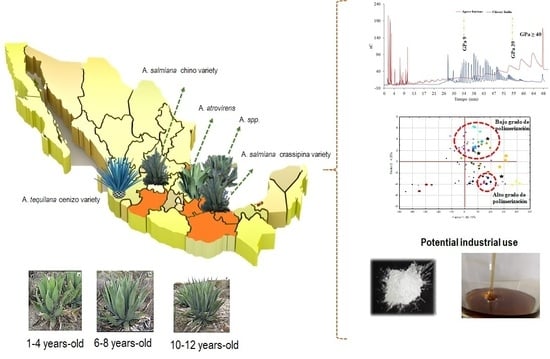
Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction
2.3. Physicochemical Properties
2.4. Mid-Infrared Spectroscopy (Mid-Ir) and Principal Component Analysis (Pca)
2.5. Fructas Distribution Profiles
High-Performance Anion-Exchange Chromatography (HPAEC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Mid-Infrared Spectroscopy (MID-IR) and Principal Component Analysis (PCA)
3.3. Distribution Profile of Fructans
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DPa | Apparent degree of polymerization |
| DRS | Direct reducing sugar |
| FEH | Fructan 1-exohydrolase |
| 1-FFT | Fructan:fructan 1-fructosyltransferase |
| 6-SFT | Sucrose:fructan 6-fructosyltransferase |
References
- Mellado-Mojica, E.; de la Vara, L.E.G.; López, M.G. Fructan active enzymes (FAZY) activities and biosynthesis of fructooligosaccharides in the vacuoles of Agave tequilana Weber blue variety plants of different age. Planta 2017, 245, 265–281. [Google Scholar] [CrossRef] [PubMed]
- García-Gamboa, R.; Ortiz-Basurto, R.I.; Calderon-Santoyo, M.; Bravo-Madrigal, J.; Ruiz-Alvarez, B.E.; González-Ávila, M. In vitro evaluation of prebiotic activity, pathogen inhibition and enzymatic metabolism of intestinal bacteria in the presence of fructans extracted from agave: A comparison based on polymerization degree. LTW Food Sci. Technol. 2018, 92, 380–387. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, D.E.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Bautista-Rosales, P.U.; Ragazzo-Sánchez, J.A. Effect of maltodextrin reduction and native agave fructans addition on the physicochemical properties of spray-dried mango and pineapple juices. Food Sci. Technol. Int. 2018, 24, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Hernández, J.A.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Prieto, C.; Lagaron, J.M. Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials 2018, 8, 868. [Google Scholar] [CrossRef] [PubMed]
- González-Ávila, M.; Prado-Ramírez, R.; Flores-Montaño, L.; Pérez-Martínez, O.; Ramírez, G.; Alonso-Segura, D. Evaluation of prebiotic potential of agave fructans from different regions of Colima and Zacatecas, Mexico. Int. J. Probiotics Prebiotics 2014, 9, 93–100. [Google Scholar]
- Moreno-Vilet, L.; García-Hernandez, M.H.; Delgado-Portales, R.E.; Corral-Fernandez, N.E.; Cortez-Espinosa, N.; Ruiz-Cabrera, M.A.; Portales-Perez, D.P. In vitro assessment of agave fructans (Agave salmiana) as prebiotics and immune system activators. Int. J. Biol. Macromol. 2014, 63, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Furlán, L.T.; Aldrete-Herrera, P.; Pérez-Padilla, A.; Ortiz-Basurto, R.I.; Campderrós, M.E. Assessment of agave fructans as lyoprotectants of bovine plasma proteins concentrated by ultrafiltration. Food Res. Int. 2014, 56, 146–158. [Google Scholar] [CrossRef]
- Zamora-Gasga, V.M.; Bello-Pérez, L.A.; Ortíz-Basurto, R.I.; Tovar, J.; Sáyago-Ayerdi, S.G. Granola bars prepared with Agave tequilana ingredients: Chemical composition and in vitro starch hydrolysis. LWT Food Sci. Technol. 2014, 56, 309–314. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Rubio-Ibarra, M.E.; Ragazzo-Sánchez, J.A.; Beristain, C.I.; Jiménez-Fernández, M. Microencapsulation of Eugenia uniflora L. juice by spray drying using fructans with different degrees of polymerization. Carbohydr. Polym. 2017, 175, 603–609. [Google Scholar] [CrossRef]
