Conjugated Linoleic Acid and Cholesterol Oxidative Products Generated in Hot Boned Beef Semimembranosus Muscle as Affected by Rigor Temperature, Ageing and Display Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Meat Sampling and Processing
2.3. Analysis of Cholesterol and Its Oxidative Stability
2.4. GC-FID Analysis
2.5. Fatty Acid Methyl Esterification (FAME) of Lipid from Beef SM Muscle
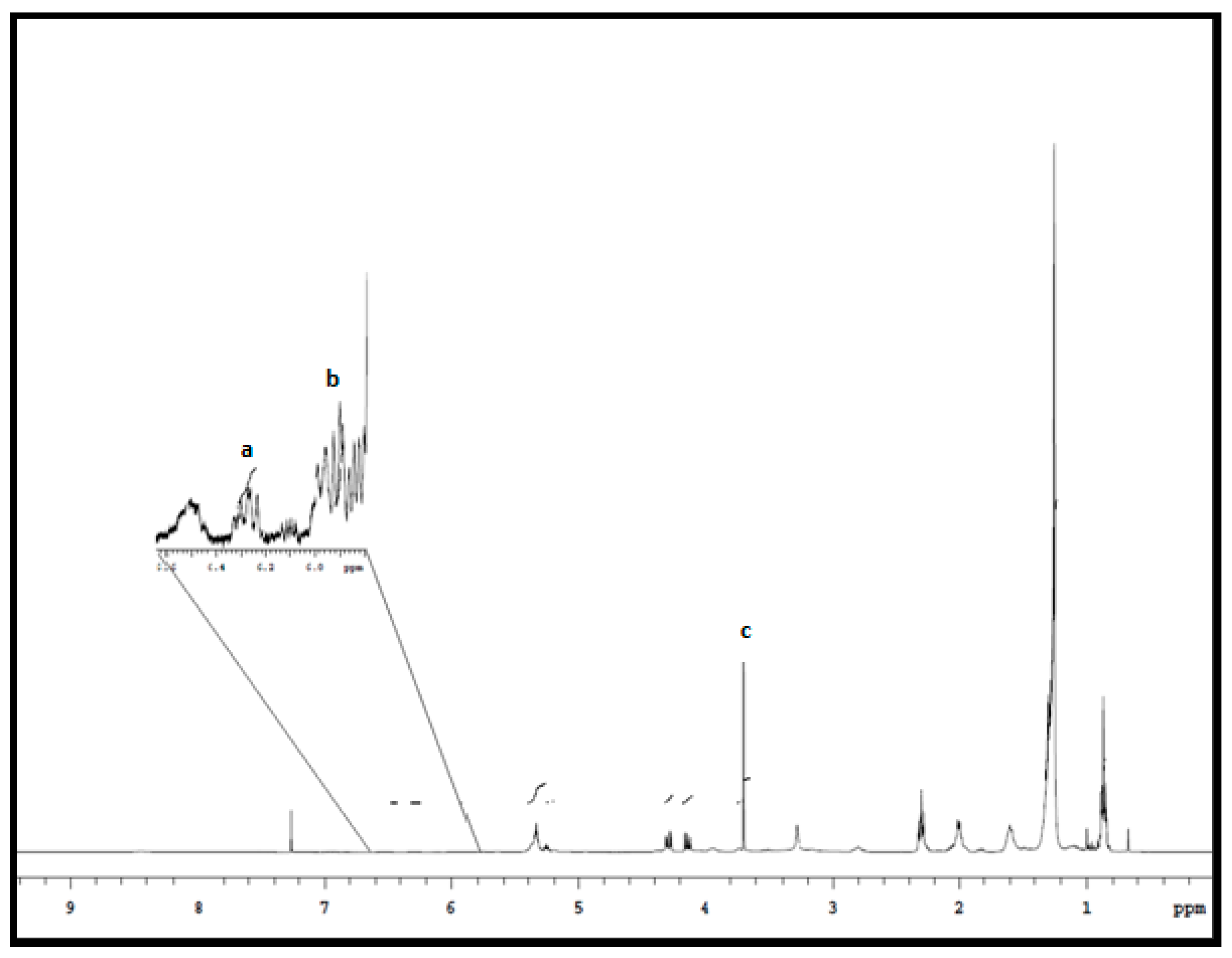
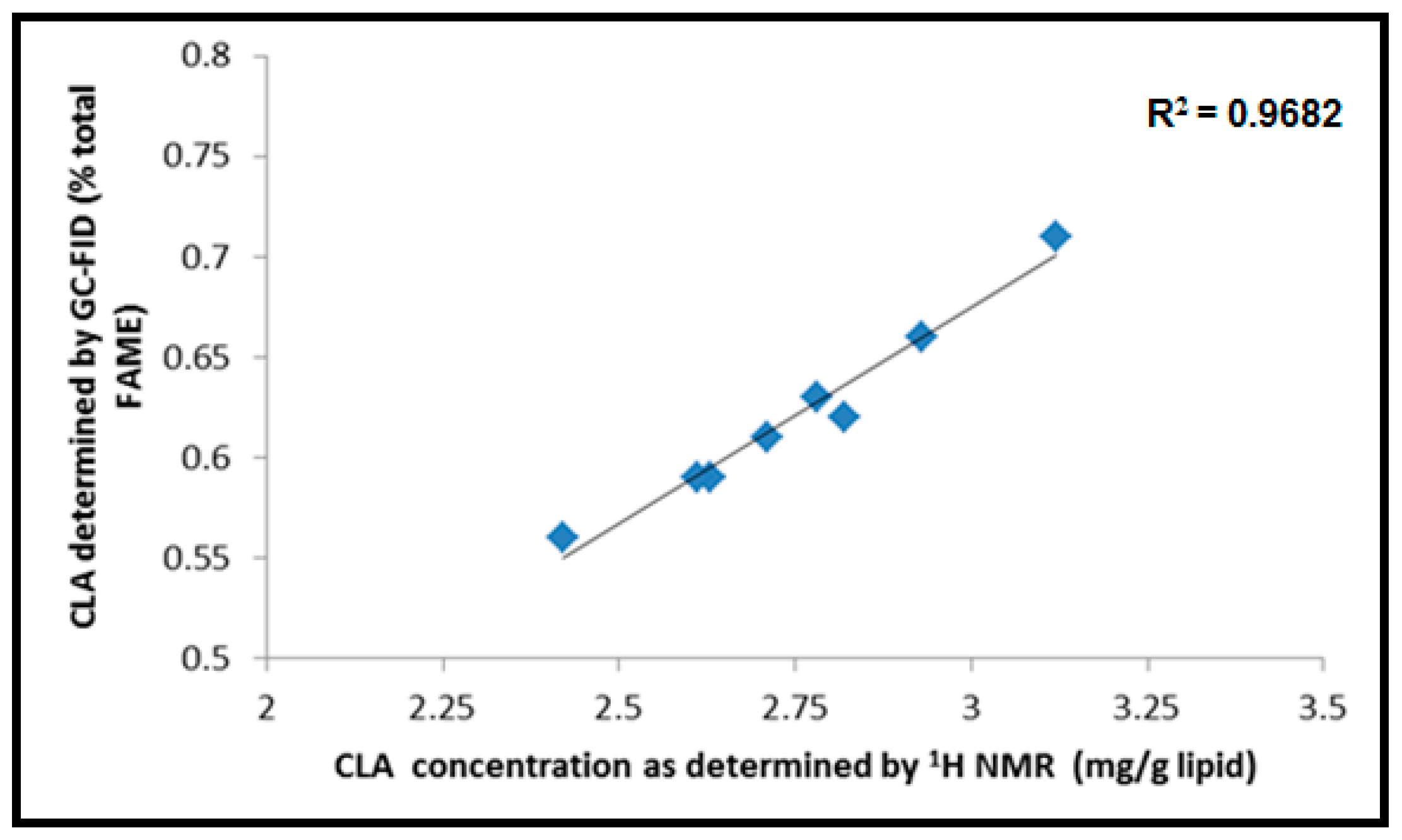
2.6. 1H NMR CLA Identification and Quantitative Measurements
2.7. Statistical Analysis
3. Results and Discussion
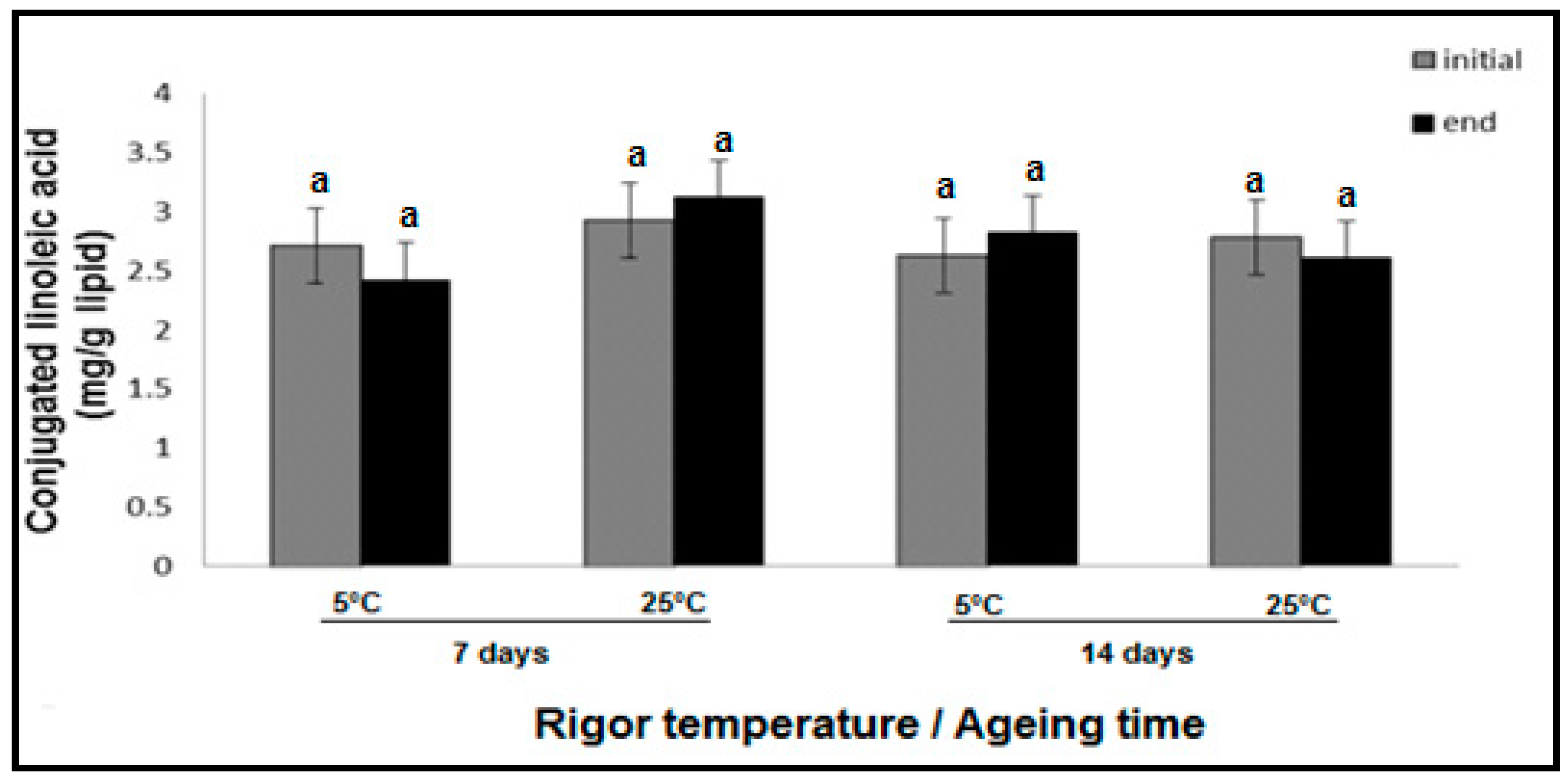
3.1. Effect of Rigor Temperature, Ageing and Display Time on CLA Concentration in Hot Boned Beef SM Muscle
3.2. Effect of Rigor Temperature, Ageing and Display Time on Cholesterol and Its Oxidative Stability in Hot Boned Beef SM Muscle
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmid, A.; Collomb, M.; Sieber, R.; Bee, G. Conjugated linoleic acid in meat and meat products: A review. Meat Sci. 2006, 73, 29–41. [Google Scholar] [CrossRef] [PubMed]
- French, P.; Stanton, C.; Lawless, F.; O’riordan, E.; Monahan, F.; Caffrey, P.; Moloney, A. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. J. Anim. Sci. 2000, 78, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Pariza, M.; Ha, Y.L. Newly recognized anticarcinogenic fatty acids. In Antimutagenesis and Anticarcinogenesis Mechanisms II; Springer: Berlin/Heidelberg, Germany, 1990; pp. 167–170. [Google Scholar]
- Manzano, M.R.; Colnago, L.A.; Aparecida Forato, L.; Bouchard, D. Fast and simple nuclear magnetic resonance method to measure conjugated linoleic acid in beef. J. Agric. Food Chem. 2010, 58, 6562–6564. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.G.; Kelsey, J.A.; Bauman, D.E. Analysis of variation in cis-9, trans-11 conjugated linoleic acid (CLA) in milk fat of dairy cows. J. Dairy Sci. 2002, 85, 2164–2172. [Google Scholar] [CrossRef] [Green Version]
