Effect of Non-Conventional Drying Methods on In Vitro Starch Digestibility Assessment of Cooked Potato Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Source Material
2.2. Cooking
2.3. Moisture Content
2.4. Drying Treatments
2.5. Starch Digestibility Assessment
2.6. Fluorescent Microscopy
2.7. Scanning Electron Microscopy (SM)
2.8. Statistical Analysis
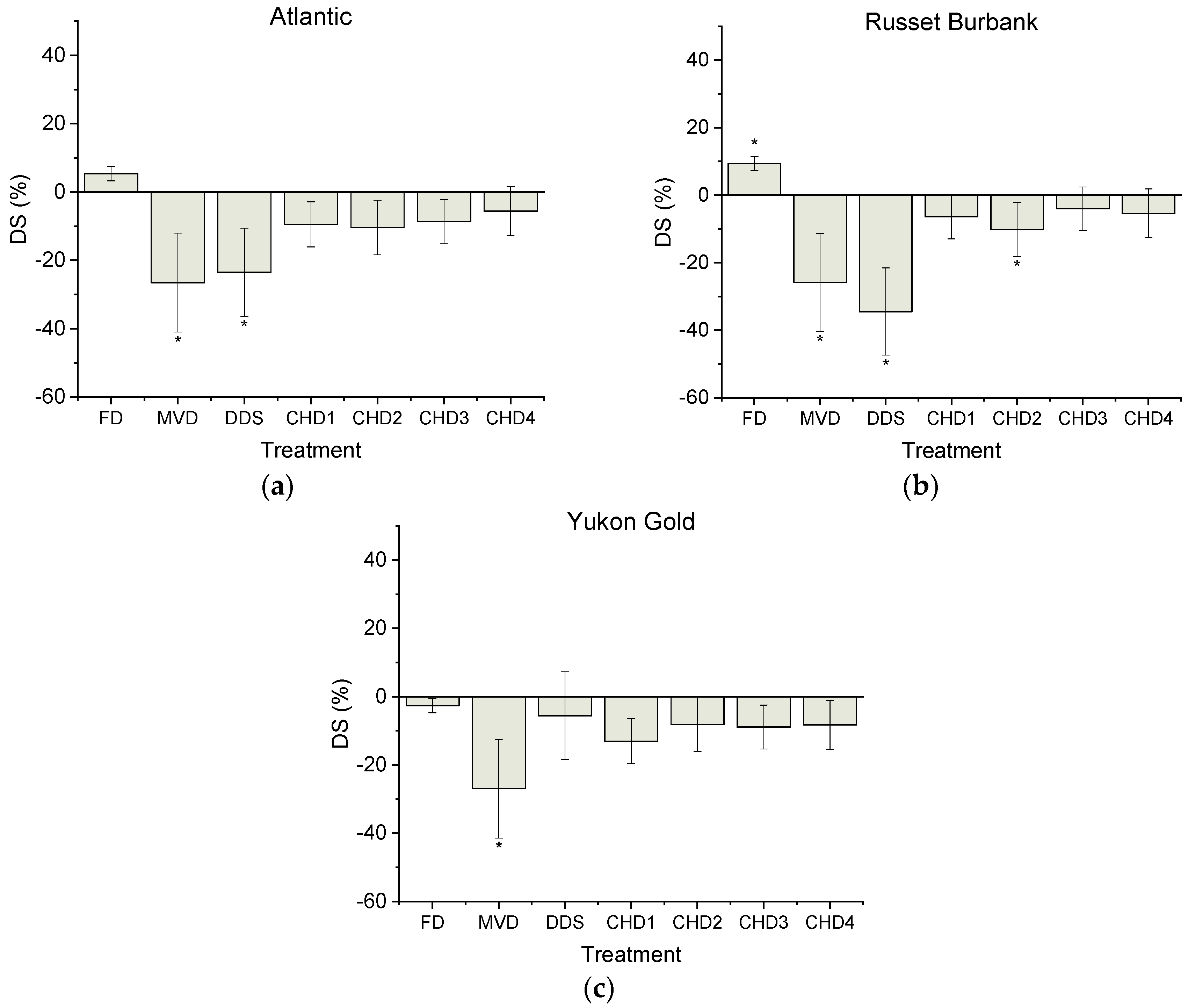
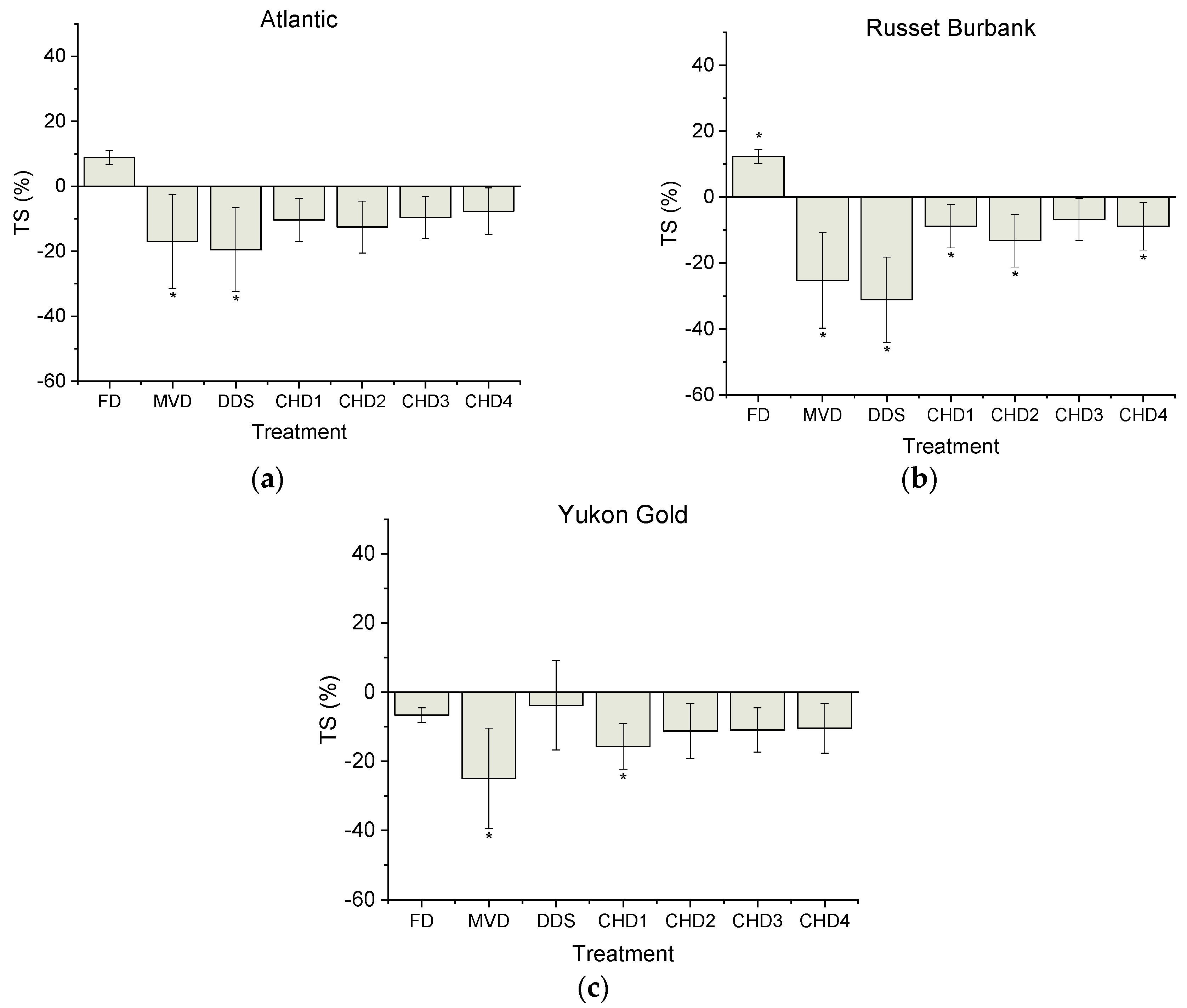
3. Results
3.1. Starch Profile
3.2. Microscopic Observations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Xu, F.; Wu, Y.; Hu, H.-H.; Dai, X.-F. Progress of potato staple food research and industry development in China. J. Integr. Agric. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Ek, K.L.; Wang, S.; Copeland, L.; Brand-Miller, J.C. Discovery of a low-glycaemic index potato and relationship with starch digestion in vitro. Br. J. Nutr. 2014, 111, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Kubow, S.; Donnelly, D. Potatoes and Human Health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Chaminda Jayawardana, B.; Liyanage, R. Health-beneficial properties of potato and compounds of interest. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Lovat, C.; Nassar, A.M.; Kubow, S.; Li, X.Q.; Donnelly, D.J. Metabolic biosynthesis of potato (Solanum tuberosum L.) antioxidants and implications for human health. Crit. Rev. Food Sci. Nutr. 2016, 56, 2278–2303. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial phytochemicals in potato—A review. Food Res. Int. 2013, 50, 487–496. [Google Scholar] [CrossRef]
- Bach, S.; Yada, R.Y.; Bizimungu, B.; Fan, M.; Sullivan, J.A. Genotype by environment interaction effects on starch content and digestibility in potato (Solanum tuberosum L.). J. Agric. Food Chem. 2013, 61, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Tang, J.; Ji, Y.; Berrios, J.J.; Powers, J.R. Colored potatoes (Solanum tuberosum L.) dried for antioxidant-rich value-added foods. J. Food Process. Preserv. 2011, 35, 571–580. [Google Scholar] [CrossRef]
- Bergthaller, W. Developments in potato starches. In Starch in Food; Eliasson, A.-C., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 241–257. [Google Scholar]