- Arrizon, J.; Hernández-Moedano, A.; Toksoy-Oner, E.; González-Ávila, M. In vitro prebiotic activity of fructans with different fructosyl linkage. Int. J. Probiotic Prebiotics 2014, 9, 69–76. [Google Scholar]
- Nagaraj, V.J.; Altenbach, D.; Galati, V.; Lüscher, M.; Meyer, A.D.; Boller, T.; Wiemken, A. Distinct regulation of sucrose: Sucrose-1-fructosyltransferase (1-SST) and sucrose: Fructan-6-fructosyltransferase (6-SFT), the key enzymes of fructan synthesis in barley leaves: 1-SST as the pacemaker. New Phytol. 2004, 161, 735–748. [Google Scholar] [CrossRef]
- García-Vieyra, M.I.; Del Real, A.; López, M.G. Agave Fructans: Their Effect on Mineral Absorption and Bone Mineral Content. J. Med. Food 2014, 17, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- De Roover, J.; Vandenbranden, K.; Van Laere, A.; Van den Ende, W. Drought induces fructan synthesis and 1-SST (sucrose: sucrose fructosyltransferase) in roots and leaves of chicory seedlings (Cichorium intybus L.). Planta 2000, 210, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Van den Ende, W.; Tillberg, J.E. Fructan accumulation induced by nitrogen deficiency in barley leaves correlates with the level of sucrose:fructan 6-fructosyltransferase mRNA. Planta 2000, 211, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Mojica, E.; Lopez, M.G. Fructan Metabolism in A. tequilana Weber Blue Variety along Its Developmental Cycle in the Field. J. Agric. Food Chem. 2012, 60, 11704–11713. [Google Scholar] [CrossRef]
- Zamora-Gasga, V.M.; Loarca-Piña, G.; Vazquez-Landaverde, P.A.; Ortiz-Basurto, R.I.; Tovar, J.; Sáyago-Ayerdi, S.G. In vitro colonic fermentation of food ingredients isolated from Agave tequilana Weber var. azul applied on granola bars. Food Sci. Technol. 2015, 60, 766–772. [Google Scholar] [CrossRef]
- Arrizon, J.; Morel, S.; Gschaedler, A.; Monsan, P. Comparison of the water-soluble carbohydrate composition and fructan structures of Agave tequilana plants of different ages. Food Chem. 2010, 122, 123–130. [Google Scholar] [CrossRef]
- Mancilla-Margalli, N.A.; López, M.G. Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Mojica, E.; López-Medina, T.L.; López, M.G. Developmental variation in Agave tequilana Weber var. azul stem carbohydrates. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2009, 3, 34–39. [Google Scholar]
- Wang, N.; Nobel, P.S. Phloem transport of fructans in the Crassulacean acid metabolism species Agave deserti. Plant Physiol. 1998, 116, 709–714. [Google Scholar] [CrossRef]
- Van den Ende, W.; Van Laere, A. Variation in the in vitro generated fructan pattern from sucrose as a function of the purified chicory root 1-SST and 1-FFT concentrations. J. Exp. Bot. 1996, 47, 1797–1803. [Google Scholar] [CrossRef]
- Cruz-García, H.; Enriquez-del Valle, J.R.; Velasco-Velasco, V.A.; Ruiz-Luna, J.; Campos-Angeles, G.V.; Aquino-García, D.E. Nutrients and carbohydrates in plants from Agave angustifolia Haw and Agave karwinskii Zucc. Rev. Mex. Cienc. Agrícolas 2013, 4, 1161–1173. [Google Scholar]