- Raes, K.; Haak, L.; Balcaen, A.; Claeys, E.; Demeyer, D.; De Smet, S. Effect of linseed feeding at similar linoleic acid levels on the fatty acid composition of double-muscled Belgian Blue young bulls. Meat Sci. 2004, 66, 307–315. [Google Scholar] [CrossRef]
- Jiang, T.; Busboom, J.R.; Nelson, M.L.; O’Fallon, J.; Ringkob, T.P.; Joos, D.; Piper, K. Effect of sampling fat location and cooking on fatty acid composition of beef steaks. Meat Sci. 2010, 84, 86–92. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Mann, N.J.; Sinclair, A.J. Effect of feeding systems on omega-3 fatty acids, conjugated linoleic acid and trans fatty acids in Australian beef cuts, potential impact on human health. Asia Pac. J. Clin. Nutr. 2006, 15, 21–29. [Google Scholar]
- Vicente, S.J.; Sampaio, G.R.; Ferrari, C.K.; Torres, E.A. Oxidation of cholesterol in foods and its importance for human health. Food Rev. Inter. 2012, 28, 47–70. [Google Scholar] [CrossRef]
- Hur, S.J.; Park, G.B.; Joo, S.T. Formation of cholesterol oxidation products (COPs) in animal products. Food Cont. 2007, 18, 939–947. [Google Scholar] [CrossRef]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Robins, S.J.; Brunengraber, H. Origin of biliary cholesterol and lecithin in the rat: Contribution of new synthesis and preformed hepatic stores. J. Lipid Res. 1982, 23, 604. [Google Scholar] [PubMed]
- Chizzolini, R.; Zanardi, E.; Dorigoni, V.; Ghidini, S. Calorific value and cholesterol content of normal and low-fat meat and meat products. Trends Food Sci. Technol. 1999, 10, 119–128. [Google Scholar] [CrossRef]
- Smith, L.L. Cholesterol autoxidation. Chem. Phys. Lipids 1987, 44, 87–125. [Google Scholar] [CrossRef]
