasmonate and ozone affect the antioxidant system and the quality of wine grape during postharvest partial dehydration. Food Res. Int. 2018, 112, 369–377. [Google Scholar] [CrossRef]
- Dettori, M.T.; Di Gaetano, R. Feijoa sellowiana: Floral biology. Adv. Hort. Sci. 1991, 5, 11–14. [Google Scholar]
- Verma, R.; Awasthi, M.; Modgil, R.; Dhaliwal, Y.S. Effect of maturity on the physico-chemical and nutritional characteristics of Kachnar (Bauhinia variegata Linn.) green buds and flowers. Indian J. Nat. Prod. Resour. 2012, 3, 242–245. [Google Scholar]
- Grzeszczuk, M.; Jadczak, D. Nutritional value of chive Edible Flowers. Acta Sci. Pol. 2011, 10, 85–94. [Google Scholar]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Changes in the phenolic concentration during flower development of rose “KORcrisett”. J. Am. Soc. Hortic. Sci. 2009, 134, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Katz, A.; Weiss, D. Protocol of chs gene expression in petúnia flowers. Physiol. Plant. 1998, 102, 210–216. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Mao, S.; Shen, C. Phenolic Compounds and Antioxidant Capacities of 10 Common Edible Flowers from China. J. Food Sci. 2014, 79, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Benvenuti, S.; Bortolotti, E.; Maggini, R. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci. Hortic. 2016, 199, 170–177. [Google Scholar] [CrossRef]
- Stefaniak, A.; Grzeszczuk, M. Nutritional and biological value of five edible flower species. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Schmitzer, V.; Osterc, G.; Veberic, R.; Stampar, F. Correlation between chromaticity values and major anthocyanins in seven Acer palmatum Thunb. cultivars. Sci. Hortic. 2009, 119, 442–446. [Google Scholar] [CrossRef]
- Ferrante, A.; Vernieri, P.; Tognoni, F.; Serra, G. Changes in abscisic acid and flower pigments during floral senescence of petunia. Biol. Plant. 2006, 50, 181. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, D.; Shinkai, Y.; Kondo, T. Change of color and components in sepals of chameleon hydrangea during maturation and senescence. Phytochemistry 2008, 69, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Vaknin, H.; Bar-Akiva, A.; Ovadia, R.; Nissim-Levi, A.; Forer, I.; Weiss, D.; Oren-Shamir, M. Active anthocyanin degradation in Brunfelsia calycina (yesterday-today- tomorrow) flowers. Planta 2005, 222, 19–26. [Google Scholar] [CrossRef]
- Noda, N.; Kanno, Y.; Kato, N.; Kazuma, K.; Suzuki, M. Regulation of gene expression involved in flavonol and anthocyanin biosynthesis during petal development in lisianthus (Eustoma grandiflorum). Physiol. Plant. 2004, 122, 305–313. [Google Scholar] [CrossRef]
- Dong, Y.H.; Beuning, L.; Davies, K.; Mitra, D.; Morris, B.; Kootstra, A. Expression of pigmentation genes and photo-regulation of anthocyanin biosynthesis in developing Royal Gala apple flowers. Aust. J. Plant. Physiol. 1998, 5, 245–252. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The Antioxidants Changes in Ornamental Flowers during Development and Senescence. Antioxidants 2013, 2, 132–155. [Google Scholar] [CrossRef] [Green Version]
- Koike, A.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Villavicencio, A.L.C.H.; Ferreira, I.C.F.R. Edible flowers of Viola tricolor L. as a new functional food: Antioxidant activity, individual phenolics and effects of gamma and electron-beam irradiation. Food Chem. 2015, 179, 6–14. [Google Scholar] [CrossRef]
- Skrajda, M.N. Phenolic compounds and antioxidant activity of edible flowers. J. Educ. Heal. Sport 2017, 7, 946–956. [Google Scholar]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, P.; Munné-Bosch, S. Photo-oxidative stress during leaf, flower and fruit development. Plant. Physiol. 2018, 176, 1004–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, N.A.; Umar, S.; Chan, M.T. Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Chapter 5; Anjum, N.A., Chan, M.-T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; p. 209. [Google Scholar]
- Takahama, U.; Oniki, T. A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol. Plant. 1997, 101, 845–852. [Google Scholar] [CrossRef]
- Petriccione, M.; Pasquariello, M.S.; Mastrobuoni, F.; Zampella, L.; Di Patre, D.; Scortichini, M. Influence of a chitosan coating on the quality and nutraceutical traits of loquat fruit during postharvest life. Sci. Hortic. 2015, 197, 287–296. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Pizzolongo, F.; Romano, R.; Di Matteo, M. Study of pomological traits and physico-chemical quality of pomegranate (Punica granatum L.) genotypes grown in Italy. Eur. Food Res. Technol. 2018, 244, 1427–1438. [Google Scholar] [CrossRef]




| Flowering Stages | TA | RS | L* | a* | b* | CHR | HUE |
|---|---|---|---|---|---|---|---|
| F1 | 0.12 ± 0.02 (e) | 3.06 ± 0.06 (a) | 28.82 ± 0.27 (a) | 46.06 ± 0.41 (e) | 20.07 ± 0.15 (d) | 50.24 ± 0.41 (e) | 23.54 ± 0.17 (b) |
| F2 | 0.08 ± 0.005 (d) | 3.87 ± 0.27 (b) | 30.30 ± 0.02 (c) | 45.31 ± 0.07 (d) | 20.62 ± 0.06 (e) | 49.78 ± 0.07 (d) | 24.47 ± 0.06 (c) |
| F3 | 0.07 ± 0.004 (c) | 4.10 ± 0.09 (c) | 29.93 ± 0.03 (b) | 37.64 ± 0.03 (c) | 7.14 ± 0.03 (c) | 38.31 ± 0.03 (c) | 10.74 ± 0.05 (a) |
| F4 | 0.05 ± 0.003 (b) | 4.45 ± 0.12 (d) | 31.65 ± 0.05 (e) | 34.38 ± 0.06 (b) | −2.93 ± 0.04 (a) | 34.50 ± 0.06 (b) | 355.13 ± 0.07 (d) |
| F5 | 0.04 ± 0.005 (a) | 4.84 ± 0.03 (e) | 31.47 ± 0.04 (d) | 24.72 ± 0.07 (a) | −1.47 ± 0.06 (b) | 24.77 ± 0.07 (a) | 356.59 ± 0.14 (e) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magri, A.; Adiletta, G.; Petriccione, M. Evaluation of Antioxidant Systems and Ascorbate-Glutathione Cycle in Feijoa Edible Flowers at Different Flowering Stages. Foods 2020, 9, 95. https://doi.org/10.3390/foods9010095
Magri A, Adiletta G, Petriccione M. Evaluation of Antioxidant Systems and Ascorbate-Glutathione Cycle in Feijoa Edible Flowers at Different Flowering Stages. Foods. 2020; 9(1):95. https://doi.org/10.3390/foods9010095
Chicago/Turabian StyleMagri, Anna, Giuseppina Adiletta, and Milena Petriccione. 2020. "Evaluation of Antioxidant Systems and Ascorbate-Glutathione Cycle in Feijoa Edible Flowers at Different Flowering Stages" Foods 9, no. 1: 95. https://doi.org/10.3390/foods9010095