- Michel-Cuello, C.; Ortiz-Cerda, I.; Moreno-Vilet, L.; Grajales-Lagunes, A.; Moscosa-Santillán, M.; Bonnin, J.; Gonzàlez-Chavez, M.M.; Ruiz-Cabrera, M. Study of enzymatic hydrolysis of fructans from Agave salmiana characterization and kinetic assessment. Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, B.; Mathieu, A.S.; Van den Ende, W.; Vergauwen, R.; Pèrilleux, C.; Javaux, M.; Lutts, S. Water stress drastically reduces root growth and inulin yield in Cichorium intybus (var. sativum) independently of photosynthesis. J. Exp. Bot. 2012, 63, 4359–4373. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, A.C.; de Carvalho, E.M.; de Lima Damasceno, B.P.G.; da Silva, P.C.D.; Converti, A.; Pessoa, A.; da Silva, J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crop. Prod. 2017, 108, 355–362. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, W.; Wang, Q.; Zhang, A.; Mu, H.; Bai, H.; Duan, J. Extraction optimization, characterization and immunity activity of polysaccharides from Fructus Jujubae. Carbohydr. Polym. 2014, 111, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Panchev, I.; Delchev, N.; Kovacheva, D.; Slavov, A. Physicochemical characteristics of inulins obtained from Jerusalem artichoke (Helianthus tuberosus L.). Eur. Food Res. Technol. 2011, 233, 889–896. [Google Scholar] [CrossRef]
- López, M.G.; Mancilla-Margalli, N.A.; Mendoza-Díaz, G. Molecular structures of fructans from Agave tequilana Weber var. azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Basurto, R.I.; Pourcelly, G.; Doco, T.; Williams, P.; Dornier, M.; Belleville, M.P. Analysis of the main components of the Aguamiel produced by the Maguey-Pulquero (Agave mapisaga) throughout the harvest period. J. Agric. Food Chem. 2008, 56, 3682–3687. [Google Scholar] [CrossRef] [PubMed]
- Praznik, W.; Löppert, R.; Cruz-Rubio, J.M.; Zangger, K.; Huber, A. Structure of fructo-oligosaccharides from leaves and stem of Agave tequilana Weber, var. azul. Carbohydr. Res. 2013, 381, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Montañez-Soto, J.; Venegas-González, J.; Vivar-Vera, M.; Ramos-Ramírez, E. Los fructanos contenidos en la cabeza y en las hojas del Agave tequilana Weber azul. Bioagro 2011, 23, 199–206. [Google Scholar]





| Agave Variety | Age (Years) | Leaf to Leaf Length (cm) | Nomenclature | Direct Reducing Sugars (g·100 g−1) | Fructans (g·100 g−1) |
|---|---|---|---|---|---|
| A. spp. | 2–4 | 57.0 ± 11.34 a | A.s1 | 23.63 ± 0.15 a | 76.40 ± 0.15 a |
| A. spp. | 6–8 | 183.0 ± 8.64 c | A.s2 | 17.33 ± 0.3 b | 82.67 ± 0.3 b |
| A. spp. | 10–12 | 255.33 ± 49.10 d | A.s3 | 14.06 ± 0.12 c | 85.90 ± 0.12 c |
| A. atrovirens | 2–4 | 51.66 ± 13.91 a | A.a1 | 24.21 ± 0.40 a | 75.80 ± 0.40 a |
| A. atrovirens | 6–8 | 246.33 ± 55.40 d | A.a2 | 12.72 ± 0.65 c | 87.28 ± 0.65 c |
| A. atrovirens | 10–12 | 322.66 ± 1.24 f | A.a3 | 13.55 ± 0.23 c | 86.45 ± 0.23 c |
| A. salmiana spp. crassipina | 2–4 | 74.0 ± 14.16 ab | A.s-c1 | 22.70 ± 1.06 a | 77.30 ± 1.06 a |
| A. salmiana spp. crassipina | 6–8 | 264.0 ± 18.49 de | A.s-c2 | 11.92 ± 0.92 c | 88.08 ± 0.92 c |