- Ek, K.L.; Brand-Miller, J.; Copeland, L. Glycemic effect of potatoes. Food Chem. 2012, 133, 1230–1240. [Google Scholar] [CrossRef]
- Bergthaller, W.; Hollmann, J.S. Starch. In Comprehensive Glycoscience; Kamerling, H., Ed.; Elsevier: Oxford, UK, 2007; Volume 2, pp. 579–612. [Google Scholar]
- Ottenhof, M.-A.; Farhat, I.A. Starch retrogradation. Biotechnol. Genet. Eng. Rev. 2004, 21, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes and cardiovascular disease. J. Am. Med. Assoc. 2002, 287, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.Z.; Ramadan, M.F. Degradability of different phosphorylated starches and thermoplastic films prepared from corn starch phosphomonoesters. Starch Stärke 2001, 53, 317–322. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Tang, J. Impact of food processing on the glycemic index (GI) of potato products. Food Res. Int. 2014, 56, 35–46. [Google Scholar] [CrossRef]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of dietary resistant starch on the human gut microbiome, metaproteome and metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.E.; Leeman, A.M.; Björck, I.M.E.; Eliasson, A.-C. Some physical and nutritional characteristics of genetically modified potatoes varying in amylose/amylopectin ratios. Food Chem. 2007, 100, 136–146. [Google Scholar] [CrossRef]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Xia, J.; Zhu, D.; Wang, R.; Cui, Y.; Yan, Y. Crop resistant starch and genetic improvement: A review of recent advances. Theor. Appl. Genet. Int. J. Plant Breed. Res. 2018, 131, 2495–2511. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, K.; Hasjim, J.; Li, E.; Flanagan, B.M.; Gidley, M.J.; Dhital, S. Freeze-drying changes the structure and digestibility of B-polymorphic starches. J. Agric. Food Chem. 2014, 62, 1482–1491. [Google Scholar] [CrossRef]
- Mishra, S.; Monro, J.; Neilson, P. Starch Digestibility and Dry Matter Roles in the Glycemic Impact of Potatoes. Am. J. Potato Res. 2012, 89, 465–470. [Google Scholar] [CrossRef]
- Larder, C.E.; Abergel, M.; Kubow, S.; Donnelly, D.J. Freeze-drying affects the starch digestibility of cooked potato tubers. Food Res. Int. 2018, 103, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Sjöö, M.; Eliasson, A.-C.; Autio, K. Comparison of different microscopic methods for the study of starch and other components within potato cells. Foods 2009, 3, 39–44. [Google Scholar]