- Ferioli, F.; Dutta, P.C.; Caboni, M.F. Cholesterol and lipid oxidation in raw and pan-fried minced beef stored under aerobic packaging. J. Sci. Food Agric. 2010, 90, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Lercker, G.; Rodriguez-Estrada, M.; Guardiola, F.; Dutta, P.; Codony, R.; Savage, G. Cholesterol Oxidation Mechanisms. Cholesterol and Phytosterol Oxidation Products: Analysis, Occurrence, and Biological Effects; American Oil Chemistry Society Press: Champaign, IL, USA, 2002; pp. 1–25. [Google Scholar]
- Schroepfer, G.J., Jr. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Cardenia, V.; Rodriguez-estrada, M.T. Cholesterol photosensitized oxidation in muscle foods. Eur. J. Lipid Sci. Technol. 2012, 114, 644–655. [Google Scholar] [CrossRef]
- Paniangvait, P.; King, A.; Jones, A.; German, B. Cholesterol oxides in foods of animal origin. J. Food Sci. 1995, 60, 1159–1174. [Google Scholar] [CrossRef]
- Cardenia, V.; Rodriguez-Estrada, M.T.; Boselli, E.; Lercker, G. Cholesterol photosensitized oxidation in food and biological systems. Biochimie 2012, 95, 473–481. [Google Scholar] [CrossRef]
- Rodriguez-Estrada, M.T.; Garcia-Llatas, G.; Lagarda, M.J. 7-Ketocholesterol as marker of cholesterol oxidation in model and food systems: When and how. Biochem. Biophy. Res. Comm. 2014, 446, 792–797. [Google Scholar] [CrossRef]
- Mungure, T.E.; Bekhit, A.E.D.A.; Birch, E.J.; Stewart, I. Effect of rigor temperature, ageing and display time on the meat quality and lipid oxidative stability of hot boned beef Semimembranosus muscle. Meat Sci. 2016, 114, 146–153. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Li, S.; Ahn, D.; Cherian, G.; Chung, T.; Sim, J. Dietary oils and tocopherol supplmentation on cholesterol oxide formation in freeze-dried chicken meat during storage. J. Food Lipids 1996, 3, 27–42. [Google Scholar] [CrossRef]
- Van Wijngaarden, D. Modified rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 1967, 39, 848–849. [Google Scholar] [CrossRef]