| A. salmiana spp. crassipina | 10–12 | 308.0 ± 14.89 ef | A.s-c3 | 13.08 ± 1.34 c | 86.90 ± 1.34 c |
| A. tequilana cenizo variety | 2–4 | 56.83 ± 4.36 a | A.t-c1 | 21.30 ± 1.16 a | 78.30 ± 1.16 a |
| A. tequilana cenizo variety | 6–8 | 97.5 ± 24.60 ab | A.t-c2 | 18.55 ± 0.45 b | 81.45 ± 0.45 b |
| A. tequilana cenizo variety | 10–12 | 85.66 ± 7.76 ab | A.t-c3 | 10.38 ± 0.87 c | 89.70 ± 0.87 c |
| A. salmiana chino variety | 2–4 | 104.33 ± 14.61 b | A.s-ch1 | 26.40 ± 1.07 a | 73.60 ± 1.07 a |
| A. salmiana chino variety | 6–8 | 163.0 ± 14.56 c | A.s-ch2 | 18.03 ± 0.84 b | 81.97 ± 0.84 b |
| A. salmiana chino variety | 10–12 | 187.83 ± 21.83 c | A.s-ch3 | 13.08 ± 0.77 c | 86.90 ± 0.77 c |
| Variety | Age (years) | DPa 1–2 | DPa 3–9 | DPa 10–39 | DPa > 40 |
|---|---|---|---|---|---|
| A. spp. | 2–4 | 48.69 ± 7.91 | 30.21 ± 8.66 | 10.13 ± 4.61 | --- |
| A. spp. | 6–8 | 41.47 ± 4.76 | 53.73 ± 22.35 | --- | --- |
| A. spp. | 10–12 | 28.21 ± 14.47 | 40.06 ± 6.86 | 6.46 ± 3.71 | --- |
| A. atrovirens | 2–4 | 38.11 ± 5.03 | 35.65 ± 4.57 | 10.82 ± 5.32 | 4.53 ± 1.19 |
| A. atrovirens | 6–8 | 34.05 ± 9.83 | 41.79 ± 6.2 | 1.41 ± 0.09 | --- |
| A. atrovirens | 10–12 | 27.99 ± 8.09 | 49.51 ± 14.19 | 2.08 ± 1.42 | --- |
| A. salmiana spp. crassipina | 2–4 | 22.16 ± 0.60 | 51.84 ± 17.53 | --- | --- |
| A. salmiana spp. crassipina | 6–8 | 44.53 ± 10.65 | 36.80 ± 8.25 | 1.43 ± 0.17 | --- |
| A. salmiana spp. crassipina | 10–12 | 25.05 ± 12.62 | 32.66 ± 9.51 | 4.3 ± 1.37 | --- |
| A. tequilana cenizo variety | 2–4 | 41.11 ± 1.56 | 49.32 ± 5.79 | 2.16 ± 1.08 | --- |
| A. tequilana cenizo variety | 6–8 | 21.06 ± 1.50 | 60.77 ± 4.59 | 10.73 ± 5.96 | 4.20 ± 1.19 |
| A. tequilana cenizo variety | 10–12 | 14.21 ± 1.45 | 28.04 ± 7.93 | 43.10 ± 1.74 | 14.53 ± 2.12 |
| A. salmiana chino variety | 2–4 | 34.55 ± 12.22 | 39.56 ± 8.53 | 4.37 ± 1.15 | --- |
| A. salmiana chino variety | 6–8 | 30.19 ± 6.23 | 50.76 ± 4.93 | 2.75 ± 1.53 | --- |
| A. salmiana chino variety | 10–12 | 22.76 ± 13.66 | 56.07 ± 14.61 | 3.46 ± 0.57 | --- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldrete-Herrera, P.I.; López, M.G.; Medina-Torres, L.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-Ávila, M.; Ortiz-Basurto, R.I. Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use. Foods 2019, 8, 404. https://doi.org/10.3390/foods8090404
Aldrete-Herrera PI, López MG, Medina-Torres L, Ragazzo-Sánchez JA, Calderón-Santoyo M, González-Ávila M, Ortiz-Basurto RI. Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use. Foods. 2019; 8(9):404. https://doi.org/10.3390/foods8090404
Chicago/Turabian StyleAldrete-Herrera, Pamela I., Mercedes G. López, Luis Medina-Torres, Juan A. Ragazzo-Sánchez, Montserrat Calderón-Santoyo, Marisela González-Ávila, and Rosa I. Ortiz-Basurto. 2019. "Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use" Foods 8, no. 9: 404. https://doi.org/10.3390/foods8090404
APA StyleAldrete-Herrera, P. I., López, M. G., Medina-Torres, L., Ragazzo-Sánchez, J. A., Calderón-Santoyo, M., González-Ávila, M., & Ortiz-Basurto, R. I. (2019). Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use. Foods, 8(9), 404. https://doi.org/10.3390/foods8090404