- Cottrell, J.E.; Duffus, C.M.; Paterson, L.; Mackay, G.R. Properties of potato starch: Effects of genotype and growing conditions. Phytochemistry 1995, 40, 1057–1064. [Google Scholar] [CrossRef]
- Jansky, S.H.; Fajardo, D.A. Tuber starch amylose content is associated with cold-induced sweetening in potato. Food Sci. Nutr. 2014, 2, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.; Juhel, F.; Louka, N.; Allaf, K. A Study of dehydration of fish using successive pressure drops (DDS) and controlled instantaneous pressure drop (DIC). Dry. Technol. 2004, 22, 457–478. [Google Scholar] [CrossRef]
- Mounir, S.; Allaf, T.; Mujumdar, A.S.; Allaf, K. Swell drying: Coupling instant controlled pressure drop DIC to standard convection drying processes to intensify transfer phenomena and improve quality—An overview. Dry. Technol. 2012, 30, 1508–1531. [Google Scholar] [CrossRef]
- Godbout, S.; Palacios, J.H.; Zegan, D.; Pacheco, S.G.; Coronado, A.P.; Marciniak, A.; Lagacé, R. Drying of wet agri-food residual matter via successive pressure drops: Effect of drying parameters. In Proceedings of the Canadian Society for Bioengineering 2016 Annual Conference, Halifax, NS, Canada, 3–6 July 2016. [Google Scholar]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Figiel, A.; Anna, M. Overall Quality of Fruits and Vegetables Products Affected by the Drying Processes with the Assistance of Vacuum-Microwaves. Int. J. Mol. Sci. 2016, 18, 71. [Google Scholar] [CrossRef]
- Lin, T.M.; Durance, T.D.; Scaman, C.H. Characterization of vacuum microwave, air and freeze dried carrot slices. Food Res. Int. 1998, 31, 111–117. [Google Scholar] [CrossRef]
- Baeghbali, V.; Niakousari, M.; Farahnaky, A. Refractance window drying of pomegranate juice: Quality retention and energy efficiency. Food Sci. Technol. 2016, 66, 34–40. [Google Scholar] [CrossRef]
- Baeghbali, V.; Niakousari, M.; Hadi Eskandari, M.; Ngadi, M.O. Combined ultrasound and infrared assisted conductive hydro-drying of apple slices. Dry. Technol. 2018. [Google Scholar] [CrossRef]
- Durance, T.D.; Fu, J.; Cao, L.B. Microwave Vacuum-Drying of Organic Materials. Patent No. 20150128442, 23 February 2016. [Google Scholar]