- Prema, D.; Pilfold, J.L.; Krauchi, J.; Church, J.S.; Donkor, K.K.; Cinel, B. Rapid Determination of Total Conjugated Linoleic Acid Content in Select Canadian Cheeses by 1H NMR Spectroscopy. J. Agric. Food Chem. 2013, 61, 9915–9921. [Google Scholar] [CrossRef] [PubMed]
- Barison, A.; Pereira da Silva, C.W.; Campos, F.R.; Simonelli, F.; Lenz, C.A.; Ferreira, A.G. A simple methodology for the determination of fatty acid composition in edible oils through 1H NMR spectroscopy. Magn. Reson. Chem. 2010, 48, 642–650. [Google Scholar] [CrossRef]
- Prema, D.; Turner, T.D.; Jensen, J.; Pilfold, J.L.; Church, J.S.; Donkor, K.K.; Cinel, B. Rapid determination of total conjugated linoleic acid concentrations in beef by 1H NMR spectroscopy. J. Food Comp. Anal. 2015, 41, 54–57. [Google Scholar] [CrossRef]
- Igarashi, T.; Aursand, M.; Hirata, Y.; Gribbestad, I.; Wada, S.; Nonaka, M. Nondestructive quantitative acid and n-3 fatty acids in fish oils by high-resolution 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 737–748. [Google Scholar] [CrossRef]
- Shantha, N.C.; Crum, A.D.; Decker, E.A. Evaluation of conjugated linoleic acid concentrations in cooked beef. J. Agric. Food Chem. 1994, 42, 1757–1760. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, Z. Oxidative stability of conjugated linoleic acids relative to other polyunsaturated fatty acids. J. Am. Oil Chem. Soc. 1997, 74, 1611–1613. [Google Scholar] [CrossRef]
- Mir, P.S.; McAllister, T.A.; Scott, S.; Aalhus, J.; Baron, V.; McCartney, D.; Charmley, E.; Goonewardene, L.; Basarab, J.; Okine, E. Conjugated linoleic acid–enriched beef production. Am. J. Clin. Nutr. 2004, 79, 1207S–1211S. [Google Scholar] [CrossRef]
