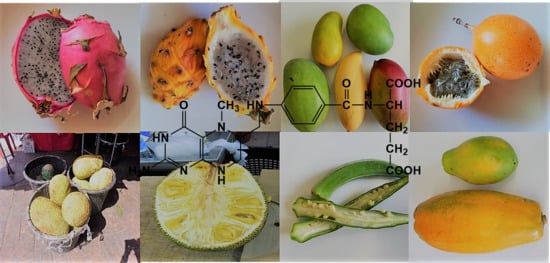
Different tropical foods, among them various fruits and several vegetables, were analyzed for their total folate content and vitamer profiles based on fresh weight. The most abundant folate vitamers in food, namely 5-CH3-H4folate, 5-CHO-H4folate, 10-CHO-PteGlu, H4folate, and PteGlu were determined. The fruits and vegetables analyzed in this study were selected according to their availability in German supermarkets and food markets. In this paper, the fruits were grouped according to their plant family whenever possible.
3.2. Folate Content in Guavas
A selection of guavas (Myrtaceae,
Psidium) of various origins, among them feijoa (
feijoa sellowiana), also named pineapple guava, were analyzed for their folate profiles (
Table 2). The different guavas were analyzed unpeeled and showed similar folate contents (43.1 ± 5.16 µg/100 g–47.9 ± 0.57 µg/100 g). Since all guavas are eaten peeled and unpeeled, whole Australian grown feijoa fruits as well as pulp and peel were analyzed separately. The whole fruit appeared to have a folate content of 91.0 ± 1.98 µg/100 g, the pulp was lower in folate with 64.4 ± 2.57 µg/100 g, and the peel showed the highest content of 103 ± 4.32 µg/100 g. By contrast, the USDA states a folate content of 49 µg/100 g for guavas [
23] and 23 µg/100 g for feijoas [
24]. However, Akilanathan et al. [
11] analyzed two different varieties of guavas and found also very different values ranging between 49.0 and 211 µg/100 g. The main vitamer in all guavas and feijoas analyzed was 5-CH
3-H
4folate.
3.3. Folate Content in Papayas
Papayas (Caricaceae,
Carica papaya) are popular fruits, originally coming from the American tropics, however, nowadays papayas are grown widely in the tropics and subtropics worldwide. We analyzed two papaya fruits as well as seeds and pulp separately. The results are shown in
Table 3. The papaya fruits revealed total folate contents of 61.6 ± 3.01 µg/100 g and 90.7 ± 1.24 µg/100 g. The folate content of the seeds (25.6 ± 5.91 µg/100 g and 41.2 ± 1.91 µg/100 g) was lower than that of the pulp (56.3 ± 1.48 µg/100 g and 90.8 ± 1.91 µg/100 g). Since the percentage share of seeds is very small compared to the pulp, the folate content of the pulp did not differ from the folate content of the whole fruit. Compared with different varieties analyzed in previous studies, our results are substantially higher. Akilanathan et al. [
11] analyzed two different papaya varieties using microbiological assays and only found 11.0 µg/100 g and 23.0 µg/100 g. In the USDA (United States Department of Agriculture) data base, papaya is listed with a folate content of 37.0 µg/100 g [
25]. The discrepancy of the analyzed folate content with Akilanathan et al. [
11] can possibly be traced back to the differing determination method. The latter group used a microbiological assay with PteGlu as calibration standard. As already discussed in the introduction usually there are no significant differences between the quantification methods, but the accuracy of the microbiological assay can vary with the chosen calibrant [
26]. As the main vitamer in papayas was 5-CH
3-H
4folate, PteGlu as a calibrant might have led to inaccuracies. Furthermore, an incomplete deconjugation could have led to underrated results, which we can exclude as we automatically test for deconjugation efficiency in each run. Apart from the methodological differences, the geographical origin and the different varieties can also be responsible for the inequality. Regarding the vitamer distribution, 5-CH
3-H
4folate was again the main vitamer in both fruits.
3.4. Folate Content in Jack Fruit
Jack fruit (Moraceae,
Artocarpus hereophyllus), with its well flavored yellow pulp, is originally from India and is now indigenous in the tropics worldwide. The folate contents of two individual fruits were 83.6 ± 5.50 (1) µg/100 g and 52.9 ± 2.61 (2) µg/100 g, and therefore in a similar range to mangos (
Table 4). Jack fruit seeds of fruit (1), which are embedded in the pulp and consisted of approximately around 10–15% of the total fruit had 51.1 ± 2.17 µg/100 g total folate. Furthermore, we analyzed commercially bought jack fruit chips and found 192 ± 3.38 µg/100 g total folate. The main vitamer in all analyzed jack fruit samples was 5-CH
3-H
4folate. The USDA specifies a total folate content for jack fruit of 24.0 µg/100 g and, consequently, we found a considerably higher folate content [
27]. Akilanathan et al. [
11] indicated a total folate content of 35 µg/100g, which is also a little lower than the analyzed content in the present study. As already discussed, the discrepancy in the total folate content can be caused by the different methods, the environmental impact and the variety of the fruits. Of note is the varying moisture content of the analyzed fruits compared to the literature, which can be caused by different ripening state or the different varieties. The moisture content of jack fruit (1) was 71.7% and that of jack fruit (2) was 79.7%, which may contribute to the rather high difference in folates of both fruits. In comparison to our samples, the moisture content of the jackfruit analyzed by Akilanathan et al. [
11] was 76.0%, and stated by USDA as being 73.5% [
27]. Due to the lack of information about the variety of the jackfruits, the reason of the different total folate content may only be assumed.