- Mishra, S.; Rana, A.; Tripathy, A.; Meda, V. Drying characteristics of carrot under microwave-vacuum condition. In Proceedings of the 2006-North Central Inter-Sectional Conference, St. Joseph, MI, USA, 5–7 October 2006. [Google Scholar]
- Mosqueda, M.R.P. Evaluation of Drying Technologies and Physico-Chemical Characterization of Wheat Distillers Dried Grain with Solubles (DDGS); University of Saskatchewan: Saskatoon, SK, Canada, 2014. [Google Scholar]
- Baeghbali, V.; Niakousari, M. Ultrasound and Infrared Assisted Conductive Hydro-Dryer. U.S. Patent 2018/0045462 A1, 15 February 2018. [Google Scholar]
- Englyst, H.; Wiggins, H.L.; Cummins, J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Cummings, J.H. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am. J. Clin. Nutr. 1985, 42, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Cummins, J.H. Digestion of the carbohydrates of banana (Musa paradisiaca sapientum) in the human small intestine. Am. J. Clin. Nutr. 1986, 44, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. lassification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar] [PubMed]
- Goni, I.; Garcia-Diz, E.; Manas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem. 1996, 56, 445–449. [Google Scholar] [CrossRef]
- Åkerberg, A.; Liljeberg, H.; Björck, I. Effects of amylose/amylopectin ratio and baking conditions on resistant starch formation and glycaemic indices. J. Cereal Sci. 1998, 2871–2880. [Google Scholar] [CrossRef]
- Champ, M. Determination of resistant starch in foods and food products: Interlaboratory study. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S51–S62. [Google Scholar]
- Pinhero, R.G.; Waduge, R.N.; Liu, Q.; Sullivan, J.A.; Tsao, R.; Bizimungu, B.; Yada, R.Y. Evaluation of nutritional profiles of starch and dry matter from early potato varieties and its estimated glycemic impact. Food Chem. 2016, 203, 356–366. [Google Scholar] [CrossRef]
- Apinan, S.; Yujiro, I.; Hidefumi, Y.; Takeshi, F.; Myllärinen, P.I.; Forssell, P.; Poutanen, K. Visual Observation of Hydrolyzed Potato Starch Granules by α-Amylase with Confocal Laser Scanning Microscopy. Starch Stärke 2007, 59, 543–548. [Google Scholar] [CrossRef]