- Wijesundera, C.; Ceccato, C.; Watkins, P.; Fagan, P.; Fraser, B.; Thienthong, N.; Perlmutter, P. Docosahexaenoic acid is more stable to oxidation when located at the sn-2 position of triacylglycerol compared to sn-1 (3). J. Am. Oil Chem. Soc. 2008, 85, 543–548. [Google Scholar] [CrossRef]
- Shantha, N.C. Comparison of methylation methods for the quantitation of conjugated linoleic acid isomers. J. Am. Oil Chem. Soc. 1993, 76, 644–649. [Google Scholar]
- Wheeler, T.; Davis, G.; Stoecker, B.; Harmon, C. Cholesterol concentration of longissimus muscle, subcutaneous fat and serum of two beef cattle breed types. J. Anim. Sci. 1987, 65, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.K.; Prusa, K.J.; Hughes, K.V. Cholesterol content and sensory analysis of ground beef as influenced by fat level, heating, and storage. J. Food Sci. 1986, 51, 1162–1165. [Google Scholar] [CrossRef]
- Alina, A.; Shazamawati, Z.; Nor’Atiqah, N.; Juhana, M.T.; Juriani, J.; Syamsul, K.; Mashitoh, A.S. Effect of storage on fatty acid methyl ester (FAME) and cholesterol oxidation products (COPs) in Different Type of Sausages. World Appl. J. 2012, 17, 45–50. [Google Scholar]
- Linseisen, J.; Hoffmann, J.; Riedl, J.; Wolfram, G. Effect of a single oral dose of antioxidant mixture (vitamin E, carotenoids) on the formation of cholesterol oxidation products after ex vivo LDL oxidation in humans. Eur. J. Med. Res. 1998, 3, 5–12. [Google Scholar]
- Du, M.; Nam, K.; Ahn, D. Cholesterol and Lipid Oxidation Products in Cooked Meat as Affected by Raw Meat Packaging and Irradiation and by Cooked Meat Packaging and Storage Time. J. Food Sci. 2001, 66, 1396–1401. [Google Scholar] [CrossRef]
- Guardiola, F.; Codony, R.; Rafecas, M.; Grau, A.; Jordán, A.; Boatella, J. Oxysterol Formation in Spray-Dried Egg Processed and Stored under Various Conditions: Prevention and Relationship with Other Quality Parameters. J. Agric. Food Chem. 1997, 45, 2229–2243. [Google Scholar] [CrossRef]