3.5. Folate Content in Other Tropical Fruits
The total folate content and vitamer profiles in a selection of mainstream and non-mainstream tropical fruits and vegetables is presented in
Table 5. Three different pitayas (Cactaceae,
Hylocereus cacti) were examined and total folate contents from 18.7 ± 0.11 µg/100 g, to 36.0 ± 0.53 µg/100 g were found. Chew et al. [
9] also analyzed the folate content of commonly consumed Malaysian foods using microbiological assays and found a much lower folate content in dragon fruit of only 3 µg/100 g. A similar folate content of 23.8 ± 0.44 µg/100 g was found in prickly pear (Cactaceae,
Opuntia ficus-indica). Salak (Arecaceae,
Salacca zalacca), mainly grown in Asia but also in European Mediterranean regions, had a total folate content of 27.3 ± 2.09 µg/100 g. However, in a previous study, Salak was found to have a very low folate content of 6 µg/100 g [
9]. The very popular fruits from the Longan-tree (Sapindaceae,
Dimorcarpus longan) were also analyzed, having 67.8 ± 0.12 µg/100 g total folate. In contrast, Longkong (Meliaceae,
Aglaia dookoo), which is present throughout South East Asia, appeared to be much lower in folate with only 15.9 ± 0.67 µg/100 g. Kaki (Ebenaceae,
Diospyros kaki) can be eaten peeled or unpeeled. A folate content of 40.5 ± 1.33 µg/100 g and 50.5 ± 0.09 µg/100 g was found for peeled and unpeeled fruit, respectively. Chew et al. [
9] analyzed a Korean persimmon (Pisang kaki) and again found a much lower folate content of only 6 µg/100 g, which is approximately seven times lower than our results. Lucuma (Sapotaceae,
Pouteria lucuma), a fruit species mainly originating from South America, was found to have 41.8 ± 5.37 µg/100 g total folate. Since the fruit is eaten fresh or as flour, we calculated the total folate content also on a dry weight basis (209 ± 5.37 µg/100 g).
Several fruits belonging to the Passifloraceae family were also analyzed and found to be very high in folates. Among them, sweet granadilla (Passiflora ligularis) had a folate content of 64.0 ± 1.70 µg/100 g, passion fruit (Passiflora edulis) of 136 ± 21.7 µg/100 g, and yellow passionfruit (Passiflora flavicarpa) with 271 ± 3.64 µg/100 g was highest in total folate.
Tamarind (Fabaceae,
Tamarindus indica) was quite low in folate with 11.4 ± 0.70 µg/100 g, whereas pete beans (Fabaceae,
Parkia speciosa) were substantially higher with 100 ± 3.26 µg/100 g. The popular fruit Chinese jujube (Rhamnaceae,
Ziziphus jujuba), mainly coming from China, had a folate content of 22.7 ± 0.23 µg/100 g. Cherimoya (Annonaceae,
Annona cherimola) was found to have 48.4 ± 0.57 µg/100 g total folate. Two different batches of okras (Malvaceae,
Abelmoschus esculentus), also known as Lady’s Finger, appeared to be a good natural source of folate with 101 ± 7.62 µg/100 g and 109 ± 3.91 µg/100 g, respectively. Okra as a good source of folate was already confirmed previously. Ismail et al. [
10] found 100 µg/100 g total folate in okra analyzed by HPLC-UV. Devi et al. [
28] found also a relative high folate content of 81 µg/100 g. Horned melons, also known as kiwano (Cucurbitaceae,
Cucumis metuliferus), contained 7.82 ± 0.17 µg/100 g and 10.2 ± 0.31 µg/100 g total folates. The USDA listed horned melon as having a total folate content of 3.00 µg/100 g [
29]. Furthermore, tamarillo, also known as tree tomato (Solanaceae,
Solanum betacea), had a relative low folate content of 16.4 ± 0.60 µg/100 g.
5-CH3-H4folate was the main vitamer in most of the analyzed fruit and vegetable samples, except in salak, tamarind and one of the horned melon samples. The relative amounts of the individual vitamers (individual vitamer vs. total folate content in %) were as follows: 5-CH3-H4folate (14.9% to 94.8%), 5-CHO-H4folate (3.17% to 41.3%), 10-CHO-PteGlu (0.48% to 48.1%), H4folate (0.71% to 13.6%), and PteGlu (0.52% to 22.9%).