- Mishra, S.; Monro, J.; Hedderley, D. Effect of processing on slowly digestible starch and resistant starch in potato. Starch Stärke 2008, 60, 500–507. [Google Scholar] [CrossRef]
- Aparicio-Saguilán, A.; Flores-Huicochea, E.; Tovar, J.; García-Suárez, F.; Gutiérrez-Meraz, F.; Bello-Pérez, L.A. Resistant starch-rich powders prepared by autoclaving of native and lintnerized banana starch: Partial characterization. Starch Stärke 2005, 57, 405–412. [Google Scholar] [CrossRef]
- Dodd, H.; Williams, S.; Brown, R.; Venn, B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am. J. Clin. Nutr. 2011, 94, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; González, M.C.; Morales, M.D.; Saura-Calixto, F. An approach to the influence of nutrients and other food constituents on resistant starch formation. Food Chem. 1997, 60, 527–532. [Google Scholar] [CrossRef]
- Odenigbo, A.; Rahimi, J.; Ngadi, M.; Amer, S.; Mustafa, A. Starch digestibility and predicted glycemic index of fried sweet potato cultivars. Funct. Foods Health Dis. 2012, 2, 280–287. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larder, C.E.; Baeghbali, V.; Pilon, C.; Iskandar, M.M.; Donnelly, D.J.; Pacheco, S.; Godbout, S.; Ngadi, M.O.; Kubow, S. Effect of Non-Conventional Drying Methods on In Vitro Starch Digestibility Assessment of Cooked Potato Genotypes. Foods 2019, 8, 382. https://doi.org/10.3390/foods8090382
Larder CE, Baeghbali V, Pilon C, Iskandar MM, Donnelly DJ, Pacheco S, Godbout S, Ngadi MO, Kubow S. Effect of Non-Conventional Drying Methods on In Vitro Starch Digestibility Assessment of Cooked Potato Genotypes. Foods. 2019; 8(9):382. https://doi.org/10.3390/foods8090382
Chicago/Turabian StyleLarder, Christina E., Vahid Baeghbali, Celeste Pilon, Michèle M. Iskandar, Danielle J. Donnelly, Sebastian Pacheco, Stephane Godbout, Michael O. Ngadi, and Stan Kubow. 2019. "Effect of Non-Conventional Drying Methods on In Vitro Starch Digestibility Assessment of Cooked Potato Genotypes" Foods 8, no. 9: 382. https://doi.org/10.3390/foods8090382
APA StyleLarder, C. E., Baeghbali, V., Pilon, C., Iskandar, M. M., Donnelly, D. J., Pacheco, S., Godbout, S., Ngadi, M. O., & Kubow, S. (2019). Effect of Non-Conventional Drying Methods on In Vitro Starch Digestibility Assessment of Cooked Potato Genotypes. Foods, 8(9), 382. https://doi.org/10.3390/foods8090382