| 7 Days Post-Mortem (µg COPs/g Lipid) | 14 Days Post-Mortem (µg COPs/g Lipid) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Display (Initial) | Post-Display (End) | Pre-Display (Initial) | Post-Display (End) | |||||
| Rigor (°C) | 5 °C | 25 °C | 5 °C | 25 °C | 5 °C | 25 °C | 5 °C | 25 °C |
| 7α- and 7β-HC | Nd | nd | 7.2 ± 0.4 ab | 10.6 ± 0.8 b | 6.2 ± 0.6 b | 8.4 ± 0.7 b | 11.8 ± 1.2 cd | 12.3 ± 1.6 d |
| 7-KC | Nd | nd | 6.3 ± 0.9 a | 8.8 ± 0.5 ab | 6.8 ± 1.5 ab | 7.8 ± 1.8 b | 13.5 ± 1.7 c | 15.6 ± 0.9 c |
| α- and β-epoxide | Nd | nd | nd | nd | nd | nd | nd | nd |
| 20α-HC | Nd | nd | nd | nd | nd | nd | nd | nd |
| Total COPs | - | - | 13.5 ± 0.5 ab | 19.4 ± 0.6 b | 13.0 ± 1.3 a | 16.2 ± 1.4 b | 25.3 ± 1.6 c | 27.9 ± 1.5 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungure, T.E.; Birch, E.J.; Ponnampalam, E.N.; Stewart, I.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Bekhit, A.E.-D.A. Conjugated Linoleic Acid and Cholesterol Oxidative Products Generated in Hot Boned Beef Semimembranosus Muscle as Affected by Rigor Temperature, Ageing and Display Time. Foods 2020, 9, 43. https://doi.org/10.3390/foods9010043
Mungure TE, Birch EJ, Ponnampalam EN, Stewart I, Ahmed IAM, Al-Juhaimi FY, Bekhit AE-DA. Conjugated Linoleic Acid and Cholesterol Oxidative Products Generated in Hot Boned Beef Semimembranosus Muscle as Affected by Rigor Temperature, Ageing and Display Time. Foods. 2020; 9(1):43. https://doi.org/10.3390/foods9010043
Chicago/Turabian StyleMungure, Tanyaradzwa E., E. John Birch, Eric N. Ponnampalam, Ian Stewart, Isam A. Mohamed Ahmed, Fahad Y. Al-Juhaimi, and Alaa El-Din A. Bekhit. 2020. "Conjugated Linoleic Acid and Cholesterol Oxidative Products Generated in Hot Boned Beef Semimembranosus Muscle as Affected by Rigor Temperature, Ageing and Display Time" Foods 9, no. 1: 43. https://doi.org/10.3390/foods9010043






